1. Introduction
Gastrointestinal adenocarcinoma (GIA) is a common
malignant disease worldwide. Despite a worldwide decline in
incidence and mortality since the second half of the 20th century,
gastric cancer still ranks as the fourth most common and the second
most frequent cause of mortality from cancer. Gastric cancer
continues to be a major health concern due to the slow decrease in
incidence in Asia and high mortality from diagnosed gastric
carcinomas in the West, even though sophisticated diagnostic and
surgical techniques are widely applied in clinical practice
(1). Colorectal cancer is one of
the most common types of cancer in the world, accounting for almost
10% of all new cases of cancer. Pathological and genetic
observations demonstrated that colorectal adenoma precedes the
majority of colorectal adenocarcinoma and may undergo malignant
transformation into adenocarcinoma (2). Tumorigenesis and progression of
gastric and colorectal carcinoma is a multistage process with the
involvement of a multifactorial etiology, which mainly results from
gene-environment interactions. Knowledge regarding altered
expression of these genes during carcinogenesis may not only
provide information about the molecular events during the
initiation and progression of cancer, but may also result in the
discovery of biological markers for the evaluation of cancer
diagnosis and prognosis, which may aid the improvement of
diagnosis, treatment and prevention of malignancies.
In the studies presented in this review, we firstly
established tissue microarray (TMA) using the tissue microarryer
and stained the slides with hematoxylin and eosin (HE) to confirm
the histological diagnosis (Fig.
1A). Although minute TMAs cannot ensure representative areas of
donor specimen, we used 2-mm diameter needles, which are large
enough to evaluate the morphological appearance if the
representative regions are carefully selected with HE slides.
Therefore, we believe that the advantages of high throughput,
identical immunohistochemical conditions, and economy of samples,
antibodies and time make this approach effective for screening in
clinicopathological practice (3).
Additionally, a rapid immunostaining approach was employed to
improve the immunoreactive quality by utilizing microwave autoclave
and intermittent microwave irradiation (MI-77, Fig. 1B) during incubation. Intermittent
microwaving causes minute vibrations more than 2.4 billion
times/sec, which increases the probability of antibodies colliding
with specific antigens. At the same time, antibodies are easily
dislodged from non-specific binding sites by the motion (4). These determine the higher quality of
immunohistochemistry and widen the antibodies without the
application of formalin-fixed and paraffin-embedded samples in TMA
(Fig. 2). Additionally, we also
prepared the digoxin-labeled probes by PCR and performed the
DNA-mRNA in situ hybridization (ISH) to detect the in
situ expression of certain mRNA markers (Fig. 3). Using these approaches, we mainly
aimed to screen for ideal markers that indicate pathogenesis,
invasion, metastasis and prognosis of gastrointestinal carcinomas.
The detailed findings of our previous studies (5–31) are
shown in Table I and II.
