Currently, cancer is one of most common causes of
mortality worldwide. Colorectal cancer (CRC) represents one of the
most frequently diagnosed cancer types and is the fourth leading
cause of cancer-related death (1).
The spreading of cells from the primary lesion to a secondary organ
and the subsequent development of distant metastases is a key
factor that limits patient survival rate. This remains one of the
most complex issues faced in medicine (2).
The liver is the main target organ for metastatic
CRC cells and the second most commonly invaded organ, after the
lymph nodes (3). In fact, 15–25% of
CRC patients present with synchronous hepatic metastases at the
time of diagnosis, and a further 30% will later develop liver
metastasis (4,5). The complex network of vessels and
microcapillaries of the hepatic microcirculation makes the liver a
target for circulating cells (6).
Indeed, cancer cells released from a primary lesion follow a
natural blood flow directly to the liver, through the specialized
microvessel network known as the liver sinusoids. Gastric cancers
also commonly metastasize to the liver (7). Circulating cells from other primary
malignancies, such as melanoma, breast or neuroendocrine tumors
(8–10), also adhere, establish and develop in
the liver, giving rise to metastases, although this is less
common.
Metastatic progression is a highly complex and
coordinated cascade of events that is influenced by a wide variety
of mediators (11,12). Among the key factors that participate
in this process, adhesion molecules expressed on cancer cells and
cells of the target organ have a crucial role (13,14).
Adhesion molecules generate the initial cell-cell contacts that
lead to cancer cell extravasation and organ colonization.
Additionally, these proteins may also act as signaling molecules to
modulate the local microenvironment, creating a pro-metastatic
environment, and trigger an angiogenic and desmoplastic response
via a complex reciprocal dialogue between the tumor cells and the
cells of the colonized organ (15,16). In
addition to the tissue cells, immune populations recruited from the
circulation during metastasis formation are also involved in
generating a favorable environment for metastatic growth (17,18).
Therefore, determining the role of adhesion molecules during the
different stages of this process remains a major goal for our
understanding of the metastatic cascade. This, in turn, will
facilitate new opportunities for therapeutic intervention.
To date, the research effort undertaken to
investigate the function of adhesion proteins expressed on the
surface of tumor cells and their implications in organ colonization
has increased our knowledge about the signaling pathways that
operate in tumor cells. The ‘seed and soil’ theory (19) postulates that host organ-specific
adhesion molecules are required for the switch towards invasion and
disease progression (20,21). Several adhesion molecules, such as
E-selectin, vascular cellular adhesion molecule (VCAM)-1 and
ICAM-1, exhibit increased expression in the liver during metastatic
invasion (22). Among them, ICAM-1
mediates several stages of the metastatic cascade, including the
adhesion of tumor cells to the endothelial wall (23–25),
endothelial cell activation of pro-metastatic signaling pathways
(26–28), tumor cell extravasation (23,29), the
recruitment of immune cell populations (28,30,31), the
pro-angiogenic response (32) and the
transdifferentiation of stellate cells during the desmoplastic
response (33,34). This review will focus on the role of
ICAM-1 during the different events of the metastatic cascade that
drives colonization of the liver by circulating tumor cells, and
how it modulates the liver microenvironment to facilitate
metastasis.
ICAM-1 is a transmembrane glycoprotein of the
immunoglobulin (Ig)-like superfamily, consisting of five
extracellular Ig-like domains, a transmembrane domain and a short
cytoplasmic tail (35). This
transmembrane domain is essential for cell-cell adhesion and
cell-extracellular matrix (ECM) interaction (36,37). In
the liver, ICAM-1 is expressed constitutively in liver sinusoidal
endothelial cells (LSECs), hepatocytes, Kupffer cells (KCs) and
hepatic stellate cells (HSCs) (33,38,39), and
is and further upregulated by inflammatory activation, such as
stimulation by TNF-α, IL-1β or IFN-γ (40). As in other organs, inflammation is
accompanied by the recruitment of multiple immune cell populations,
such as neutrophils, lymphocytes and monocytes. Leukocytes invade
the tissue after crossing the liver endothelium via interaction
between endothelial ICAM-1 (41,42) and
its main counter-receptor, lymphocyte function-associated antigen
(LFA)-1, on lymphocytes.
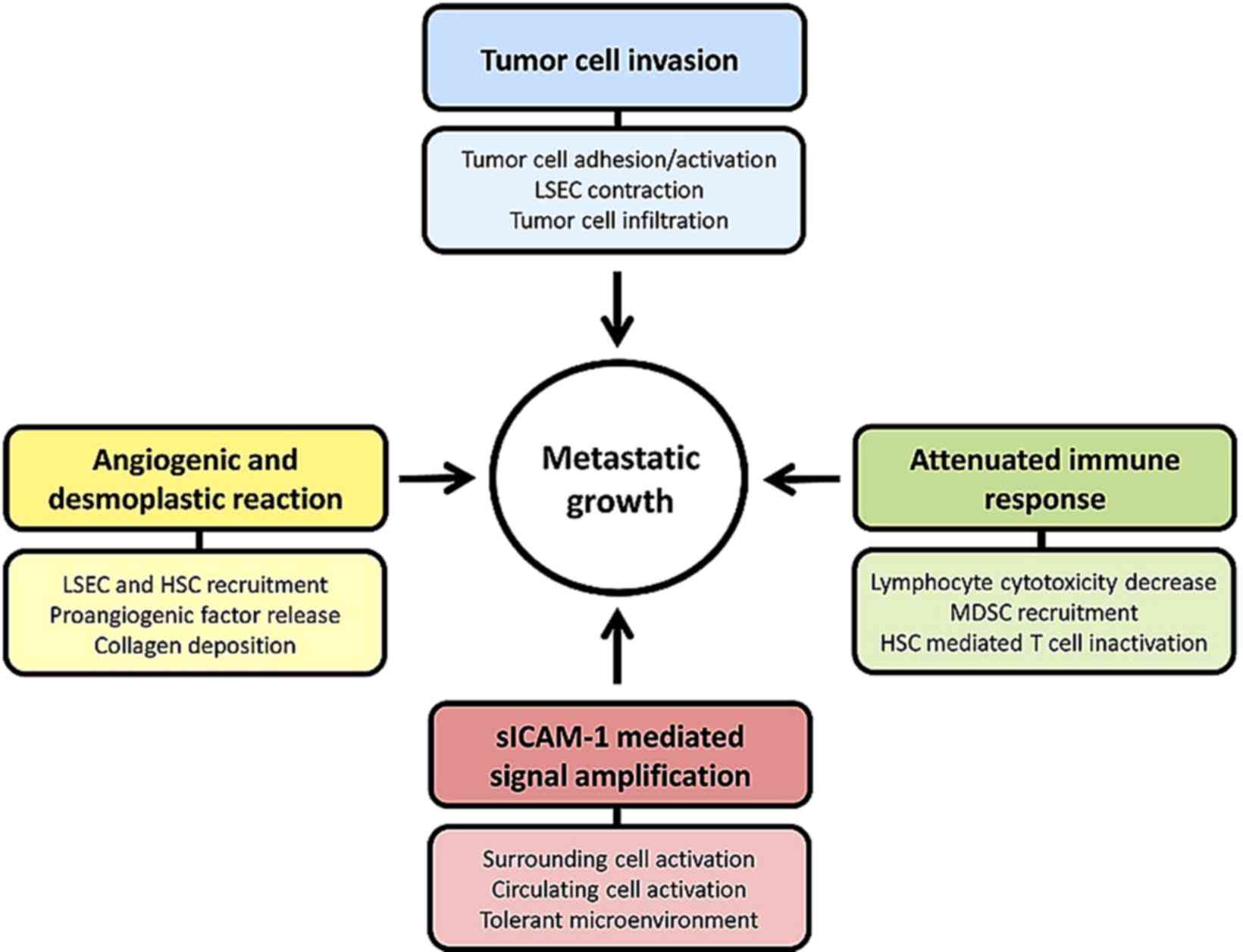
ICAM-1 appears to have a major role during the
initiation of the metastatic cascade driving tumor progression. The
expression of ICAM-1 protein by liver parenchymal and
non-parenchymal cells enhances its potential to facilitate disease
progression (Fig. 1). In fact, ligand
binding to ICAM-1 in LSECs and KCs, and ICAM-1 overexpression in
HSCs and hepatocytes (26,37,43,44)
contribute to the activation of multiple signaling pathways with
key roles in different stages of metastatic progression.
Furthermore, the soluble form, sICAM-1, enhances the pro-metastatic
phenotype and the pro-inflammatory and pro-tumoral signaling
(45). sICAM-1 has been demonstrated
to be elevated in the serum of patients with liver metastasis from
lung or gastric cancer (46,47), and has been identified as a marker for
metastatic stage, disease recurrence and prognosis in non-Hodgkin's
lymphoma, hepatocellular carcinoma (HCC), lung cancer, and other
cancer types (48–50).
