Introduction
Salvia miltiorrhiza roots have traditionally
been used for the treatment of gynecological disorders in Chinese
medicine (1). S. miltiorrhiza
extracts (SME) have been suggested to decrease lipid levels and
inhibit inflammation (2–4), and have demonstrated cytotoxic effects
on cells derived from various types of human cancer, including the
lung, colon and pancreas (5–7). Previously, Tanshinone II-A, extracted
from S. miltiorrhiza, inhibited invasion and metastasis of
human carcinoma cells (8,9). More notably, certain studies have
demonstrated that SME exhibits antitumor effects in breast cancer
cells (10,11). However, the inhibitory effect of SME
on breast cancer invasion was not known.
Breast cancer is a malignant tumor with the ability
to spread beyond the breast tissue. The patients with breast cancer
exhibited a high mortality rate (12), primarily caused by metastasis and
invasion of cancer cells. Consequently, the primary approach for
breast cancer treatment has been the development of effective
anti-metastasis and anti-invasion drugs (13,14).
Invasion and metastasis of cancer cells are
characterized by the degradation of the extracellular matrix (ECM)
by proteases secreted from cancer cells (15,16). A
total of ~24 types of human matrix metalloproteinases (MMPs) are
synthesized and secreted from cells (17,18). Of
those, MMP-9 is well known as a key enzyme that regulates breast
cancer cell invasion (19). High
concentrations of MMP-9 were identified in breast cancer tissue
compared with those in normal breast tissue (20). A variety of stimulators such as tissue
plasminogen activator (TPA), tumor necrosis factor-α and epidermal
growth factor may increase the expression of MMP-9 in cancer cells
(21–24).
Cytokine and TPA-mediated MMP-9 expression is
controlled by the transcription factors, nuclear factor-κB (NF-κB)
and activator protein-1 (AP-1) in cancer cells (19,25,26). AP-1
is the collective name for referring to dimeric transcription
factor proteins composed of Jun proto-oncogene, Fos proto-oncogene
and the activated transcription factor protein families (27). Dimerized Jun/Fos bind to specific AP-1
sites of DNA, but their functions depend on cell type and
activating agent (28–31). NF-κB forms a complex with its cellular
protein inhibitor (inhibitory κBα; IκBα) and thereby remains
inactive in the cytoplasm (32). When
TPA phosphorylates IκB this complex dissociates, releasing NF-κB,
which then translocates to the nucleus where it interacts with DNA
binding sites on the MMP-9 promoter (33,34).
Previously, Hsieh and Wu (28)
suggested that S. miltiorrhiza roots have capacity to
regulate NF-κB.
In the present study, it has been hypothesized that
SME may exhibit anticancer properties through the inhibition of
cell invasion. Therefore, the molecular mechanism by which SME
affects the invasiveness of the breast cancer MCF-7 cell line was
investigated. SME reduced TPA-induced cell invasion via the
mitogen-activated protein kinase (MAPK)/AP-1 signaling pathways,
and decreased MMP-9 expression was associated with the extent of
the inhibition of breast cancer cell invasion.
Materials and methods
Cell and reagents
MCF-7 cells were acquired from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
Dulbecco's modified Eagle medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 1% antibiotics (10,000 U/ml
penicillin, 10,000 µg/ml streptomycin, 25 µg/ml amphotericin B) and
10% fetal bovine serum (Thermo Fisher Scientific, Inc.) at 37°C in
an incubator with 5% CO2 saturation. DAPI, TPA, dimethyl
sulfoxide and anti-β-actin antibody were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Antibodies against
MMP-9 (catalog no. 12759), inhibitory κ B kinase (IKK)α (catalog
no. 2682), IKKβ (catalog no. 2678), proliferating cell nuclear
antigen (PCNA; catalog no. 7907), IκBα (catalog no. 371),
transcription factor p65 (catalog no. 372), and horseradish
peroxidase (HRP)-conjugated immunoglobulin G (IgG) (catalog no.
2004, 2005) were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Antibodies against c-Jun N-terminal kinase (JNK;
catalog no. 9252), p38 (catalog no. 9212), extracellular
signal-regulated kinase (ERK; catalog no. 9102), phosphorylated
(p)-JNK (catalog no. 9252), p-p38 (catalog no. 9211), p-ERK
(catalog no. 9101), p-c-Jun (catalog no. 9261), p-IκBα (catalog no.
2859) and p-IKKα/β (catalog no. 2697) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Goat anti-rabbit
Alexa Fluor 568 [IgG heavy and light chains (H&L)] (catalog no.
A-11036) was obtained from Invitrogen (Thermo Fisher Scientific,
Inc.).