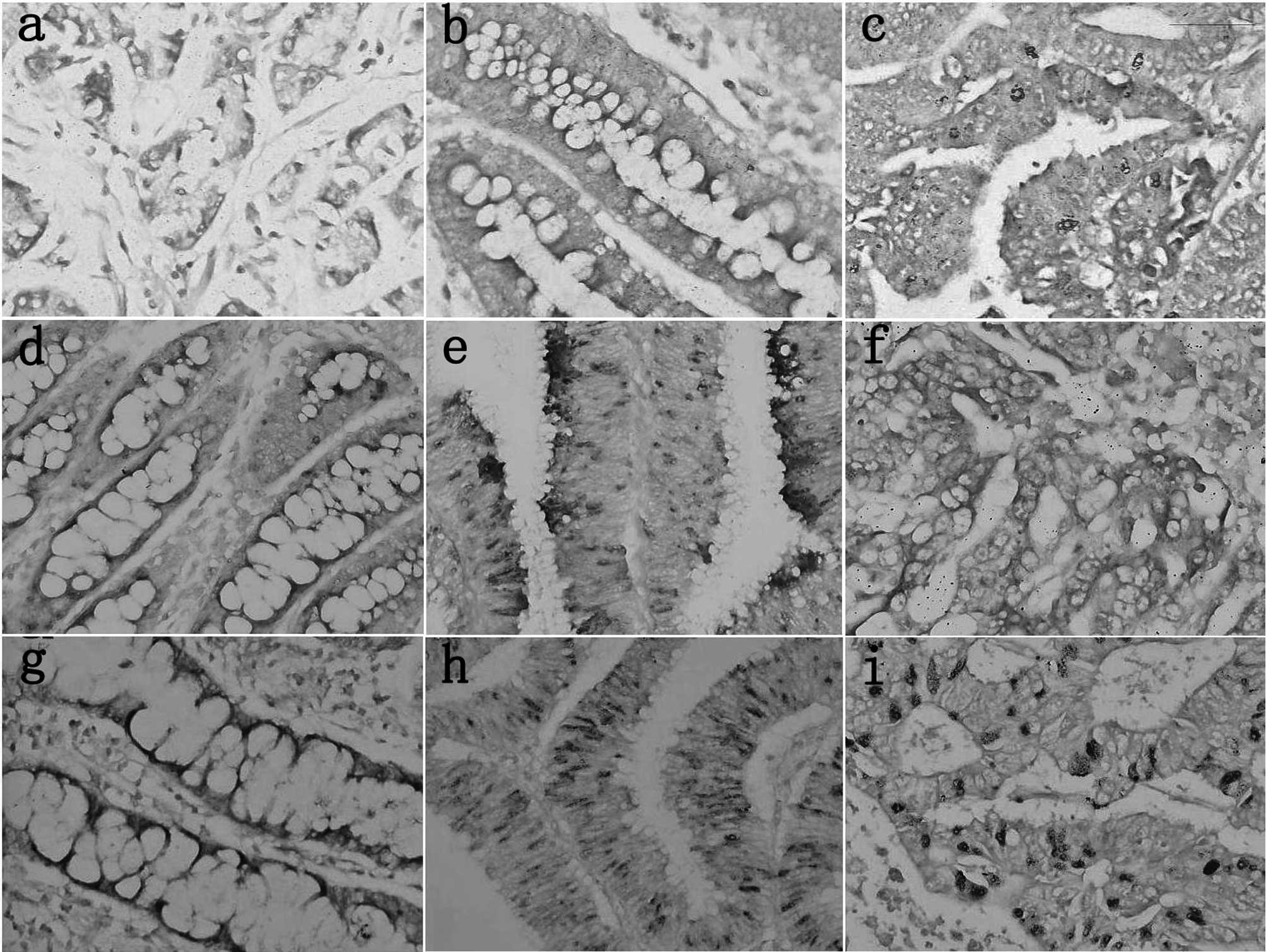
 | Figure 2Immunohistochemical staining of
various markers in gastrointestinal carcinomas. (a) p53, (b) PTEN,
(c) FHIT, (d) ING5, (e) Parafibromin, (f) KAI1, (g) maspin, (h)
MMP-2, (i) MMP-7, (j) MMP-9, (k) EMMPRIN, (l) VEGF, (m) tenascin,
(n) CD34, (o) Arp2, (p) Arp3, (q) cortactin, (r) fascin, (s) GRP78,
(t) RP94, (u) GSK3β-ser9, (v) Pim-3, (w) MUC-1, (x)
MUC-2, (y) MUC-4, (z) MUC-5AC, (a1) MUC-6, (b1) REG Iα (c1) REG Iβ,
(d1) REGIII, (e1) HIP/PAP, (f1) REG IV, (g1) CD44, (h1) E-cadherin,
(i1) β-catenin. |
 | Table IThe protein expression in
gastrointestinal carcinogenesis. |
Table I
The protein expression in
gastrointestinal carcinogenesis.