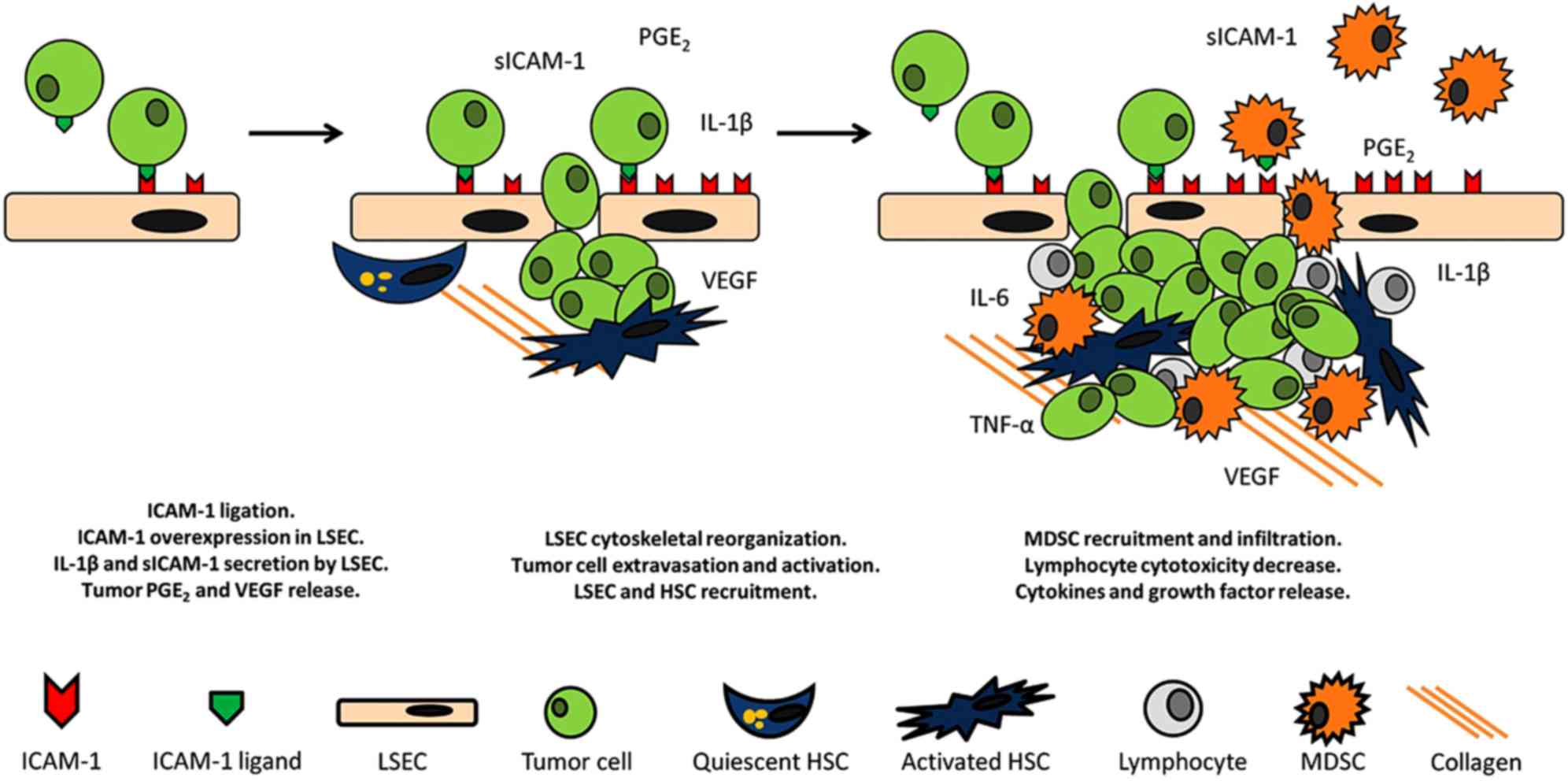
The adhesion to LSECs and the ulterior extravasation
of the malignant tumor cells across this endothelial line into the
liver represents the first step in liver metastatic colonization
(20). Invading cells initially
require an anchor to adhere to and help them escape from the blood
stream (Fig. 2). At this time,
endothelial ICAM-1 is involved in tumor cell adhesion to the
endothelium; this is a particularly important phenomenon
considering that tumor adherence to vessel walls is a common
feature across numerous types of cancer (51). Ghislin et al reported that
ICAM-1 expressed on the surface of endothelial cells is crucial for
the adhesion of melanoma cells to the endothelial monolayer in
vitro. Under these conditions, ICAM-1 expression is increased
after tumor stimulation, in parallel with an increase in tumor cell
adhesion, and this effect can be abrogated by the treatment of the
endothelial cells with specific anti-ICAM-1 antibodies (29). These results are consistent with
another report that showed that tumor cell interaction with
endothelial cells increases ICAM-1 expression on the endothelial
cell surface (52). Furthermore,
expression of ICAM-1 was shown to be correlated with the production
of pro-tumoral cytokines, such as IL-8 and IL-6. IL-8 and IL-6
facilitate tumor cell attachment to endothelial cells and enhance
vascular permeability, as observed in models of brain and lung
metastasis (53,54). Additionally, a previous study used
atomic-force microscopy to show that endothelial ICAM-1 mediates
the adhesion of different invasive bladder cancer cells to
endothelial cells (24). In line with
these results, a reduction in ICAM-1 expression via siRNAs or by
using ICAM-1-blocking antibodies significantly decreased the
adhesion of fibrosarcoma cells to ECV304 human endothelial cells
in vitro (55), and of C26 CRC
cells to LSECs in vivo (56).
Furthermore, the adhesion of different tumor cells may be reduced
by blocking the expression of ICAM-1 in the endothelium of either
brain or lung, leading to the abrogation of metastasis to these
organs, which further confirms the role of the ICAM-1 in tumor
cells (53,54). Moreover, ICAM-1 can cooperate with
other adhesion molecules, such as VCAM-1, in the adhesion of
malignant cells. ICAM-1 and VCAM-1 are both upregulated in a
TNF-α-dependent manner (TNF-α being predominantly produced by
macrophages) (57). Notably, LSECs
lack the ability to express selectins, which are basally expressed
and inducible in the endothelium of the portal tract and central
vein (58). Likewise, this may have
implications for the pathophysiology of the liver and may affect
the normal distribution of tumor cell adhesion during the first
steps of infiltration. In parallel with the upregulation of
E-selectin, ICAM-1 and VCAM-1 in the liver, the production of
pro-inflammatory cytokines is also increased in HCC (59). Among them, IL-6 is implicated in the
attraction of tumor cells, initiating a positive feedback response,
and thus, not only promotes the progression of the metastasis, but
also increases the risk of recurrence. Furthermore, the adhesion of
CRC cells to KCs through carcinoembryonic antigen (CEA), increases
the release of cytokines from KCs, including IL-1, TNF and IL-6,
which induces ICAM-1 upregulation in ECV304 human endothelial cells
and increases tumor cell adhesion to these cells in vitro
(40).
All these results support that endothelial ICAM-1
has a crucial role in the adhesion of cancer cells to the
endothelium in target organs and, thus, in the progression of
cancer. Interestingly, ICAM-1 expression has been recently linked
to a unique mechanism of leukocyte adhesion that specifically
occurs in the liver (60), in
contrast to the classic rolling-adhesion-diapedesis mechanisms used
by leukocytes in many other organs. It is tempting to hypothesize
that leukocyte adhesion molecules expressed on tumor cells and
ICAM-1 in LSECs may also been involved in the events leading to
cancer cell colonization of the liver. If so, ICAM-1 expressed on
LSECs could be crucial for the development of liver metastasis, and
a potential candidate for the development of new and improved
targeted therapies.
The majority of the studies conducted to investigate
the role of ICAM-1 in liver metastasis have mainly focused on its
expression on cancer cells. Studies involving surface ligands on
the tumor, such as MUC-1 and the β2 integrin of the LFA-1 receptor,
known to bind to ICAM-1 specifically (61), have provided evidence of the role of
this Ig superfamily molecule in metastasis when expressed on the
host cells. The expression of different ICAM-1 ligands has been
detected in a wide variety of cancer types. In fact, β2 integrin is
expressed in melanoma, lymphoma, myeloma, gastrointestinal
carcinomas, and was recently reported in breast cancer (29,62–65).
Another ICAM-1 ligand, MUC-1, is expressed on breast cancer cells
(23), as well as ovarian, prostate,
gastric and pancreatic cancer cells and liver metastases (66). The hyaluronan receptor CD44 and its
isoforms, which can be used alongside others markers for cancer
stem cell identification (67), are
expressed on breast, colorectal and pancreatic cancers cells
(68–70). The increasing evidence of tumor cell
expression of ICAM-1 ligands, and of the interaction between tumor
cells and host ICAM-1, demonstrate that the significance of host
cell ICAM-1 expression during the course of liver metastasis, and
knowledge of role of ICAM-1 expression, may increase in the coming
years. Consistently with this idea, the adhesion of
LFA-1-expressing C26 CRC cells to LSECs was shown to be reduced by
blocking ICAM-1 with specific antibodies (27,32). The
same result was observed when the expression of β2 integrin in C26
cells was reduced via siRNA (Benedicto et al, submitted).