Preparation of S. miltiorrhiza
extract
Dried roots of S. miltiorrhiza Bunge were purchased
from Kwangmyungdang Medicinal Herbs Co., Ltd. (Ulsan, Korea) and
authenticated by Professor Guem-San Lee (Department of Herbology,
Wonkwang University School of Korean Medicine, Iksan, Korea): A
voucher specimen (WKU120302-SM201406A) was deposited at the
Department of Herbology, College of Korean Medicine, Wonkwang
University (Iksan, Korea). S. miltiorrhiza Radix powder (50
g) was extracted by sonication in 500 ml of 70% aqueous ethanol for
2 h, and then filtered through paper. This procedure was repeated
twice. Liquid from the extracted solution was evaporated under 40
mmHg by rotary evaporator, and the resulting product was
freeze-dried. The final extraction yield was 6.54% (w/w).
Determination of cell viability
MCF-7 cells were seeded on 96-well plates
(1.5×104 cells/well) and treated with 1, 5, 10, 25 and
50 µg/ml SME for 24 h at 37°C. Then, 100 µl EZ-CyTox assay reagent
(Daeil Lab Service Co., Ltd., Seoul, Republic of Korea) was added
100 times diluted to the plate wells. Next, the cells were
incubated for 30 min at 37°C prior to measuring the absorbance with
a 540-nm filter in an ELISA reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Zymography
To analyze the proteolytic activity of MMP-9,
zymography was performed as previously described (35). Areas of gelatinase activity were
detected as a white zone in a dark blue field.
Western blot analysis
Total protein extracts were prepared using an
ice-cold M-PER Mammalian Protein Extraction Reagent (Pierce; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Proteins were quantified using a BioSpec-nano Micro-volume
Spectrophotometer (Shimadzu; Columbia, USA). For each lane, 20 µg
protein was used. The protein samples were separated using SDS-PAGE
(10% gel) and transferred to Hybond™ polyvinylidene fluoride
membranes. The membranes were blocked with 2% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) or 5% skim milk for 2 h at 4°C.
Membranes were then incubated overnight at 4°C with primary
antibodies. β-actin (catalog no. A2228) was purchased from
Sigma-Aldrich (Merck KGaA). MMP-9 (catalog no. 12759), IKKα
(catalog no. 2682), IKKβ (catalog no. 2678), PCNA (catalog no.
7907), IκBα (catalog no. 371) and p65 (catalog no. 372) were
obtained from Santa Cruz Biotechnology, Inc. JNK (catalog no.
9252), p38 (catalog no. 9212), ERK (catalog no. 9102),
phosphorylated (p)-JNK (catalog no. 9252), p-p38 (catalog no.
9211), p-ERK (catalog no. 9101), p-c-Jun (catalog no. 9261), p-IκBα
(catalog no. 2859) and p-IKKα/β (catalog no. 2697) were purchased
from Cell Signaling Technology, Inc. All Antibodies used were
diluted at 1:2,000. Protein expression levels were measured by Mini
HD6 image analyzer using Alliance 1D software (UVItec, Cambridge,
UK) with Immobilon™ Western Chemilumi nescent HRP Substrate
(enhanced chemiluminescence) kit (EMD Millipore, Billerica, MA,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA isolation from cells was performed using the
FastPure™ RNA kit (Takara Bio, Inc., Otsu, Japan). Complementary
DNA was synthesized using a PrimeScript™ RT Reagent kit (Takara
Bio, Inc.). qPCR analysis was performed using the StepOnePlus™
Real-Time PCR System and SYBR Green (both Applied Biosystems;
Thermo Fisher Scientific, Inc.) to determine mRNA levels. The
thermocycling conditions were as follows: 50°C for 2 min and 95°C
for 10 min, followed by 50 cycles of 95°C for 15 sec and 60°C for 1
min. The primers used were as follows: MMP-9 (NM 004994) sense,
5′-CCTGGAGACCTGAGAACCAATCT-3′ and antisense,
5′-CCACCCGAGTGTAACCATAGC-3′; and GAPDH (NM 002046) sense,
5′-ATGGAAATCCCATCACCATCTT-3′ and antisense,
5′-CGCCCCACTTGATTTTGG-3′. The mRNA levels were normalized to the
GAPDH housekeeping gene expression levels. The method of
quantification was 2−ΔΔCq method (36).
Automated image acquisition and
processing
Cells were fixed with 4% paraformaldehyde at room
temperature for 30 min, and then washed three times with cold PBS.
Next, the cells were incubated for 45 min at room temperature with
blocking buffer to prevent nonspecific antibody binding. Then,
anti-p-c-Jun (red) antibodies (dilution, 1:1,000) were added and
the cells were incubated for 24 h at 4°C. Subsequently, the cells
were washed three times and then incubated for 1 h at room
temperature with DAPI (blue) and 1:1,000 diluted goat anti-rabbit
Alexa Fluor 568 (IgG H&L) in 0.1% triton X-100 for nuclear and
p-c-Jun staining. Images were captured by an ArrayScan™ VTI system
using Cellomics VHCS: View Software, version 1.6.30 (Cellomics,
Inc., Pittsburgh, PA, USA).