| Groups | Up-regulated
molecules | Down-regulated
molecules |
|---|
| Intestinal
metaplasia | Maspin, EMMPRIN,
Arp2 | - |
| Adenoma | p53, maspin, EMMPRIN,
fascin, GRP78, GRP94, Pim-3, MUC-6, REG IV | PTEN,
parafibromin |
| Carcinoma | p53, KAI1, maspin,
EMMPRIN, Arp2, Arp3, cortactin, fascin, GRP78, GRP94,
GSK3β-ser9, Pim-3, MUC-1, REG Iα, REG III, HIP/PAP, REG
IV | PTEN, FHIT, ING5,
parafibromin, MUC-6, REG Iβ |
 | Table IIThe relationship between the protein
expression and clinicopathological parameters of gastrointestinal
carcinomas. |
Table II
The relationship between the protein
expression and clinicopathological parameters of gastrointestinal
carcinomas.
| Groups | Upregulated
molecules | Downregulated
molecules |
|---|
| Age (young) | | Nuclear ING5,
parafibromin, maspin, fascin, GRP94 |
| Gender (Male) | Cortactin, GRP94,
GSK3β-ser9, Pim-3, FHIT | - |
| Tumor size
(small) | Nuclear ING5,
parafibromin, MUC-6 | MMP-2, MMP-7, MMP-9,
EMMPRIN, VEGF, Arp2, Arp3, cortactin, fascin, GRP78, GRP94 |
| Depth of invasion
(superficial) | PTEN, FHIT, nuclear
ING5, parafibromin, MUC-6, REG Iα, REG Iβ, REG III, HIP/PAP | p53, cytoplasmic
ING5, maspin, MMP-2, MMP-7, MMP-9, EMMPRIN, VEGF, Arp2, Arp3,
cortactin, fascin, GRP78, GRP94, GSK3β-ser9, MUC-1 |
| Lymphatic invasion
(+) | PTEN, FHIT,
parafibromin, MUC-6 | p53, maspin, MMP-2,
MMP-9, EMMPRIN, VEGF, cortactin, fascin, GRP78, GRP94,
GSK3β-ser9, Pim-3, MUC-1 |
| Venous invasion
(+) | FHIT, MUC-6, REG
Iβ, HIP/PAP | p53, cytoplasmic
ING5, MMP-2, MMP-9, EMMPRIN, VEGF, Arp2, Arp3, cortactin, fascin,
GRP78, GRP94, GSK3β-ser9, Pim-3, MUC-1 |
| Lymph node
metastasis (+) | PTEN, FHIT, nuclear
ING5, parafibromin, MUC-6, REG Iα, HIP/PAP | p53, cytoplasmic
ING5, maspin, MMP-2, MMP-7, MMP-9, VEGF, cortactin, fascin, GRP78,
GRP94, GSK3β-ser9, MUC-1 |
| Liver metastasis
UICC staging (low) | PTEN, FHIT, nuclear
ING5, parafibromin, MUC-6 | PTEN, FHIT, KAI1,
tenascin cytoplasmic ING5, maspin, MMP-2, MMP-7, MMP-9, VEGF, Arp2,
Arp3, cortactin, fascin, GRP78, GRP94, MUC-1 |
| Lauren's
classification (Intestinal) | p53, FHIT, nuclear
ING5, parafibromin, maspin, MMP-2, MMP-7, MMP-9, EMMPRIN, VEGF,
cortactin, Pim-3, MUC-1, MUC-6, VEGF, GSK3β-ser9, MUC-2,
MUC-4, CD44, E-cadherin, membrane β-catenin | Cytoplasmic
ING5 |
| Favorable
prognosis | PTEN, FHIT, nuclear
ING5, parafibromin, MUC-6 | Maspin, EMMPRIN,
fascin, GRP78, GRP94, GSK3β-ser9, Pim-3, MUC-1 |
2. Tumor suppressor genes
Malignant transformation is a biologically
complicated process, resulting from frequent genetic alterations
influencing the expression of oncogenes and tumor suppressor genes
(TSG). Generally, chromosomal deletion may lead to loss of TSG
causing uncontrolled proliferation and immortal survival, critical
for initiation, promotion and tumor development.
p53 is thought to play a central role in protecting
against the development of cancer. Its encoding protein is a master
switch that coordinates and concentrates a plethora of stress
signals and transforms them into a series of responses, such as
apoptosis or cell cycle arrest in response to DNA damage, thereby
maintaining genetic stability in the organism. Although p53
inactivation in human cancer is a complex process, depending on the
tissue type, p53 dysfunction may disorder the biological events of
cancer cells and give rise to their aggressive phenotypes.