Additionally, the expression of other ICAM-1 ligands, such as
MUC-1, in breast cells was shown to mediate the adhesion of the
malignant cells to HUVECs (25).
Interestingly, the vessels located at the invasive
front of CRC tumors exhibit higher expression of ICAM-1. These
vessels are considered to be a gateway for the entry of
inflammatory cells into cancer tissue (71). Along with endothelial cells, ICAM-1
expression by other resident liver cells has been also studied. The
resident macrophages of the liver, KCs, also express ICAM-1
(72). Some reports in other cancer
types have suggested that the adhesion of macrophages to tumor
cells promotes metastasis (73). It
is tempting to hypothesize that expression of specific ligands may
mediate the adhesion of tumor cells to macrophages expressing
ICAM-1, further increasing liver colonization by circulating cancer
cells. Additionally, tumor-activated KCs, and HSCs, which also
express ICAM-1 when activated by inflammatory factors (74), further stimulate the expression of
ICAM-1 on the surface of endothelial cells, increasing vascular
permeability, which is also associated with an increase in tumor
cell invasion into a target organ (75).
Endothelial cells are the first barrier that tumor
cells encounter when invading the liver (51), since they act as gatekeepers, allowing
the infiltration of cells only when required. Once adhered to
LSECs, cancer cells must pass across the endothelium through a
process called diapedesis in order to extravasate (76,77). The
contraction of endothelial cells through actin cytoskeletal
reorganization is essential for the infiltration of invading cells
(78,79). The remodeling of the cytoskeleton is
also observed after the binding of endothelial ICAM-1 to leukocyte
LFA-1 during transmigration of immune cells, including leukocytes,
lymphocytes and neutrophils (44,80,81). In
this scenario, ICAM-1 expression has also been reported to be
correlated with efficient transmigration across the endothelial
cell layer and the subsequent extravasation of tumor cells, which
in turn, facilitates the colonization of the organ (29,82,83). While
elucidating the mechanisms of the metastatic process, it has been
proposed that tumor cells mimic the route of immune cells, by
binding to endothelial ICAM-1 and forcing their way through the
endothelial wall fenestrations to invade the tissue parenchyma. In
contrast to other organs, where leukocyte recruitment depends on
the expression of selectins on post-capillary venules, leukocyte
adhesion to the hepatic sinusoidal endothelium depends on ICAM-1
(84). This is consistent with the
lack of selectins observed in LSECs and the limited expression of
ICAM-1 in portal tracts under basal conditions. In a previous
study, neutralization of endothelial ICAM-1 and VCAM-1 by specific
antibodies in a leukemia model was reported to abrogate cancer cell
diapedesis across human coronary artery endothelial cell wall,
which further supports the idea that endothelial ICAM-1 is involved
in cancer cell invasion to the liver through regulation of
transmigration and extravasation (85). Furthermore, in a melanoma model,
Ghislin et al showed that blocking the LFA-1/ICAM-1
interaction using specific antibodies either to ICAM-1 on
endothelial cells, or to the CD18 subunit of the LFA-1 integrin in
tumor cells, reduced transendothelial migration (29). Moreover, transendothelial migration of
breast carcinoma cells is promoted by the interaction between tumor
MUC-1 and ICAM-1 expressed on the endothelial surface (23). Together these observations suggest
that ICAM-1 has an important role in organ invasion by circulating
tumor cells, although the downstream mechanisms remain unknown. As
for leukocytes, during sterile inflammation (86), tumor cells may utilize ICAM-1 to
infiltrate the liver parenchyma in order to colonize the organ.
Recent studies from our group revealed that there is a direct link
between the tumor LFA-1/endothelial ICAM-1 interaction and the
transmigration of CRC cells through the endothelial cell lining of
the liver sinusoids, since a decrease in the number of
transmigrated cells was observed when either the β2 integrin of
tumor cells (Benedicto et al, submitted) or the ICAM-1 of
endothelial cells are silenced. The perturbation of this
interaction reduced tumor cell migration through an endothelial
monolayer in vitro, and demonstrates the complexity of
metastasis and the signaling mechanisms involved in this process in
the liver.
Due to its physiological location, the liver has a
chronic inflammatory microenvironment, where the immune system must
continuously fight against pathogens from within the intestinal
tract. In fact, the cells residing in the liver, namely
hepatocytes, KCs, LSECs and HSCs, are involved in the inflammatory
cascade (87) and maintaining
homeostasis within the organ. However, this inflammatory status is
a double-edged sword, since cancer development has been shown to be
profoundly enhanced by pro-inflammatory signals (88). The creation of a pro-tumor
microenvironment is required for cancer cell proliferation and for
tumors to evade immune surveillance, leading to a decreased immune
recognition and/or elimination of reactive immune cells. Modulation
of the target organ pro-metastatic response is initiated by
interaction of tumor cells with endothelial cells, leading to the
activation of these vascular cells and secretion of
pro-inflammatory cytokines and growth factors (89). The role of ICAM-1 in the creation of
such an environment was reported by Arteta et al (27). The authors reported that ICAM-1
binding with tumor LFA-1 triggered the production of IL-1β by
LSECs, which in turn promoted mannose receptor (ManR)-mediated
endocytosis. The increase in the activity and expression of ManR
was correlated with a decreased cytotoxic effect of liver
sinusoidal lymphocytes on the C26 murine CRC cell line (27). This defective immune response
facilitates the establishment and development of metastasis, as one
of the hallmarks of tumor progression (90).
During liver metastasis, liver tissue is replaced by
the continuously growing tumor mass, along with the activated
resident liver cells, creating a dynamic network that has been
termed a ‘wound that never heals’ (91). During this ‘healing’ process after
liver damage, liver myofibroblast-like cells, also known as HSCs,
have a key role (92). This
contractile cell population are particularly important in relation
to cancer, since cancer-associated fibroblasts (CAFs) have also
been identified as a key cell type that favor tumor growth and
development (93). Intriguingly, the
expression of ICAM-1 has been shown to be induced in activated HSCs
(94), in a mechanism mediated by
cytokines and growth factors, such as TNF-α (74), as well as by cell-cell contacts. It is
interesting to note that the stellate cells present in other organs
with tumors that mainly metastasize to the liver, such as
pancreatic carcinomas, also express ICAM-1 when activated (95). Furthermore, the increased expression
of ICAM-1 on the surface of HSCs, activated by infiltrating
lymphocytes, leads to tumor growth and immune evasion (33). On the one hand, HSCs are able to
promote tumor proliferation via the secretion of a wide array of
soluble mediators (96), which
potentially modulate the tumor microenvironment. Additionally, HSC
activation by melanoma-derived soluble factors results in the
recruitment of LSECs, promoting the development of new vessels
(97). On the other hand, activated
HSCs have the ability to phagocytose lymphocytes during liver
inflammation (98), to abrogate
CD8+ T cell-mediated immunity (99), and to enhance the recruitment of
immunosuppressive cells (100), thus
impeding the appropriate anti-tumor function in an ICAM-1-dependent
pathway, and facilitating tumor expansion.
Cancer cells that are able to evade immune
surveillance require stimulation by cytokines and growth factors
derived from numerous cell sources in order to proliferate. Aside
from the factors secreted by HSCs and LSECs, tumor cells are
stimulated by KCs present in the liver sinusoids, which are known
to promote tumor progression at multiple levels, including
promoting adhesion, proliferation, immune tolerance and
angiogenesis (40). Ligand binding to
ICAM-1 activates KC secretion of IL-6 (41), which induces cell proliferation via
the STAT3 pathway, and stimulates the degradation of surrounding
ECM via increased secretion of matrix metalloproteinases (MMPs)
(101). IL-6 has been also
implicated in the differentiation of human monocytes to M2
macrophages (102), a mechanism that
probably also occurs in liver and is related to tumor progression
(103). Interestingly, the
infiltration of macrophages was reduced in ICAM-1 knockout mice in
a model of renal injury (104),
which is consistent with another report linking ICAM-1 blockage
with a reduction in macrophage infiltration in precancerous
pancreatic lesions (105). It is
tempting to speculate that the same phenomenon may occur in the
liver, as macrophages express LFA-1 and MUC-1, which are well known
ICAM-1 ligands, suggesting that ICAM-1 is a key mediator in the
recruitment of tumor-promoting macrophages. In fact, ICAM-1
silencing significantly reduced F4/80-expressing cells within the
liver metastasis foci of C26 tumor-bearing mice (56).