Cytoplasmic and nuclear
fractionation
MCF-7 cells (2×106) were treated with SME
and/or TPA for 4 h at 37°C. Following this, the cells were
centrifuged at 1,500 × g for 5 min at 4°C. Nuclear and cytoplasmic
extracts were separated by using NE-PER Nuclear and Cytoplasmic
Extraction Reagent (Pierce; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol.
Invasion and migration assays
The invasion (35) and
migration assays (37) were performed
as previously described. Cell growth medium (0.5 ml) with cells was
added to the upper chamber, and medium with TPA alone or with SME
was added to bottom wells.
Statistical analysis
The data were analyzed by one-way analysis of
variance and Duncan's multiple range tests using SAS software,
version 9.1; (SAS Institute Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of SME on TPA-induced MMP-9
expression and secretion in MCF-7 cells
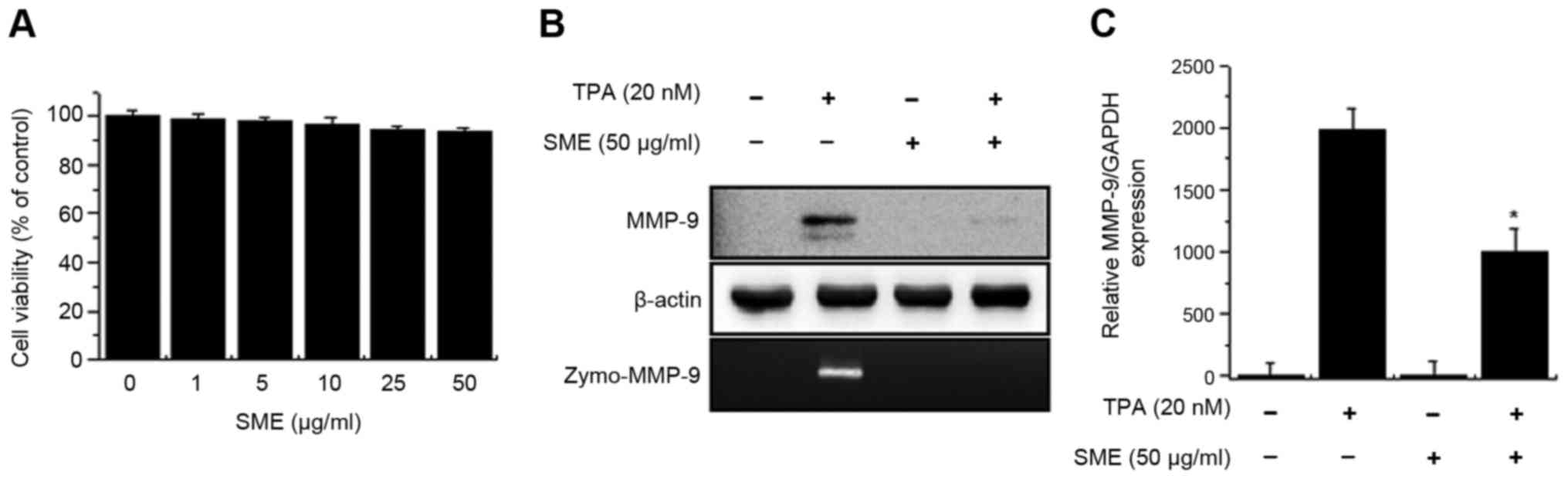
The treatment of cells with SME did not affect cell
viability until concentrations reached 50 µg/ml (Fig. 1A). On that basis, an SME concentration
of 50 µg/ml was used for subsequent experiments. In order to
investigate the effect of SME on the expression of MMP-9, cells
were pretreated with SME for 1 h and then incubated with TPA for 24
h at 37°C. TPA-mediated expression/secretion of MMP-9 was decreased
by pre-treatment with SME (Fig. 1B).
qPCR analysis revealed that SME significantly reduced the levels of
MMP-9 mRNA expression induced by TPA (Fig. 1C).