Currently, no antibody can discriminate between the mutant and
wild-type p53 protein, although the anti-p53 antibody is widely
applied in clinical practice. In our study, the p53 expression was
gradually increased from gastrointestinal mucosa to adenoma to
adenocarcinoma, and showed a positive association with depth of
invasion, local invasion via vessels and lymph node metastasis of
GIA, suggesting that the majority of accumulated p53 proteins may
be of the mutant subtype in GIA or adenocarcinoma (5).
The PTEN gene (phosphatase and tensin
homology deleted from human chromosome 10) inhibits Shc
phosphorylation and therefore blocks the activation of the
Ras/MAP-kinase pathway. PTEN also dephosphorylates focal adhesion
kinase (FAK), affecting cell adhesion, spreading and recognition.
Furthermore, PTEN acts as a phospholipid phosphatase with
phosphatidylinositol 3, 4, 5-trisphosphate (PIP3) as a substrate
and one down-stream target of PIP3, protein kinase (Akt/PKB), is
continually activated by phosphorylation in cells lacking in
functional PTEN. PTEN expression was lower in gastric carcinoma
than that in non-neoplastic mucosa (NNM), adenoma or carcinoma of
the stomach and colorectum, and inversely correlated with tumor
size, depth of invasion, lymphatic invasion, venous invasion, lymph
node metastasis, TNM staging, lower caspase-3 expression, and worse
prognosis of gastric and colorectal carcinoma. The mutation
analysis revealed only one synonymous mutation in exon 8 (codon 312
Asp: GAC→GAT) in colorectal carcinoma using high fidelity
polymerase and direct DNA sequencing. In our studies, the anti-PTEN
antibody can recognize the nuclear protein, although PTEN functions
as a phosphatase in the cytosol. PTEN has nuclear localization
signal-like sequences for nuclear import mediated by a major vault
protein and is required for cell cycle arrest in the nucleus
(6,7).
FHIT (fragile histine triad) was isolated by
positional cloning, and encompassed the most common human fragile
site FRA3B at 3p14.2, a region with frequent hemizygous and
homozygous deletion in a variety of human tumors. Studies on
protein-protein interactions, cell lines, tumorigenicity tests and
knockout mice suggest that the FHIT protein is involved in cell
proliferation and apoptosis, and may act as a tumor suppressor
independent of its hydrolase activity. FHIT was less expressed in
gastric NNM and adenoma than gastritis. FHIT expression showed a
significantly negative association with depth of invasion,
lymphatic invasion, lymph node metastasis, liver metastasis, UICC
staging and worse prognosis of gastric carcinoma, but its functions
have not been sufficiently explored (6).
Inhibitor of growth 5 (ING5) interacts with histone
H3K4me3 and is involved in the formation of two different histone
acetyl transferase (HAT) complexes, which have an important role
during DNA replication in cooperation with the mini-chromosome
maintenance complex. ING5 was reported to activate the
cyclin-dependent kinase inhibitor p21/waf1 promoter to
induce p21/waf1 expression, enhance p53 acetylation at Lys-382
residues, and physically interact with p300, a member of the HAT
complexes, and p53 in vivo. In our GIAs, ING5 protein has
nuclear-cytoplasmic translocation with aggressive transformation in
the colorectal adenoma-adenocarcinoma sequence. Downregulated
nuclear ING5 expression may be employed to indicate worse behaviors
or prognosis, while it was the converse for the cytoplasmic
counterpart in the two carcinomas. The subcellular distribution of
ING5, its biological effects on cell phenotypes and related
molecular mechanisms should be further investigated in the future
(8,9).