An additional key and rate-limiting step in the
metastatic process of a wide variety of tumors is the development
of new blood vessels in order to fulfill the metabolic requirements
of the tumor mass. The process of de novo formation of tumor
blood vessels, tumor angiogenesis, determines the viability and
survival of tumor cells and, thus, the establishment and
development of the disease. The generation of new capillaries is
favored by the stimuli present in the tumor microenvironment,
produced by the tumor itself, by liver cells and by recruited
immune cells. LSECs are required to form new capillary structures,
with contractile HSCs necessary to provide support for new vessel
formation (97). Our group revealed
that LFA-1-expressing C26 cells interact with LSECs via ICAM-1, and
that this interaction was the initial angiogenic stimulus during
the early stage of liver metastasis, promoting tumor release of
vascular endothelial growth factor (VEGF) (27,32), and
driving the directional migration of LSECs and HSC recruitment to
tumor areas (97). To further amplify
this reaction, tumor cells, tumor-associated macrophages (TAMs) and
myeloid-derived suppressor cells (MDSCs) contribute to VEGF
secretion in response to their stimulation by TNF-α, IL-6 and NO,
which are released through ICAM-1-mediated signaling pathways in
KCs and LSECs (106,107). Wang and Doerschuk reported that upon
ICAM-1 ligand binding, pulmonary endothelial cells undergo
cytoskeletal reorganization (108),
a process required for the formation of new vessels, which may also
occur in the liver when interacting with ICAM-1 ligands expressed
on tumor cells. Recruitment of HSCs is crucial for the support of
new vessels. HSCs respond to VEGF produced by tumor-activated cells
and migrate to the sites of angiogenesis (109). The p38 mitogen-activated protein
kinase (MAPK) pathway has been recently proposed as a mediator of
endothelial cell activation, cytoskeletal reorganization and
migration of HSCs (110,111). Interestingly, ICAM-1 activation has
been shown to stimulate the p38 MAPK pathway in endothelial cells,
astrocytes and renal fibroblasts (112–114).
Moreover, this MAPK pathway is also activated by platelet-derived
growth factor, the most potent mitogen for HSCs (115), suggesting that ligand binding to
ICAM-1 is a possible mitogenic stimulus responsible for the
proliferative phenotype of these myofibroblast-like cells,
expanding the number of activated cells ready to support
angiogenesis (Fig. 2).
In addition to the angiogenic response, a
desmoplastic reaction is observed during metastatic invasion of the
liver. HSCs are the main producers of ECM proteins in the liver. In
fact, the progression of CRC is associated with the desmoplastic
reaction (116). In fibroblasts
isolated from human CRC samples, ICAM-1 was increased in
tumor-associated fibroblasts and was associated with an increase in
desmoplasia in tissue sections (117). In liver metastasis induced by
sICAM-1-activated C26 cells, an increase in collagen deposition is
correlated with the infiltration of HSCs and with CD31-positive
cells, indicating that the desmoplastic reaction is coupled to the
angiogenic response and mediated by ICAM-1 (Arteta et al,
Abstract in 12th International Symposium on Cells of the hepatic
sinusoids, Bilbao, spain, 2004).
As mentioned previously, recruited immune
populations infiltrate the liver via ICAM-1 adhesion. In order to
efficiently fight against infiltrating tumor cells, either resident
or recruited lymphocyte populations are required in order to mount
an adequate immune response against the invading tumor.
CD8+ T lymphocytes are able to control metastatic growth
upon activation; however, when depleted, disease progression
continues (118,119). On the other hand, CD4+ T
lymphocytes also contribute to immune defense against cancer
development (120). However, among
the immune cell populations recruited from the circulation, MDSCs
are able to suppress T cell cytotoxic activity towards the tumor
and promote immune suppression (106). In this context, TAMs and MDSCs also
contribute to tumor progression by decreasing anti-tumor immunity
and generating a pro-tumor microenvironment (106,121–124).
In fact, macrophages induce apoptosis of peripheral T cells
following binding of LFA-1 to ICAM-1 (125), therefore promoting tumor survival
through depletion of cytotoxic cells. Increased numbers of these
immune cell populations, known to express LFA-1 receptor and MUC-1,
are associated with the metastatic development of different
cancers. In fact, the involvement of ICAM-1 in the adhesion of
myeloid cell populations in vitro was reported by Makgoba
et al in 1988 (126).
Subsequently, other studies have reported the direct role of ICAM-1
in the adhesion of myeloid cells to dermal fibroblasts (127). Other authors have linked the
increased expression of ICAM-1 with the infiltration of TAMs in
renal carcinoma (128). Furthermore,
this ICAM-1-mediated infiltration was also augmented in pancreatic
cancer and correlated with the formation of precancerous lesions
(105). Moreover, ICAM-1 silencing
in C26 tumor-bearing mice resulted in decreased myeloid
infiltration in metastatic liver lesions (56). Consequently, the expression of ICAM-1
may represent a potential therapeutic target for treating immune
suppression during liver metastasis.
Recently, interest in the role of neutrophils as
promoters of metastatic progression has grown. In ductal pancreatic
adenocarcinoma, a significant association between the neutrophil
count and the overall survival of the patients was reported, along
with a correlation between the presence of neutrophils and distant
metastasis after surgery (129). A
relationship between neutrophils and metastasis formation in
several organs, including the liver, has also been observed in
other primary tumor types, such as breast carcinoma and CRC
(130,131). However, the role of host ICAM-1 in
this process is unknown. In chronic inflammation of the liver, an
increase of ICAM-1 on LSECs is known to mediate their interaction
with neutrophils through CD18 integrin, favoring transmigration and
adhesion to hepatocytes (132).
Thus, it is feasible to consider that the same mechanism may be
involved in neutrophil recruitment after CRC cell invasion in the
liver. In fact, it has been shown that neutrophils bind to the
liver endothelium through ligation to ICAM-1, facilitating the
adhesion of melanoma cells (133), a
process that may also be mediated by KCs. The fact that ICAM-1
expression on LSECs induced by KC-derived TNF-α leads to neutrophil
adhesion and migration into the hepatic sinusoids after liver
injury (134) supports the previous
observation. Additionally, neutrophils take an active role during
angiogenesis by secreting high levels of MMP-9. In fact, in gastric
cancer, neutrophils promote the immune evasion of tumor cells and
also the formation of new vessels (135).
The influence of ICAM-1 goes beyond its
membrane-bound form. In 1991, a soluble form of ICAM-1 was detected
in human serum (136). Subsequently,
chromatography and antibody detection revealed the significance of
this finding, demonstrating the ability of this soluble isoform to
interact with the membrane-bound ligands, such as LFA-1, as sICAM-1
contains a large fragment of the extracellular region of ICAM-1
(137). The serum level of
circulating sICAM-1 is considered as a biomarker for several
vascular inflammatory diseases, as well as in several different
cancer types, such as breast, lung and colon cancers, and for lung
and liver metastases (138–140). Interestingly, serum levels of
sICAM-1 in patients with liver metastasis were found to be the
highest among patients with different organ metastases (140). In the liver sICAM-1 is secreted from
diverse cell types, including mononuclear cells, endothelial cells,
fibroblasts, KCs and hepatocytes. During liver metastasis, several
pathways are mediated by this soluble factor. Tumor-activated LSECs
secrete sICAM-1 in response to various inflammatory stimuli, such
as IL-1β (27), and thereby further
enhance the metastatic capacity of tumor cells (40). It is tempting to hypothesize that
sICAM-1 may promote the expression of other adhesion molecules in
LSECs, as reported in human micro-vascular lung endothelial cells
using different cancer cell lines (141). Among the responses to sICAM-1,
proliferation of tumor and stromal cells has been observed in other
cancer models (142). In fact, COX-2
was demonstrated to be activated in tumor cells upon sICAM-1
binding with LFA-1, resulting in an increase in the production of
prostaglandin E2 (PGE2); PGE2 is a potent inflammatory, pro-immune
tolerance and pro-angiogenic mediator, which stimulates the
secretion of IL-1β by LSECs in an autocrine and paracrine loop
(27), and ultimately increases the
expression of membrane-bound ICAM-1 and vascular permeability, as
mentioned previously (Fig. 2). Both
IL-1β and PGE2 act as chemoattractants for MDSCs (143). Therefore, tumor cell binding to
sICAM-1 results in an amplifying strategy for the recruitment of
immune populations to develop an immune tolerant microenvironment
in the liver. This immune tolerant status is further supported by
the fact that sICAM-1 can interfere in the anti-tumor response, by
inhibiting the interaction between patrolling T cells and cancer
cells, and abrogating anti-tumor activity in natural killer cells
(144,145). Consequently, immune recognition
leading to tumor cell clearance may be reduced by the increased
level of circulating sICAM-1. Therefore, sICAM-1 acts as a
messenger of the membrane-bound ICAM-1 activation signals, moving
across the tumor vasculature to further enhance the pro-metastatic
response of the liver.
ICAM-1 has been unequivocally shown to have a key
role during tumor progression and metastasis formation in different
organs. ICAM-1 is involved in the rearrangement of the actin
cytoskeleton, the activation of pro-inflammatory cascades, and the
mediation of multiple signaling pathways that regulate metastasis,
such as tumor cell adhesion and transmigration, immune escape,
desmoplasia and angiogenesis. However, even though the mechanisms
by which the malignant cell expression of ICAM-1 mediates the
aggressiveness of tumor cells have received much research
attention, and are better understood, the role of ICAM-1 expression
on host organ cells has not been a major focus of investigation.