Effects of SME on the TPA-mediated
MAPK signaling pathway
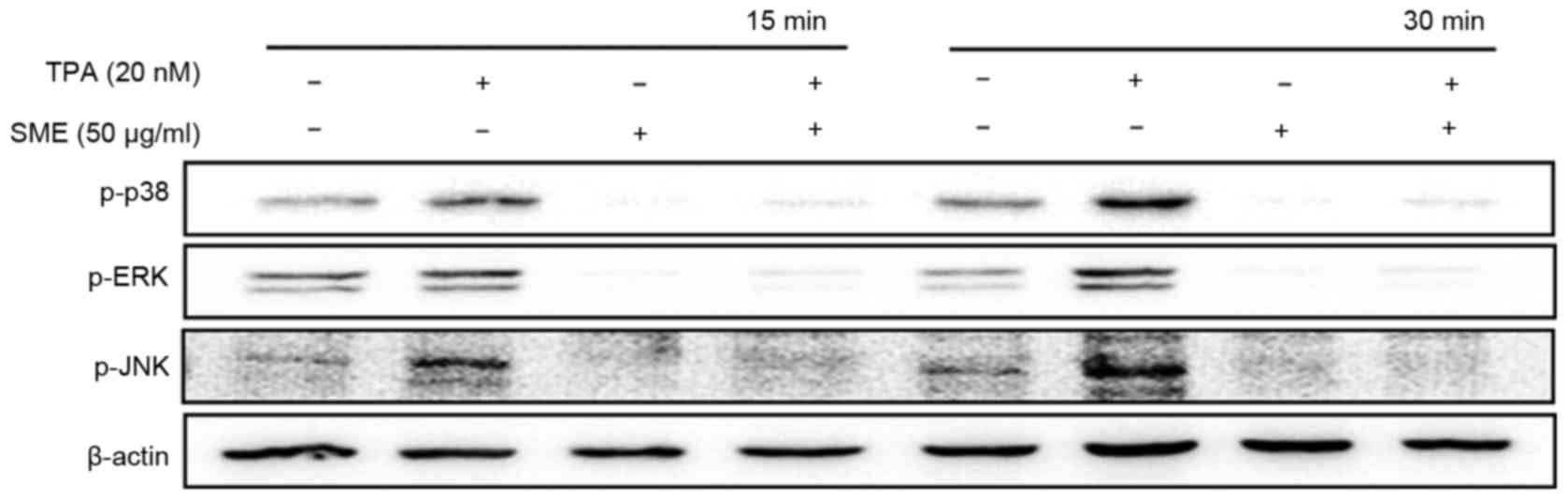
To confirm whether SME is involved in MAPK
activation, western blot analysis was performed. Exposure to TPA
for 15 or 30 min markedly elevated the phosphorylation levels of
p38/JNK/ERK, and pre-treatment with SME markedly decreased them
(Fig. 2).
Effects of SME on TPA-induced NF-κB
and AP-1 activation
To confirm this TPA-induced activation of NF-κB
(p65/p50) and AP-1 (c-Jun/c-Fos), immunofluorescence and western
blotting were used. TPA induced substantial p-c-Jun expression,
whereas pre-treatment with SME blocked it (Fig. 3A and B). Pre-treatment with SME did
not affect the translocation of p65 into the nucleus by TPA
(Fig. 3B). Additionally, SME did not
affect the levels of p-IKKαβ, p-IκBα or IκBα induced by TPA
(Fig. 3C). These results suggested
that SME inhibited TPA-induced MMP-9 expression by blocking AP-1
activation.
 | Figure 3.SME suppresses TPA-induced
transcriptional activation of MMP-9 by inhibiting AP-1. (A) Cells
were pretreated with SME in the presence of TPA. Following 4 h of
incubation, the expression of p-c-Jun in the nucleus was assessed
by immunofluorescence analysis (magnification, ×10). (B) Cells were
pretreated with SME for 1 h and then exposed to TPA for 4 h.
Western blotting was performed to determine the nuclear levels of
p65 and activator protein 1 (p-c-Jun) subunits. PCNA was used as
loading control. (C) Cells were pretreated with SME for 1 h and
then stimulated with TPA for 4 h. Western blotting was performed to
determine the cytoplasmic levels of IκBα, p-IκBα, IKKα, IKKβ and
p-IKKαβ. SME, Salvia miltiorrhiza extract; TPA,
12-O-tetradecanoylphorbol-13-acetate; p, phosphorylated; PCNA,
proliferating cell nuclear antigen; IKK, inhibitory κ B kinase;
IκBα, inhibitory κ B α; p65, nuclear factor-κB p65 subunit. |
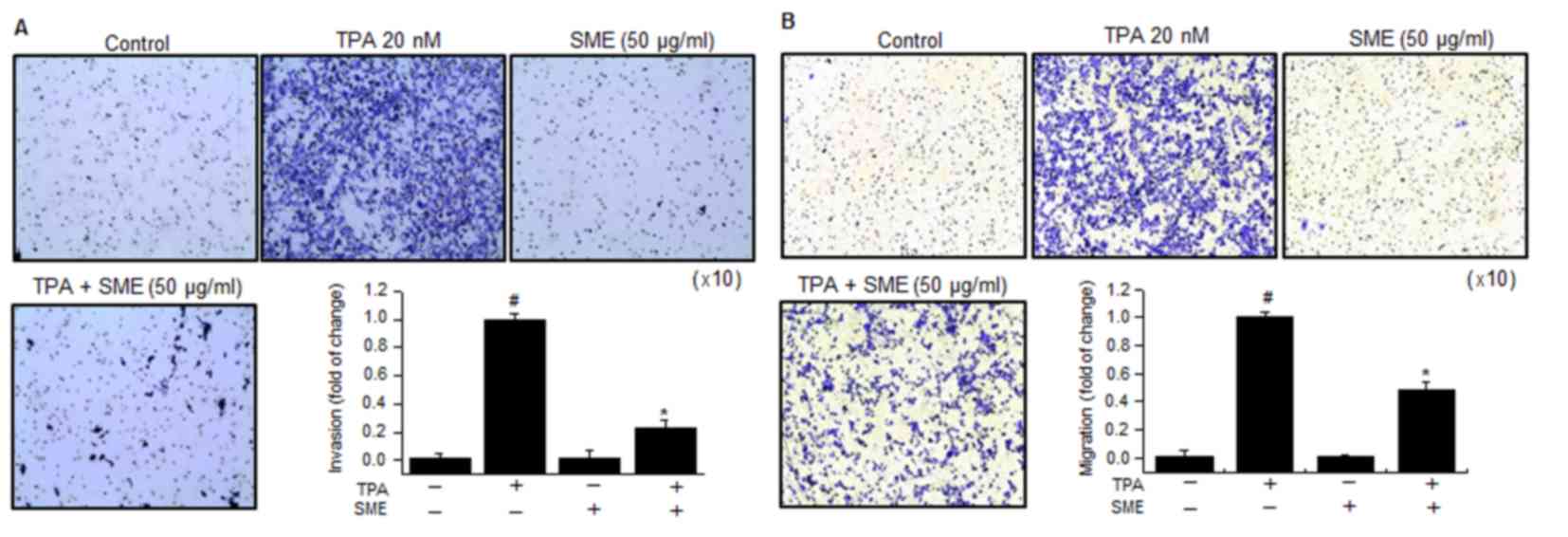
Effect of SME on TPA-induced MCF-7
cell invasion/migration in vitro
To demonstrate whether SME inhibits the invasion and
migration abilities of MCF-7 cells, in vitro invasion and
migration assays were performed. Subsequent to treatment of the
cells with TPA, the levels of cell invasion and migration were
significantly increased. However, the invasion and migration
abilities of cells treated with 50 µg/ml SME and TPA was
considerably lower compared with that of cells treated with TPA
(Fig. 4).
Discussion
In the present study, it was revealed that SME
blocked TPA-induced cell invasion and MMP-9 expression through