Parafibromin is be involved in the formation of the
Paf1 complex, which is associated with RNA polymerase II and
involved in transcript site selection, transcriptional elongation,
histone H2B ubiquitination, histone H3 methylation, poly (A) length
control, and coupling of transcriptional and post-transcriptional
events. Parafibromin overexpression was found to inhibit colony
formation and cellular proliferation and induce cell cycle arrest
in the G1 phase, indicating that parafibromin has a critical role
in cell growth. RNA interference with the expression of
parafibromin or Paf1 was proved to stimulate cell proliferation and
increase the c-myc level by stabilizing c-myc protein and
activating the c-myc promoter. We found that parafibromin
expression was gradually decreased from gastric and colorectal
carcinogenesis regardless of protein or mRNA levels. Its
downregulated expression was inversely correlated with tumor size,
depth of invasion, lymphatic invasion, lymph node metastasis, UICC
staging or shorter survival time. In addition, parafibromin
overexpression caused G1 arrest and enhanced differentiation of
DLD-1 cells. A high expression of p21, p27 and cyclin E, but a low
expression of cyclin D1 mRNA, phospho-cdc2 and
phospho-cdc25c proteins was observed in parafibromin-overexpressing
DLD-1. Parafibromin inhibited c-myc mRNA expression by
binding to the c-myc promoter in colorectal carcinoma cells.
The signal pathways of nuclear parafibromin are being established
by phenotype, protein-protein or protein-DNA analyses in GIAs
(10,11).
3. Metastasis suppressor genes
KAI1 (CD82/C33/R2/IA4) was initially
identified as a tumor metastasis suppressor gene and encodes
transmembrane glycoproteins of the tetraspanins family (TM4SF).
TM4SF proteins have cytoplasmic N- and C-termini and traverse the
cell membrane four times forming one small and one large
extracellular loop with residues susceptible to post-translational
phosphorylation and/or N-linked glycosylation. KAI1 interacts with
integrin α4β1, other TM4SF proteins, and cell surface molecules
including CD4, CD8, CD19, CD21 and MHC class I and II, forming ‘the
tetraspanin web’. Several recent reports suggested that a complex
combination between KAI1 and specific proteins is involved in a
number of biological processes, such as cellular adhesion,
mobility, proliferation and apoptosis (12,13).
In our study, KAI1 expression was higher in GIAs than that in their
adjacent NNM and showed a significantly negative association with
the liver metastasis of GIAs, which is useful and may aid in
predicting the risk of liver metastasis (14).
Maspin exhibits significant homology to the
super-family of such serine protease inhibitors as the plasminogen
activator inhibitors 1 and 2, and α1-antitrypsin, which is closely
linked to the degradation of extracellular matrix (ECM). Maspin has
been shown to inhibit tumor cell motility and invasion in mammary
and prostate carcinoma cell lines in vitro, and to reduce
the capacity for tumorigenesis and metastasis of cancer cell lines
in animal models. However, in vivo experiments have
indicated a decrease and an increase of maspin levels in tumors,
and levels of the protein have paradoxically been described to
parallel tumor progression in spite of being regarded as a tumor
suppressor gene (15–19). In this study, it was also found that
cytoplasmic and nuclear maspin expression paralleled each other and
decreased from gastric intestinal metaplasia, adenoma and carcinoma
to normal mucosa. A significant positive association was noted with
depth of invasion, lymphatic invasion, lymph node metastasis, TNM
stage and cumulative patient survival in gastric carcinoma. By
contrast, maspin expression showed a significant increase from
colorectal NNM to adenocarcinoma through adenoma, and correlated
negatively with the liver metastasis of colorectal carcinomas.
Therefore, the tissue specificity of maspin and its function in the
nucleus should be studied in future investigations (20,21).