The expression of ICAM-1 on the surface of various resident liver
cells with roles in different events during tumor invasion and
colonization indicates that ICAM-1 could be a potential target for
complementary and personalized therapies, as well as a powerful
diagnostic and prognostic marker. As shown in this review, ICAM-1
acts as a transducer molecule, able to initiate a strong
inflammatory response, which in turn amplifies the reactions,
leading to increased adhesion, extravasation and tumor foci
formation. Moreover, the production of immune cell chemoattractants
promotes the recruitment of immunoregulatory cell populations that
reduce the immune surveillance of the tumor. Additionally, the
activation of pro-angiogenic and pro-desmoplastic stromal cells
though ICAM-1-mediated signaling pathways triggers the development
of a pro-tumor stroma and new vessel development, which further
favor the growth and ultimate colonization of the liver.
Thus, tissue ICAM-1 expression may reflect the
growth and metastatic status of multiple cancer types, and may be a
factor to predict cancer metastasis to the liver. ICAM-1 may be
involved in various stages, from the very initial stages of
inflammation, tumor and leukocyte adhesion to sinusoidal
endothelial cells, and the evasion of immune destruction, to the
very late stages of directional migration, differentiation and
colonization. In light of the present knowledge, host ICAM-1
interactions with ligands on tumor and host cells during the
different steps of metastatic progression remain an attractive
target for the development of anti-cancer strategies. This will
open new possibilities for treatments based on ICAM-1 as a
therapeutic target. However, further investigations into its
biological effects and the underlying mechanisms are required in
order to develop effective therapies that can block interactions
mediated by ICAM-1 without interfering with the normal functions of
the organism.
The present study was financially supported in part
by a postdoctoral fellowship from the University of the Basque
Country to A.B. and by funds from the Basque Government-Saiotek to
B.A.
|
1
|
Malietzis G, Lee GH, Bernardo D, Blakemore
AI, Knight SC, Moorghen M, Al-Hassi HO and Jenkins JT: The
prognostic significance and relationship with body composition of
CCR7-positive cells in colorectal cancer. J Surg Oncol. 112:86–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altendorf-Hofmann A and Scheele J: A
critical review of the major indicators of prognosis after
resection of hepatic metastases from colorectal carcinoma. Surg
Oncol Clin N Am. 12:165–192. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vidal-Vanaclocha F: The prometastatic
microenvironment of the liver. Cancer Microenviron. 1:113–129.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scheele J, Stangl R and Altendorf-Hofmann
A: Hepatic metastases from colorectal carcinoma: Impact of surgical
resection on the natural history. Br J Surg. 77:1241–1246. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donadon M, Ribero D, Morris-Stiff G,
Abdalla EK and Vauthey JN: New paradigm in the management of
liver-only metastases from colorectal cancer. Gastrointest Cancer
Res. 1:20–27. 2007.PubMed/NCBI
|
|
6
|
Haier J, Korb T, Hotz B, Spiegel HU and
Senninger N: An intravital model to monto steps of metastatic tumor
cell adhesion within the hepatic microcirculation. J Gastrointest
Surg. 7:507–514. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van den Eyden GG, Majeed AW, Illemann M,
Vermeulen PB, Bird NC, Høyer-Hansen G, Eefsen RL, Reynolds AR and
Brodt P: The multifaceted role of the microenvironment in liver
metastasis: Biology and clinical implications. Cancer Res.
73:2031–2043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rose DM, Essner R, Hughes TM, Tang PC,
Bilchik A, Wanek LA, Thompson JF and Morton DL: Surgical resection
for metastatic melanoma to the liver: The John Wayne cancer
institute and sydney melanoma unit experience. Arch Surg.
136:950–955. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eichbaum MH, Kaltwasser M, Bruckner T, de
Rossi TM, Schneeweiss A and Sohn C: Prognostic factors for patients
with liver metastases from breast cancer. Breast Cancer Res Treat.
96:53–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang TX, Chua TC and Morris DL:
Radioembolization and chemoembolization for unresectable
neuroendocrine liver metastases-a systematic review. Surg Oncol.
21:299–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klein CA: Cancer. The metastasis cascade.
Science. 321:1785–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stasinopoulos I, Penet MF, Krishnamachary
B and Bhujwalla ZM: Molecular and functional imaging of invasion
and metastasis: Windows into the metastatic cascade. Cancer
Biomark. 7:173–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paschos KA, Canovas D and Bird NC: The
role of cell adhesion molecules in the progression of colorectal
cancer and the development of liver metastasis. Cell Signal.
21:665–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawaguchi T: Organ preference of cancer
metastasis and metastasis-related cell adhesion molecules including
carbohydrates. Cardiovasc Hematol Disord Drug Targets. 15:164–186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Francavilla C, Maddaluno L and Cavallaro
U: The functional role of cell adhesion molecules in tumor
angiogenesis. Semin Cancer Biol. 19:298–309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mueller MM and Fusenig NE: Friends or
foes-bipolar effects of the tumour stroma in cancer. Nat Rev
Cancer. 4:839–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fridman WH, Remark R, Goc J, Giraldo NA,
Becht E, Hammond SA, Damotte D, Dieu-Nosjean MC and Sautès-Fridman
C: The immune microenvironment: A major player in human cancers.
Int Arch Allergy Immunol. 164:13–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McDonald PC, Chafe SC and Dedhar S:
Overcoming hypoxia-mediated tumor progression: Combinatorial
approaches targeting pH Regulation, angiogenesis and immune
dysfunction. Front Cell Dev Biol. 4:272016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited-the role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi H, Boelte KC and Lin PC:
Endothelial cell adhesion molecules and cancer progression. Curr
Med Chem. 14:377–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arabzadeh A, Chan C, Nouvion AL, Breton V,
Benlolo S, DeMarte L, Turbide C, Brodt P, Ferri L and Beauchemin N:
Host-related carcinoembryonic antigen cell adhesion molecule 1
promotes metastasis of colorectal cancer. Oncogene. 32:849–860.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khatib AM, Auguste P, Fallavollita L, Wang
N, Samani A, Kontogiannea M, Meterissian S and Brodt P:
Characterization of the host proinflammatory response to tumor
cells during the initial stages of liver metastasis. Am J Pathol.
167:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahn JJ, Chow JW, Horne GJ, Mah BK,
Emerman JT, Hoffman P and Hugh JC: MUC1 mediates transendothelial
migration in vitro by ligating endothelial cell ICAM-1. Clin Exp
Metastasis. 22:475–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laurent VM, Duperray A, Rajan Sundar V and
Verdier C: Atomic force microscopy reveals a role for endothelial
cell ICAM-1 expression in bladder cancer cell adherence. PLoS One.
9:e980342014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Palange AL, Di Mascolo D, Carallo C,
Gnasso A and Decuzzi P: Lipid-polymer nanoparticles encapsulating
curcumin for modulating the vascular deposition of breast cancer
cells. Nanomedicine. 10:991–1002. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clayton A, Evans RA, Pettit E, Hallett M,
Williams JD and Steadman R: Cellular activation through the
ligation of intercellular adhesion molecule-1. J Cell Sci.
111:443–453. 1998.PubMed/NCBI
|
|
27
|
Arteta B, Lasuen N, Lopategi A,
Sveinbjörnsson B, Smedsrød B and Vidal-Vanaclocha F: Colon
carcinoma cell interaction with liver sinusoidal endothelium
inhibits organ-specific antitumor immunity through
interleukin-1-induced mannose receptor in mice. Hepatology.
51:2172–2182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Delfortrie S, Pinte S, Mattot V, Samson C,
Villain G, Caetano B, Lauridant-Philippin G, Baranzelli MC,
Bonneterre J, Trottein F, et al: Egfl7 promotes tumor escape from
immunity by repressing endothelial cell activation. Cancer Res.
71:7176–7186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghislin S, Obino D, Middendorp S, Boggetto
N, Alcaide-Loridan C and Deshayes F: LFA-1 and ICAM-1 expression
induced during melanoma-endothelial cell co-culture favors the
transendothelial migration of melanoma cell lines in vitro. BMC
Cancer. 12:4552012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HT, Lee HI, Guo JH, Chen SH, Liao ZK,
Huang KW, Torng PL and Hwang LH: Calreticulin promotes tumor
lymphocyte infiltration and enhances the antitumor effects of
immunotherapy by up-regulating the endothelial expression of
adhesion molecules. Int J Cancer. 130:2892–2902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akeichi T, Mocevicius P, Deduchovas O,
Salnikova O, Castro-Santa E, Büchler MW, Schmidt J and Ryschich E:
αL β2 integrin is indispensable for CD8+ T-cell
recruitment in experimental pancreatic and hepatocellular cancer.
Int J Cancer. 130:2067–2076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Valcárcel M, Arteta B, Jaureguibeitia A,
Lopategi A, Martínez I, Mendoza L, Muruzabal FJ, Salado C and
Vidal-Vanaclocha F: Three-dimensional growth as multicellular
spheroid activates the proangiogenic phenotype of colorectal
carcinoma cells via LFA-1-dependent VEGF: Implications on hepatic
micrometastasis. J Transl Med. 6:572008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin Z, Jiang G, Fung JJ, Lu L and Qian S:
ICAM-1 expressed on hepatic stellate cells plays an important role
in immune regulation. Microsurgery. 27:328–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bruns T, Zimmermann HW, Pachnio A, Li KK,
Trivedi PJ, Reynolds G, Hubscher S, Stamataki Z, Badenhorst PW,
Weston CJ, et al: CMV infection of human sinusoidal endothelium
regulates hepatic T cell recruitment and activation. J Hepatol.