inhibition of the MAPK/AP-1 signaling pathway. Concurrently, SME
did not affect NF-κB signaling. These results suggest that SME
blocks cell invasion by suppressing MMP-9 expression, mediated by
the activity of the MAPK/AP-1 signaling pathway, in MCF-7 breast
cancer cells.
The ECM provides biochemical and physical barriers
to the proliferation and spread of cancer cells, and cancer cell
invasion requires its degradation (38,39).
Basement membranes consist of various proteins such as collagen,
gelatin and other ECM components that are degraded by MMPs. The
production of MMPs is tightly controlled by non-specific protease
inhibitors or tissue-specific MMP inhibitors (40–43). When
this regulation becomes impaired, MMP expression in cancer cells
increases abnormally in response to various factors (21–24). In
previous studies, this expression was increased by growth factors,
inflammatory cytokines, phorbol esters and different signaling
pathways in breast cancer cells (44–46). MMP-9
decomposes gelatin and accelerates breast cancer cell invasion
(47). Therefore, regulation of MMP-9
expression may be important in treatment strategies for tumor
metastasis. Songyou Yin, an herbal compound including S.
miltiorrhiza, inhibits tumor invasion via the downregulation of
MMPs in hepatocellular carcinoma (48). A previous study reported that SME
extracts inhibit the proliferation of MCF-7 cells at a
concentration of 5 µg/ml (49).
However, the present study confirmed that the inhibitory effect of
SME on cell invasion at a concentration of 50 µg/ml does not affect
the toxicity of MCF-7 cells. The difference in the concentration of
SME for cytotoxicity is considered to be caused by the change of
the components depending on the difference of purification
processes. The present study suggested that SME inhibited
TPA-induced expression of MMP-9 in MCF-7 breast cancer cells
(Fig. 1). These results demonstrated
that SME has the ability to regulate MMPs.
It has been indicated in several previous studies
that the active component of S. miltiorrhiza modulates the
MAPK families, including ERK, JNK and p38 kinase. MAPK is involved
in cell invasion and protease secretion processes (50,51). In
MCF-7 breast cancer cells, the MAPK signaling pathway is activated
by protein phosphorylation, which in turn is modulated by TPA
(22,52). Previous studies have implicated MAPK
in cancer metastasis (19,21,45). In
the present study, SME suppressed the TPA-induced phosphorylation
of ERK, JNK and p38 in MCF-7 breast cancer cells (Fig. 2).