4. Angiogenesis
Growth of solid tumors depends on angiogenesis,
which facilitates metastasis formation, and provides nutrients and
oxygen for growth at the metastasis site. Critical steps during
tumor angiogenesis are the outgrowth of endothelial cells from
preexisting capillary vessels and their migration from parental
vessels under the stimulation of vascular endothelial growth factor
(VEGF). Proliferating endothelial cells subsequently remodel ECM
via matrix metalloproteinases (MMPs), align into tube-like
structures, and eventually form new functional blood vessels. In
cancer cells, overexpression of angiogenic factors (e.g., VEGF and
MMPs) results in their increased secretion into the ECM to
stimulate the proliferation and mobility of vascular epithelial
cells, closely linked to invasion and metastasis.
MMPs are a family of enzymes that proteolytically
degrade various components of the ECM. A high expression of MMPs by
the tumor cells is closely correlated with tumor invasive and
metastatic potential. MMP-2 (72 kDa), −7 (19 kDa) and −9 (92 kDa)
have been shown to play critical roles in the ‘angiogenic switch’
and tumor cells synthesize and secret large amounts of MMP-2 and −9
in a paracrine and/or autocrine manner to stimulate angiogenesis
and increase VEGF release. VEGF is a 45-kDa homodimeric
glycoprotein, which acts as a potent and selective endothelial
mitogen. Our group found that the expression of MMP-2, −7, −9 and
VEGF were positively correlated with tumor size, depth of invasion,
lymphatic and venous invasion, lymph node metastasis, TNM staging
and microvessel density (MVD) of gastric carcinomas. VEGF
expression was also positively correlated with the levels of MMP-2
and −9, but negatively with PTEN. The latter was also inversely
associated with the MVD in gastric carcinomas (22,23).
The extracellular region of EMMPRIN (extracellular
matrix metalloproteinase inducer) contains three N-linked
glycosylation sites, and this extracellular region is responsible
for the MMP-stimulating activity. EMMPRIN is expressed in highly-
(HG 45–65 kDa), poorly-glycosylated (LG 32–44 kDa) and core protein
(approximately 27 kDa) forms. EMMPRIN was reported to stimulate
tumor angiogenesis by elevating VEGF and MMP expression in
neighboring fibroblasts and epithelial cells. In our study, it was
found that EMMPRIN expression was gradually increased from normal
mucosa to carcinomas through hyperplastic or metaplastic mucosa of
the stomach, and positively correlated with tumor size, depth of
invasion, lymphatic invasion, expression of ki-67, MMP-2, −9 and
VEGF, MVD and poor prognosis of gastric carcinoma. In colorectal
carcinoma, EMMPRIN expression was immunohistochemically stronger in
colorectal high-grade adenoma, adenocarcinoma and metastatic
carcinoma compared to non-neoplastic superficial epithelium and
low-grade adenoma, and positively correlated with tumor size, depth
of invasion, vascular or lymphatic invasion, grade of infiltration
and VEGF expression of carcinomas. However, the manner in which
these angiogenesis-related factors regulate each other at the
transcriptional level is likely to become a crucial issue as the
hydrolytic effects of MMPs on transmembrane proteins are clarified
(24,25).
5. Cell adhesion
Tenascin is a large ECM glycoprotein with a
six-armed disulfide-bonded macromolecular structure, consisting of
tenascin-C (formerly known under various synonyms, such as
cytotactin and hexabrachion), tenascin-R (for restrictin), -X, -Y
and -W. All family members share a modular structure, consisting of
a cysteine-rich N-terminal domain involved in the oligomerization
of tenascin-C, -R, and possibly -X, as well as a series of
epidermal growth factor-like repeats, followed by a number of
fibronectin type III-like domains and a carboxy-terminal
fibrinogen-like domain. Tenascin has a number of biological
functions likely to affect tumor development, such as the
regulation of tumor cell-cell interaction, proliferation, invasion
and metastasis, and involvement in angiogenesis. Tenascin
expression showed a significantly negative association with liver
metastasis of GIA. There was a significantly negative relationship
between EMMPRIN and tenascin expression in GIA, indicating that
EMMPRIN induced the production of MMPs, which degrade the tenascin
(14).