63:38–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Den Engel NK, Heidenthal E, Vinke A,
Kolb H and Martin S: Circulating forms of intercellular adhesion
molecule (ICAM)-1 in mice lacking membranous ICAM-1. Blood.
95:1350–1355. 2000.PubMed/NCBI
|
|
36
|
Pluskota E and D'Souza SE: Fibrinogen
interactions with ICAM-1 (CD54) regulate endothelial cell survival.
Eur J Biochem. 267:4693–4704. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen Q, Rahn JJ, Zhang J, Gunasekera N,
Sun X, Shaw AR, Hendzel MJ, Hoffman P, Bernier A and Hugh JC: MUC1
initiates Src-CrkL-Rac1/Cdc42-mediated actin cytoskeletal
protrusive motility after ligating intercellular adhesion
molecule-1. Mol Cancer Res. 6:555–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gulubova MV: Expression of cell adhesion
molecules and their beta1 and beta2 integrin ligands in human liver
peliosis. Pathol Res Pract. 201:503–511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oudar O, Moreau A, Feldmann G and Scoazec
JY: Expression and regulation of intercellular adhesion molecule-1
(ICAM-1) in organotypic cultures of rat liver tissue. J Hepatol.
29:901–909. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gangopadhyay A, Lazure DA and Thomas P:
Adhesion of colorectal carcinoma cells to the endothelium is
mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp
Metastasis. 16:703–712. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang L, Froio RM, Sciuto TE, Dvorak AM,
Alon R and Luscinskas FW: ICAM-1 regulates neutrophil adhesion and
transcellular migration of TNF-α-activated vascular endothelium
under flow. Blood. 106:584–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lalor PF, Shields P, Grant A and Adams DH:
Recruitment of lymphocytes to the human liver. Immunol Cell Biol.
80:52–64. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lawson C, Ainsworth M, Yacoub M and Rose
M: Ligation of ICAM-1 on endothelial cells leads to expression of
VCAM-1 via a nuclear factor-kappaB-independent mechanism. J
Immunol. 162:2990–2996. 1999.PubMed/NCBI
|
|
44
|
Selzner N, Selzner M, Odermatt B, Tian Y,
Van Rooijen N and Clavien PA: ICAM-1 triggers liver regeneration
through leukocyte recruitment and Kupffer cell-dependent release of
TNF-alpha/IL-6 in mice. Gastroenterology. 124:692–700. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Witkowska AM and Borawska MH: Soluble
intercellular adhesion molecule-1 (sICAM-1): An overview. Eur
Cytokine Netw. 15:91–98. 2004.PubMed/NCBI
|
|
46
|
Sprenger A, Schardt C, Rotsch M, Zehrer M,
Wolf M, Havemann K and Heymanns J: Soluble intercellular adhesion
molecule-1 in patients with lung cancer and benign lung diseases. J
Cancer Res Clin Oncol. 123:632–638. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maruo Y, Gochi A, Kaihara A, Shimamura H,
Yamada T, Tanaka N and Orita K: ICAM-1 expression and the soluble
ICAM-1 level for evaluating the metastatic potential of gastric
cancer. Int J Cancer. 100:486–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Christiansen I, Gidlof C, Kälkner KM,
Hagberg H, Bennmarker H and Tötterman T: Elevated serum levels of
soluble ICAM-1 in non-Hodgkin's lymphomas correlate with tumour
burden, disease activity and other prognostic markers. Br J
Haematol. 92:639–646. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu XW and Gong JP: Expression and role of
icam-1 in the occurrence and development of hepatocellular
carcinoma. Asian Pac J Cancer Prev. 14:1579–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kotteas EA, Gkiozos I, Tsagkouli S, Bastas
A, Ntanos I, Saif MW and Syrigos KN: Soluble ICAM-1 levels in
small-cell lung cancer: Prognostic value for survival and
predictive significance for response during chemotherapy. Med
Oncol. 30:6622013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gassmann P, Kang ML, Mees ST and Haier J:
In vivo tumor cell adhesion in the pulmonary microvasculature is
exclusively mediated by tumor cell-endothelial cell interaction.
BMC Cancer. 10:1772010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang P, Goodrich C, Fu C and Dong C:
Melanoma upregulates ICAM-1 expression on endothelial cells through
engagement of tumor CD44 with endothelial E-selectin and activation
of a PKCα-p38-SP-1 pathway. FASEB J. 28:4591–4609. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sipos E, Chen L, András IE, Wrobel J,
Zhang B, Pu H, Park M, Eum SY and Toborek M: Proinflammatory
adhesion molecules facilitate polychlorinated biphenyl-mediated
enhancement of brain metastasis formation. Toxicol Sci.
126:362–371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gong L, Mi HJ, Zhu H, Zhou X and Yang H:
P-selectin-mediated platelet activation promotes adhesion of
non-small cell lung carcinoma cells on vascular endothelial cells
under flow. Mol Med Rep. 5:935–942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Park JS, Kim KM, Kim MH, Chang HJ, Baek
MK, Kim SM and Jung YD: Resveratrol inhibits tumor cell adhesion to
endothelial cells by blocking ICAM-1 expression. Anticancer Res.
29:355–362. 2009.PubMed/NCBI
|
|
56
|
Benedicto A, Marquez J, Olaso E and Arteta
B: LFA-1/ICAM-1 interaction switches on an orchestrated
prometastatic microenvironmental shift during experimental liver
metastasis of colon C26 cancer cells. abstract. Cancer Res. 75:B10.
2015. View Article : Google Scholar
|
|
57
|
Eaton KV, Yang HL, Giachelli CM and
Scatena M: Engineering macrophages to control the inflammatory
response and angiogenesis. Exp Cell Res. 339:300–309. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Steinhoff G, Behrend M, Schrader B,
Duijvestijn AM and Wonigeit K: Expression patterns of leukocyte
adhesion ligand molecules on human liver endothelia. Lack of ELAM-1
and CD62 inducibility on sinusoidal endothelia and distinct
distribution of VCAM-1, ICAM-1, ICAM-2 and LFA-3. Am J Pathol.
142:481–488. 1993.PubMed/NCBI
|
|
59
|
Kong J, Kong L, Kong J, Ke S, Gao J, Ding
X, Zheng L, Sun H and Sun W: After insufficient radiofrequency
ablation, tumor-associated endothelial cells exhibit enhanced
angiogenesis and promote invasiveness of residual hepatocellular
carcinoma. J Transl Med. 10:2302012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee WY and Kubes P: Leukocyte adhesion in
the liver: Distinct adhesion paradigm from other organs. J Hepatol.
48:504–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Salas A, Shimaoka M, Phan U, Kim M and
Springer TA: Transition from rolling to firm adhesion can be
mimicked by extension of integrin alphaLbeta2 in an intermediate
affinity state. J Biol Chem. 281:10876–10882. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Roosien FF, de Kuiper PE, de Rijk D and
Roos E: Invasive and metastatic capacity of revertants of
LFA-1-deficient mutant T-cell hybridomas. Cancer Res. 50:3509–3513.
1990.PubMed/NCBI
|
|
63
|
Tatsumi T, Shimazaki C, Goto H, Araki S,
Sudo Y, Yamagata N, Ashihara E, Inaba T, Fujita N and Nakagawa M:
Expression of adhesion molecules on myeloma cells. Jpn J Cancer
Res. 87:837–842. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gulubova MV: Expression of cell adhesion
molecules, their ligands and tumour necrosis factor alpha in the
liver of patients with metastatic gastrointestinal carcinomas.
Histochem J. 34:67–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Soto MS, Serres S, Anthony DC and Sibson
NR: Functional role of endothelial adhesion molecules in the early
stages of brain metastasis. Neuro-Oncol. 16:540–551. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Horm TM and Schroeder JA: MUC1 and
metastatic cancer: Expression, function and therapeutic targeting.
Cell Adh Migr. 7:187–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Williams K, Motiani K, Giridhar PV and
Kasper S: CD44 integrates signaling in normal stem cell, cancer
stem cell and (pre)metastatic niches. Exp Biol Med (Maywood).