MAPK families serve an important role in the
activation of AP-1. ERK enhances AP-1 activation through c-Fos,
whereas JNK leads to the phosphorylation of c-Jun (53). The overexpression of c-Jun enhances
MMP-9 expression and in vitro chemo-invasion (54). In the present study, SME blocked
TPA-induced c-Jun phosphorylation (Fig.
3). NF-κB is a transcription factor that serves a pivotal role
in inducing the expression of MMP-9 (47,55). In
the present study, treatment with SME did not inhibit TPA-induced
phosphorylation of IKKαβ/IκBα, degradation of IκBα or nuclear
translocation of p65NF-κB. These data suggest that SME specifically
inhibits the MAPK/AP-1 signaling pathway in TPA-induced MCF-7
breast cancer cells. Previously, Tanshinone II-A demonstrated an
inhibitory effect on the invasion and metastasis of human carcinoma
cells (8,9). However, the mechanism for that effect
was not known in breast cancer. Furthermore, Tanshinone II-A is an
alcohol extract of the root of the traditional Chinese medicinal
plant S. miltiorrhiza Bunge. These results indicate that
S. miltiorrhiza may regulate the invasion abilities of
breast cancer. Therefore, the present study assessed the effect of
SME on TPA-stimulated cell metastasis in MCF-7 breast cancer cells,
and the results indicated that SME inhibited cell invasion
(Fig. 4). These findings demonstrated
that SME attenuated TPA-induced MMP-9 expression and invasion in
MCF-7 breast cancer cells by inhibiting MAPK/AP-1 activation, and
revealed the potential use of SME as a traditional therapeutic
agent in inhibiting breast cancer metastasis.
Acknowledgements
The present study was supported by a grant from the
National Research Foundation of Korea (NRF) funded by the Nuclear
Research & Development Program of the National Research
Foundation (grant no. NRF-2012M2A2A6011335) and by the Korean
government (the Ministry of Education, Science Technology); grant
no. 2011-0030130), Republic of Korea.
References
|
1
|
Yan X: Dan Shen (Salvia miltiorrhiza) in
Medicine. 1. Springer; The Netherlands: 2016
|
|
2
|
Park CH, Kim DH, Park MH, Kim MK, Kim ND,
Kim CM, Tanaka T, Yokozawa T, Chung HY and Moon HR: Chinese
prescription Kangen-karyu and Salviae Miltiorrhizae Radix improve
age-related oxidative stress and inflammatory response through the
PI3K/Akt or MAPK pathways. Am J Chin Med. 42:987–1005. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiping Z, Yan P, Xinmei H, Guanghua F,
Meili M, Jie N and Fangjie Z: Effects of dexamethasone and Salvia
miltiorrhizae on the small intestine and immune organs of rats with
severe acute pancreatitis. Inflammation. 33:259–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu J, Li JC, Tan R, Fan LN and Zhang X:
Effects of combination of Puerariae lobatae radix and Salviae
miltiorrhizae radix et rhizoma on lipid metabolism in
atherosclerotic quails. Zhongguo Zhong Yao Za Zhi. 38:3939–3942.
2013.(In Chinese). PubMed/NCBI
|
|
5
|
Wu CF, Bohnert S, Thines E and Efferth T:
Cytotoxicity of Salvia miltiorrhiza against multidrug-resistant
cancer cells. Am J Chin Med. 44:871–894. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ge Y, Yang B, Chen Z and Cheng R:
Cryptotanshinone suppresses the proliferation and induces the
apoptosis of pancreatic cancer cells via the STAT3 signaling
pathway. Mol Med Rep. 12:7782–7788. 2015.PubMed/NCBI
|
|
7
|
Gao H, Sun W, Zhao W, Hao W, Leung CH, Lu
J and Chen X: Total tanshinones-induced apoptosis and autophagy via
reactive oxygen species in lung cancer 95D Cells. Am J Chin Med.
43:1265–1279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuxian X, Feng T, Ren L and Zhengcai L:
Tanshinone II-A inhibits invasion and metastasis of human
hepatocellular carcinoma cells in vitro and in vivo. Tumori.
95:789–795. 2009.PubMed/NCBI
|
|
9
|
Shan YF, Shen X, Xie YK, Chen JC, Shi HQ,
Yu ZP, Song QT, Zhou MT and Zhang QY: Inhibitory effects of
tanshinone II-A on invasion and metastasis of human colon carcinoma
cells. Acta Pharmacol Sin. 30:1537–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nizamutdinova IT, Lee GW, Lee JS, Cho MK,
Son KH, Jeon SJ, Kang SS, Kim YS, Lee JH, Seo HG, et al: Tanshinone
I suppresses growth and invasion of human breast cancer cells,
MDA-MB-231, through regulation of adhesion molecules.