6. Cell mobility
The aggressive phenotype depends on cell
adhesiveness, motility and deformability, which are thought to
result from quantitative alterations and the rearrangement of
various cytoskeletal components, disassembly of actin filaments and
actin polymerization. The cytoskeleton is composed of actin
filaments, intermediate filaments and microtubules. In particular,
actin-binding proteins such as cortactin and fascin are involved in
the cytoskeleton formation, cell migration and signaling pathways.
The actin-related protein (Arp)2/3 complex has been identified to
bind to itself and directly regulates the actin polymerization
reaction by interacting with Wiskott-Aldrich syndrome-related
protein family proteins. Cortactin is a filamentous actin-binding
monomer and consists of an amino-terminal acidic (NTA) region,
37-residue-long segments, a proline-rich region and an SH3 domain.
The NTA region harbors a short motif called DDW, which is necessary
for binding to the Arp2/3 complex. Arp2 and −3 closely resemble the
structure of monomeric actin and serve as nucleation sites for new
actin filaments to stimulate actin polymerization. In vitro
experimental evidence indicated that fascin overexpression
correlated with increased proliferation, altered β1 integrin
distribution, enhanced the invasive capacity and altered the
differentiation status in colonic adenocarcinoma.
The expression of cortactin and fascin was higher in
gastric carcinomas than adenoma and NNMs, and positively correlated
with tumor size, depth of invasion, lymphatic and venous invasion,
lymph node metastasis and TNM staging. Arp2 and −3 proteins were
expressed at low levels in gastritis, compared with carcinomas.
Arp2 was more frequently expressed in intestinal metaplasia than in
carcinoma or gastritis. Arp2 and −3 proteins were positively
correlated with tumor size, depth of invasion, venous invasion,
UICC staging and expression of cortactin or fascin. Univariate
analysis indicated that the cumulative survival rate of patients
with positive fascin expression was lower than that without its
expression, even when stratified according to the depth of
invasion. Lamellipodia and invadopodia formation of gastric
carcinoma cells have yet to be adequately investigated. If the
effects of these mobility-related proteins on invasion and
migration are clarified, the anti-metastatic therapy is likely to
be improved for patients with advanced gastrointestinal carcinomas
(26,27).
7. Endoplasmic reticulum stress
The endoplasmic reticulum (ER) is important in
regulating the synthesis, folding and targeting of secretory and
membrane proteins. Oxidative stress, glucose deprivation, chemical
toxicity, alterations in intracellular Ca2+ levels,
blockade of glycosylation and hypoxia induce ER stress, in which
the expression of glucose-related proteins (GRPs) is activated.
GRPs are ubiquitously expressed in ER and is capable of assisting
in protein folding and assembly, and are consequently considered as
molecular chaperones.
There was more expression of the two proteins in
gastric carcinoma and adenoma than in NNM. The two proteins were
positively correlated with tumor size, depth of invasion, lymphatic
and venous invasion, lymph node metastasis, TNM staging and worse
prognosis of gastric carcinoma. In colorectal carcinoma, there was
a gradually increased GRP78 expression from NNMs and carcinomas, to
low-grade and high-grade adenomas, while up-regulated GRP94
expression occurred from NNM, low-grade adenoma and high-grade
adenoma to carcinoma. GRP78 expression was negatively correlated
with lymphatic invasion or a low GRP94 expression of colorectal
carcinomas. The significance of GRPs in gastric and colorectal
carcinoma may be explained by the distinct histogenesis and
behaviors of the two carcinomas (28,29).
8. Threonine protein kinase
Glycogen synthase kinase-3β (GSK3β) is a
serine/threonine protein kinase, which may be involved in protein
synthesis, cell proliferation, cell differentiation, microtubule
dynamics and cell motility by phosphorylating initiation factors,
components of the cell-division cycle, transcription factors and
proteins involved in microtubule function and cell adhesion. The
activity of GSK3β is inhibited via ser-9 phosphorylation by p70 S6
kinase, p90Rsk, Akt, certain isoforms of protein kinase, and cyclic
AMP-dependent protein kinase. Inactive GSK3β phosphorylated at
ser-9 was more expressed in gastric carcinomas than in NNM, and
positively correlated with depth of invasion, lymphatic and venous
invasion, lymph node metastasis, TNM staging, expression of VEGF
and EMMPRIN, and poor prognosis in gastric carcinoma (30).