238:324–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Olsson E, Honeth G, Bendahl PO, Saal LH,
Gruvberger-Saal S, Ringnér M, Vallon-Christersson J, Jönsson G,
Holm K, Lövgren K, et al: CD44 isoforms are heterogeneously
expressed in breast cancer and correlate with tumor subtypes and
cancer stem cell markers. BMC Cancer. 11:4182011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ohtani H: Pathophysiologic significance of
host reactions in human cancer tissue: Desmoplasia and tumor
immunity. Tohoku J Exp Med. 187:193–202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Essani NA, McGuire GM, Manning AM and
Jaeschke H: Differential induction of mRNA for ICAM-1 and selectins
in hepatocytes, Kupffer cells and endothelial cells during
endotoxemia. Biochem Biophys Res Commun. 211:74–82. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Usami Y, Ishida K, Sato S, Kishino M,
Kiryu M, Ogawa Y, Okura M, Fukuda Y and Toyosawa S: Intercellular
adhesion molecule-1 (ICAM-1) expression correlates with oral cancer
progression and induces macrophage/cancer cell adhesion. Int J
Cancer. 133:568–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ohira H, Ueno T, Shakado S, Sakamoto M,
Torimura T, Inuzuka S, Sata M and Tanikawa K: Cultured rat hepatic
sinusoidal endothelial cells express intercellular adhesion
molecule-1 (ICAM-1) by tumor necrosis factor-alpha or interleukin-1
alpha stimulation. J Hepatol. 20:729–734. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tacconi C, Correale C, Gandelli A,
Spinelli A, Dejana E, D'Alessio S and Danese S: Vascular
endothelial growth factor C disrupts the endothelial lymphatic
barrier to promote colorectal cancer invasion. Gastroenterology.
148:1438–1451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Weber MR, Zuka M, Lorger M, Tschan M,
Torbett BE, Zijlstra A, Quigley JP, Staflin K, Eliceiri BP, Krueger
JS, et al: Activated tumor cell integrin αvβ3 cooperates with
platelets to promote extravasation and metastasis from the blood
stream. Thromb Res. 140:(Suppl 1). S27–S36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Locard-Paulet M, Lim L, Veluscek G,
McMahon K, Sinclair J, van Weverwijk A, Worboys JD, Yuan Y, Isacke
CM and Jørgensen C: Phosphoproteomic analysis of interacting tumor
and endothelial cells identifies regulatory mechanisms of
transendothelial migration. Sci Signal. 9:ra152016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Skau CT, Fischer RS, Gurel P, Thiam HR,
Tubbs A, Baird MA, Davidson MW, Piel M, Alushin GM, Nussenzweig A,
et al: FMN2 makes perinuclear actin to protect nuclei during
confined migration and promote metastasis. Cell. 167:1571–1585.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Freeman SA, McLeod SJ, Dukowski J, Austin
P, Lee CC, Millen-Martin B, Kubes P, McCafferty DM, Gold MR and
Roskelley CD: Preventing the activation or cycling of the Rap1
GTPase alters adhesion and cytoskeletal dynamics and blocks
metastatic melanoma cell extravasation into the lungs. Cancer Res.
70:4590–4601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sato T, Habtezion A, Beilhack A, Schulz S,
Butcher E and Thorlacius H: Short-term homing assay reveals a
critical role for lymphocyte function-associated antigen-1 in the
hepatic recruitment of lymphocytes in graft-versus-host disease. J
Hepatol. 44:1132–1140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gorina R, Lyck R, Vestweber D and
Engelhardt B: β2 integrin-mediated crawling on endothelial ICAM-1
and ICAM-2 is a prerequisite for transcellular neutrophil
diapedesis across the inflamed blood-brain barrier. J Immunol.
192:324–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fu C, Tong C, Wang M, Gao Y, Zhang Y, Lü
S, Liang S, Dong C and Long M: Determining beta2-integrin and
intercellular adhesion molecule 1 binding kinetics in tumor cell
adhesion to leukocytes and endothelial cells by a gas-driven
micropipette assay. J Biol Chem. 286:34777–34787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Haddad O, Chotard-Ghodsnia R, Verdier C
and Duperray A: Tumor cell/endothelial cell tight contact
upregulates endothelial adhesion molecule expression mediated by
NFkappaB: Differential role of the shear stress. Exp Cell Res.
316:615–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wong J, Johnston B, Lee SS, Bullard DC,
Smith CW, Beaudet AL and Kubes P: A minimal role for selectins in
the recruitment of leukocytes into the inflamed liver
microvasculature. J Clin Invest. 99:2782–2790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ronald JA, Ionescu CV, Rogers KA and
Sandig M: Differential regulation of transendothelial migration of
THP-1 cells by ICAM-1/LFA-1 and VCAM-1/VLA-4. J Leukoc Biol.
70:601–609. 2001.PubMed/NCBI
|
|
86
|
Kubes P and Mehal WZ: Sterile inflammation
in the liver. Gastroenterology. 143:1158–1172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ramadori G, Moriconi F, Malik I and Dudas
J: Physiology and pathophysiology of liver inflammation, damage and
repair. J Physiol Pharmacol. 59:(Suppl 1). S107–S117. 2008.
|
|
88
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Whiteside TL: Immune suppression in
cancer: Effects on immune cells, mechanisms and future therapeutic
intervention. Semin Cancer Biol. 16:3–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Madar S, Goldstein I and Rotter V: ‘Cancer
associated fibroblasts’-more than meets the eye. Trends Mol Med.
19:447–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hellerbrand Wang SC, Tsukamoto H, Brenner
DA and Rippe RA: Expression of intracellular adhesion molecule 1 by
activated hepatic stellate cells. Hepatology. 24:670–676. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Masamune A, Sakai Y, Kikuta K, Satoh M,
Satoh A and Shimosegawa T: Activated rat pancreatic stellate cells
express intercellular adhesion molecule-1 (ICAM-1) in vitro.
Pancreas. 25:78–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kang N, Gores GJ and Shah VH: Hepatic
stellate cells: Partners in crime for liver metastases? Hepatology.
54:707–713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Olaso E, Salado C, Egilegor E, Gutierrez
V, Santisteban A, Sancho-Bru P, Friedman SL and Vidal-Vanaclocha F:
Proangiogenic role of tumor-activated hepatic stellate cells in
experimental melanoma metastasis. Hepatology. 37:674–685. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Muhanna N, Doron S, Wald O, Horani A, Eid
A, Pappo O, Friedman SL and Safadi R: Activation of hepatic
stellate cells after phagocytosis of lymphocytes: A novel pathway
of fibrogenesis. Hepatology. 48:963–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Schildberg FA, Wojtalla A, Siegmund SV,
Endl E, Diehl L, Abdullah Z, Kurts C and Knolle PA: Murine hepatic
stellate cells veto CD8 T cell activation by a CD54-dependent
mechanism. Hepatology. 54:262–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhao W, Zhang L, Xu Y, Zhang Z, Ren G,
Tang K, Kuang P, Zhao B, Yin Z and Wang X: Hepatic stellate cells
promote tumor progression by enhancement of immunosuppressive cells
in an orthotopic liver tumor mouse model. Lab Invest. 94:182–191.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Fisher DT, Appenheimer MM and Evans SS:
The two faces of IL-6 in the tumor microenvironment. Semin Immunol.
26:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Roca H, Varsos ZS, Sud S, Craig MJ, Ying C
and Pienta KJ: CCL2 and interleukin-6 promote survival of human
CD11b+ peripheral blood mononuclear cells and induce
M2-type macrophage polarization. J Biol Chem. 284:34342–34354.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Okada S, Shikata K, Matsuda M, Ogawa D,
Usui H, Kido Y, Nagase R, Wada J, Shikata Y and Makino H:
Intercellular adhesion molecule-1-deficient mice are resistant
against renal injury after induction of diabetes. Diabetes.
52:2586–2593. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liou GY, Döppler H, Necela B, Edenfield B,
Zhang L, Dawson DW and Storz P: Mutant KRAS-induced expression of
ICAM-1 in pancreatic acinar cells causes attraction of macrophages
to expedite the formation of precancerous lesions. Cancer Discov.
5:52–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lee BR, Chang SY, Hong EH, Kwon BE, Kim
HM, Kim YJ, Lee J, Cho HJ, Cheon JH and Ko HJ: Elevated endoplasmic
reticulum stress reinforced immunosuppression in the tumor
microenvironment via myeloid-derived suppressor cells. Oncotarget.
5:12331–12345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sander LE, Sackett SD, Dierssen U, Beraza
N, Linke RP, Müller M, Blander JM, Tacke F and Trautwein C: Hepatic
acute-phase proteins control innate immune responses during
infection by promoting myeloid-derived suppressor cell function. J
Exp Med. 207:1453–1464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang Q and Doerschuk CM: The p38
mitogen-activated protein kinase mediates cytoskeletal remodeling
in pulmonary microvascular endothelial cells upon intracellular
adhesion molecule-1 ligation. J Immunol. 166:6877–6884. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Novo E, Cannito S, Zamara E, di Valfrè
Bonzo L, Caligiuri A, Cravanzola C, Compagnone A, Colombatto S,
Marra F, Pinzani M and Parola M: Proangiogenic cytokines as
hypoxia-dependent factors stimulating migration of human hepatic
stellate cells. Am J Pathol. 170:1942–1953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li X, Wang X, Han C, Wang X, Xing G, Zhou
L, Li G and Niu Y: Astragaloside IV suppresses collagen production
of activated hepatic stellate cells via oxidative stress-mediated
p38 MAPK pathway. Free Radic Biol Med. 60:168–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cui X, Zhang X, Yin Q, Meng A, Su S, Jing
X, Li H, Guan X, Li X, Liu S and Cheng M: F-actin cytoskeleton
reorganization is associated with hepatic stellate cell activation.