Carcinogenesis. 29:1885–1892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nizamutdinova IT, Lee GW, Son KH, Jeon SJ,
Kang SS, Kim YS, Lee JH, Seo HG, Chang KC and Kim HJ: Tanshinone I
effectively induces apoptosis in estrogen receptor-positive (MCF-7)
and estrogen receptor-negative (MDA-MB-231) breast cancer cells.
Int J Oncol. 33:485–491. 2008.PubMed/NCBI
|
|
12
|
Redig AJ and McAllister SS: Breast cancer
as a systemic disease: A view of metastasis. J Intern Med.
274:113–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leber MF and Efferth T: Molecular
principles of cancer invasion and metastasis (Review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
15
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woodhouse EC, Chuaqui RF and Liotta LA:
General mechanisms of metastasis. Cancer. 80:(8 Suppl).
S1529–S1537. 1997. View Article : Google Scholar
|
|
17
|
Yan C and Boyd DD: Regulation of matrix
metalloproteinase gene expression. J Cell Physiol. 211:19–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Overall CM and Dean RA: Degradomics:
Systems biology of the protease web. Pleiotropic roles of MMPs in
cancer. Cancer Metastasis Rev. 25:69–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong S, Park KK, Magae J, Ando K, Lee TS,
Kwon TK, Kwak JY, Kim CH and Chang YC: Ascochlorin inhibits matrix
metalloproteinase-9 expression by suppressing activator
protein-1-mediated gene expression through the ERK1/2 signaling
pathway: Inhibitory effects of ascochlorin on the invasion of renal
carcinoma cells. J Biol Chem. 280:25202–25209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu JF, Crépin M, Liu JM, Barritault D and
Ledoux D: FGF-2 and TPA induce matrix metalloproteinase-9 secretion
in MCF-7 cells through PKC activation of the Ras/ERK pathway.
Biochem Biophys Res Commun. 293:1174–1182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Przybylowska K, Kluczna A, Zadrozny M,
Krawczyk T, Kulig A, Rykala J, Kolacinska A, Morawiec Z, Drzewoski
J and Blasiak J: Polymorphisms of the promoter regions of matrix
metalloproteinases genes MMP-1 and MMP-9 in breast cancer. Breast
Cancer Res Treat. 95:65–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim S, Choi JH, Kim JB, Nam SJ, Yang JH,
Kim JH and Lee JE: Berberine suppresses TNF-alpha-induced MMP-9 and
cell invasion through inhibition of AP-1 activity in MDA-MB-231
human breast cancer cells. Molecules. 13:2975–2985. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim S, Choi JH, Lim HI, Lee SK, Kim WW,
Cho S, Kim JS, Kim JH, Choe JH, Nam SJ, et al: EGF-induced MMP-9
expression is mediated by the JAK3/ERK pathway, but not by the
JAK3/STAT-3 pathway in a SKBR3 breast cancer cell line. Cell
Signal. 21:892–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung TW, Moon SK, Chang YC, Ko JH, Lee
YC, Cho G, Kim SH, Kim JG and Kim CH: Novel and therapeutic effect
of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma
cells: Complete regression of hepatoma growth and metastasis by
dual mechanism. FASEB J. 18:1670–1681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noh EM, Chung EY, Youn HJ, Jung SH, Hur H,
Lee YR and Kim JS: Cis-guggulsterone inhibits the IKK/NF-kappaB
pathway, whereas trans-guggulsterone inhibits MAPK/AP-1 in MCF-7
breast cancer cells: Guggulsterone regulates MMP-9 expression in an
isomer-specific manner. Int J Mol Med. 31:393–399. 2013.PubMed/NCBI
|
|
27
|
Hess J, Angel P and Schorpp-Kistner M:
AP-1 subunits: Quarrel and harmony among siblings. J Cell Sci.
117:5965–5973. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SO, Jeong YJ, Kim M, Kim CH and Lee
IS: Suppression of PMA-induced tumor cell invasion by capillarisin
via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem
Biophys Res Commun. 366:1019–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh JH, Chung AS, Steinbrenner H, Sies H
and Brenneisen P: Thioredoxin secreted upon ultraviolet A
irradiation modulates activities of matrix metalloproteinase-2 and
tissue inhibitor of metalloproteinase-2 in human dermal
fibroblasts. Arch Biochem Biophys. 423:218–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Himelstein BP, Lee EJ, Sato H, Seiki M and
Muschel RJ: Tumor cell contact mediated transcriptional activation
of the fibroblast matrix metalloproteinase-9 gene: Involvement of
multiple transcription factors including Ets and an alternating
purine-pyrimidine repeat. Clin Exp Metastasis. 16:169–177. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jacobs MD and Harrison SC: Structure of an
IkappaBalpha/NF-kappaB complex. Cell. 95:749–758. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HR, Kim JM, Kim MS, Hwang JK, Park YJ,
Yang SH, Kim HJ, Ryu DG, Lee DS, Oh H, et al: Saussurea lappa
extract suppresses TPA-induced cell invasion via inhibition of
NF-kappaB-dependent MMP-9 expression in MCF-7 breast cancer cells.
BMC Complement Altern Med. 14:1702014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee YR, Noh EM, Oh HJ, Hur H, Kim JM, Han
JH, Hwang JK, Park BH, Park JW, Youn HJ, et al:
Dihydroavenanthramide D inhibits human breast cancer cell invasion
through suppression of MMP-9 expression. Biochem Biophys Res
Commun. 405:552–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JM, Noh EM, Kim HR, Kim MS, Song HK,
Lee M, Yang SH, Lee GS, Moon HC, Kwon KB and Lee YR: Suppression of
TPA-induced cancer cell invasion by peucedanum japonicum thunb.
Extract through the inhibition of PKCα/NF-κB-dependent MMP-9
expression in MCF-7 cells. Int J Mol Med. 37:108–114.
2016.PubMed/NCBI
|
|
36
|
Livak KL and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noh EM, Lee YR, Hong OY, Jung SH, Youn HJ
and Kim JS: Aurora kinases are essential for PKC-induced invasion
and matrix metalloproteinase-9 expression in MCF-7 breast cancer
cells. Oncol Rep. 34:803–810. 2015.PubMed/NCBI
|
|
38
|
Woessner JF Jr: Matrix metalloproteinases
and their inhibitors in connective tissue remodeling. FASEB J.
5:2145–2154. 1991.PubMed/NCBI
|
|
39
|
Zwiefel K and Janni W: Current standards
in the treatment of breast cancer. Med Monatsschr Pharm.
34:280–288; quiz 289–290. 2011.(In German). PubMed/NCBI
|
|
40
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Köhrmann A, Kammerer U, Kapp M, Dietl J
and Anacker J: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer and breast cancer cell lines: New
findings and review of the literature. BMC Cancer. 9:1882009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lombard C, Saulnier J and Wallach J:
Assays of matrix metalloproteinases (MMPs) activities: A review.
Biochimie. 87:265–272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Duffy MJ and McCarthy K: Matrix
metalloproteinases in cancer: Prognostic markers and targets for
therapy (Review). Int J Oncol. 12:1343–1348. 1998.PubMed/NCBI
|
|
44
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993.PubMed/NCBI
|
|
45
|
Cho HJ, Kang JH, Kwak JY, Lee TS, Lee IS,
Park NG, Nakajima H, Magae J and Chang YC: Ascofuranone suppresses
PMA-mediated matrix metalloproteinase-9 gene activation through the
Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis.
28:1104–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mo N, Li ZQ, Li J and Cao YD: Curcumin
inhibits TGF-β1-induced MMP-9 and invasion through ERK and Smad
signaling in breast cancer MDA- MB-231 cells. Asian Pac J Cancer
Prev. 13:5709–5714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lungu G, Covaleda L, Mendes O,
Martini-Stoica H and Stoica G: FGF-1-induced matrix
metalloproteinase-9 expression in breast cancer cells is mediated
by increased activities of NF-kappaB and activating protein-1. Mol
Carcinog. 47:424–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang XY, Wang L, Huang ZL, Zheng Q, Li QS
and Tang ZY: Herbal extract ‘Songyou Yin’ inhibits tumor growth and
prolongs survival in nude mice bearing human hepatocellular
carcinoma xenograft with high metastatic potential. J Cancer Res
Clin Oncol. 135:1245–1255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang W, Ju JH, Jeon MJ, Han X and Shin I:
Danshen (Salvia miltiorrhiza) extract inhibits proliferation of
breast cancer cells via modulation of Akt activity and p27 level.
Phytother Res. 24:198–204. 2010.PubMed/NCBI
|
|
50
|
Cobb MH and Goldsmith EJ: How MAP kinases
are regulated. J Biol Chem. 270:14843–14846. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Seger R, Biener Y, Feinstein R, Hanoch T,
Gazit A and Zick Y: Differential activation of mitogen-activated
protein kinase and S6 kinase signaling pathways by
12-O-tetradecanoylphorbol-13-acetate (TPA) and insulin. Evidence
for involvement of a TPA-stimulated protein-tyrosine kinase. J Biol
Chem. 270:28325–28330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yao J, Xiong S, Klos K, Nguyen N, Grijalva
R, Li P and Yu D: Multiple signaling pathways involved in
activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1
in human breast cancer cells. Oncogene. 20:8066–8074. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Smith LM, Wise SC, Hendricks DT, Sabichi
AL, Bos T, Reddy P, Brown PH and Birrer MJ: cJun overexpression in
MCF-7 breast cancer cells produces a tumorigenic, invasive and
hormone resistant phenotype. Oncogene. 18:6063–6070. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappa B
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000. View Article : Google Scholar : PubMed/NCBI
|