Mol Med Rep. 9:1641–1647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang Q, Pfeiffer GR II and Gaarde WA:
Activation of SRC tyrosine kinases in response to ICAM-1 ligation
in pulmonary microvascular endothelial cells. J Biol Chem.
278:47731–47743. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lee SJ, Drabik K, Van Wagoner NJ, Lee S,
Choi C, Dong Y and Benveniste EN: ICAM-1-induced expression of
proinflammatory cytokines in astrocytes: Involvement of
extracellular signal-regulated kinase and p38 mitogen-activated
protein kinase pathways. J Immunol. 165:4658–4666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Blaber R, Stylianou E, Clayton A and
Steadman R: Selective regulation of ICAM-1 and RANTES gene
expression after ICAM-1 ligation on human renal fibroblasts. J Am
Soc Nephrol. 14:116–127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Borkham-Kamphorst E, van Roeyen CR,
Ostendorf T, Floege J, Gressner AM and Weiskirchen R:
Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol.
46:1064–1074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Vermeulen PB, Colpaert C, Salgado R,
Royers R, Hellemans H, Van Den Heuvel E, Goovaerts G, Dirix LY and
Van Marck E: Liver metastases from colorectal adenocarcinomas grow
in three patterns with different angiogenesis and desmoplasia. J
Pathol. 195:336–342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Schellerer VS, Langheinrich M, Hohenberger
W, Croner RS, Merkel S, Rau TT, Stürzl M and Naschberger E:
Tumor-associated fibroblasts isolated from colorectal cancer
tissues exhibit increased ICAM-1 expression and affinity for
monocytes. Oncol Rep. 31:255–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Brackett CM, Kojouharov B, Veith J, Greene
KF, Burdelya LG, Gollnick SO, Abrams SI and Gudkov AV: Toll-like
receptor-5 agonist, entolimod, suppresses metastasis and induces
immunity by stimulating an NK-dendritic-CD8+ T-cell
axis. Proc Natl Acad Sci USA. 113:E874–E883. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Poczobutt JM, Nguyen TT, Hanson D, Li H,
Sippel TR, Weiser-Evans MC, Gijon M, Murphy RC and Nemenoff RA:
Deletion of 5-Lipoxygenase in the tumor microenvironment promotes
lung cancer progression and metastasis through regulating T cell
recruitment. J Immunol. 196:891–901. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zanetti M: Tapping CD4 T cells for cancer
immunotherapy: The choice of personalized genomics. J Immunol.
194:2049–2056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Umansky V, Blattner C, Gebhardt C and
Utikal J: The Role of Myeloid-Derived Suppressor Cells (MDSC) in
Cancer Progression. Vaccines (Basel). 4:E362016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Umansky V, Blattner C, Fleming V, Hu X,
Gebhardt C, Altevogt P and Utikal J: Myeloid-derived suppressor
cells and tumor escape from immune surveillance. Semin
Immunopathol. 39:295–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Baay M, Brouwer A, Pauwels P, Peeters M
and Lardon F: Tumor cells and tumor-associated macrophages:
Secreted proteins as potential targets for therapy. Clin Dev
Immunol. 2011:5651872011. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ostrand-Rosenberg S and Sinha P:
Myeloid-derived suppressor cells: Linking inflammation and cancer.
J Immunol. 182:4499–4506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zen K, Masuda J and Ogata J:
Monocyte-derived macrophages prime peripheral T cells to undergo
apoptosis by cell-cell contact via ICAM-1/LFA-1-dependent
mechanism. Immunobiology. 195:323–333. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Makgoba MW, Sanders ME, Ginther Luce GE,
Dustin ML, Springer TA, Clark EA, Mannoni P and Shaw S: ICAM-1 a
ligand for LFA-1-dependent adhesion of B, T and myeloid cells.
Nature. 331:86–88. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Rabquer BJ, Hou Y, Del Galdo F, Haines GK
III, Gerber ML, Jimenez SA, Seibold JR and Koch AE: The proadhesive
phenotype of systemic sclerosis skin promotes myeloid cell adhesion
via ICAM-1 and VCAM-1. Rheumatology (Oxford). 48:734–740. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hemmerlein B, Scherbening J, Kugler A and
Radzun HJ: Expression of VCAM-1, ICAM-1, E- and P-selectin and
tumour-associated macrophages in renal cell carcinoma.
Histopathology. 37:78–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tao L, Zhang L, Peng Y, Tao M, Li L, Xiu
D, Yuan C, Ma Z and Jiang B: Neutrophils assist the metastasis of
circulating tumor cells in pancreatic ductal adenocarcinoma: A new
hypothesis and a new predictor for distant metastasis. Medicine
(Baltimore). 95:e49322016. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tabariès S, Ouellet V, Hsu BE, Annis MG,
Rose AA, Meunier L, Carmona E, Tam CE, Mes-Masson AM and Siegel PM:
Granulocytic immune infiltrates are essential for the efficient
formation of breast cancer liver metastases. Breast Cancer Res.
17:452015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hirai H, Fujishita T, Kurimoto K, Miyachi
H, Kitano S, Inamoto S, Itatani Y, Saitou M, Maekawa T and Taketo
MM: CCR1-mediated accumulation of myeloid cells in the liver
microenvironment promoting mouse colon cancer. Clin Exp Metastasis.
31:977–989. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ramaiah SK and Jaeschke H: Hepatic
neutrophil infiltration in the pathogenesis of alcohol-induced
liver injury. Toxicol Mech Methods. 17:431–440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Slattery MJ, Liang S and Dong C: Distinct
role of hydrodynamic shear in leukocyte-facilitated tumor cell
extravasation. Am J Physiol Cell Physiol. 288:C831–C839. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sakamoto S, Okanoue T, Itoh Y, Nakagawa Y,
Nakamura H, Morita A, Daimon Y, Sakamoto K, Yoshida N, Yoshikawa T
and Kashima K: Involvement of Kupffer cells in the interaction
between neutrophils and sinusoidal endothelial cells in rats.
Shock. 18:152–157. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Li TJ, Jiang YM, Hu YF, Huang L, Yu J,
Zhao LY, Deng HJ, Mou TY, Liu H, Yang Y, et al:
Interleukin-17-producing neutrophils link inflammatory stimuli to
disease progression by promoting angiogenesis in gastric cancer.
Clin Cancer Res. 23:1575–1585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Seth R, Raymond FD and Makgoba MW:
Circulating ICAM-1 isoforms: Diagnostic prospects for inflammatory
and immune disorders. Lancet. 338:83–84. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Rothlein R, Mainolfi EA, Czajkowski M and
Marlin SD: A form of circulating ICAM-1 in human serum. J Immunol.
147:3788–3793. 1991.PubMed/NCBI
|
|
138
|
Tesarova P, Kalousova M, Zima T, Suchanek
M, Malikova I, Kvasnicka J, Duskova D, Tesar V, Vachek J,
Krupickova-Kasalova Z and Malik J: Endotelial activation and
flow-mediated vasodilation in young patients with breast cancer.
Neoplasma. 60:690–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Guney N, Soydinc HO, Derin D, Tas F,
Camlica H, Duranyildiz D, Yasasever V and Topuz E: Serum levels of
intercellular adhesion molecule ICAM-1 and E-selectin in advanced
stage non-small cell lung cancer. Med Oncol. 25:194–200. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Dymicka-Piekarska V, Guzinska-Ustymowicz
K, Kuklinski A and Kemona H: Prognostic significance of adhesion
molecules (sICAM-1, sVCAM-1) and VEGF in colorectal cancer
patients. Thromb Res. 129:e47–e50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Chen C, Duckworth CA, Zhao Q, Pritchard
DM, Rhodes JM and Yu LG: Increased circulation of galectin-3 in
cancer induces secretion of metastasis-promoting cytokines from
blood vascular endothelium. Clin Cancer Res. 19:1693–1704. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Takahara M, Nagato T, Komabayashi Y,
Yoshino K, Ueda S, Kishibe K and Harabuchi Y: Soluble ICAM-1
secretion and its functional role as an autocrine growth factor in
nasal NK/T cell lymphoma cells. Exp Hematol. 41:711–718. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Draghiciu O, Lubbers J, Nijman HW and
Daemen T: Myeloid derived suppressor cells-An overview of combat
strategies to increase immunotherapy efficacy. Oncoimmunology.
4:e9548292015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Becker JC, Dummer R, Hartmann AA, Burg G
and Schmidt RE: Shedding of ICAM-1 from human melanoma cell lines
induced by IFN-gamma and tumor necrosis factor-alpha. Functional
consequences on cell-mediated cytotoxicity. J Immunol.
147:4398–4401. 1991.PubMed/NCBI
|
|
145
|
Becker JC, Termeer C, Schmidt RE and
Bröcker EB: Soluble intercellular adhesion molecule-1 inhibits
MHC-restricted specific T cell/tumor interaction. J Immunol.
151:7224–7232. 1993.PubMed/NCBI
|