Pim-3, a member of the proto-oncogene Pim family
with serine/threonine kinase activity, was aberrantly expressed in
cancer lesions of endoderm-derived organs. The ablation of Pim-3
expression induced the apoptosis of human hepatocellular, pancreas
and colon carcinoma cell lines. Moreover, Pim-3 can inactivate a
potent pro-apoptotic molecule, Bad, in human pancreas and colon
carcinoma cells, by phosphorylating its Ser112, thereby preventing
apoptosis. Pim-3 expression was enhanced in adenoma and metastasis
sites of gastric carcinoma and, to a lesser degree, in primary
sites of gastric carcinoma, compared with NNM. Pim-3 expression was
positively correlated with lymphatic and venous invasion, a high
expression of VEGF and EMMPRIN, a low PTEN expression and poor
prognosis. However, the biological functions of Pim-3 should be
further studied in gastric carcinoma, particularly regarding
differentiating induction (31).
9. Mucin production
The mucin components of the gastric gel layer
function as a protective and lubricating factor against luminal
acid and proteolytic enzymes, and also hinder access of carcinogens
causing DNA damage. When the stomach suffers from infection with
Helicobacter pylori (HP), a group I carcinogen, HP
lipopolysaccharides decrease mucin synthesis by the
phosphatidylinositol 3-kinase/ERK pathway and via the inhibition of
galactosyltransferase.
In malignancies, MUC-1 may function as an
anti-adhesion molecule facilitating the release of cells from tumor
nests and may mask extracellular domains of cancer cells from
immune surveillance. MUC-6 is essential in epithelial
cytoprotection against a wide range of substances. In gastric
carcinoma, MUC-1 was found to be highly expressed in gastric
carcinomas in comparison with NNM and positively correlated with
depth of invasion, lymphatic and venous invasion, lymph node
metastasis, TNM staging, poor prognosis, and MUC-4 expression,
while it was the converse for MUC-6. It is of great significance to
characterize the mucin production of gastric carcinoma,
particularly in signet ring cell carcinoma (SCR) (32,33).
10. Reg family
The Regenerating (Reg) gene family belongs to the
calcium-dependent lectin (C-type lectin) gene superfamily, which
encodes a group of small multifunctional secretory proteins. Reg
family proteins function as acute phase reactants, lectins,
anti-apoptotic factors and growth agents. These proteins are
primarily involved in cell proliferation and differentiation,
inflammation, diabetes and carcinogenesis. Three subtypes of
REG gene have been identified in humans, including REG
I (Iα and Iβ), REG III (III and
HIP/PAP: hepatocellular carcinoma-intestine-pancreas
gene/pancreatitis-associated protein) and REG IV. REG IV
has been reported to be a potent activator of the epidermal growth
factor receptor/Akt/activator protein-1 signaling pathway in colon
cancer cells and increases the expression of BCL-2, Bcl-xL, and
survivin proteins. REG IV treatment protects normal intestinal
crypt cells from irradiation-induced apoptosis by increasing the
expression of BCL-2, Bcl-xL and survivin.
Our group found that REG IV protein expression was
gradually decreased from gastric IM, adenoma and carcinoma to
gastritis. The positive rate of its mRNA was higher in gastric IM
than carcinoma or NNM. Reg IV expression was significantly
correlated with the MUC-2 and MUC-5AC expression. In colorectal
carcinoma, the expression of REG Iα, III and HIP/PAP was more
frequently observed in colorectal carcinomas than in adjacent NNM,
while it was the converse for REG Iβ and IV. The expression of REG
Iα, Iβ, III and HIP/PAP was negatively correlated with the depth of
invasion of CRCs. REG Iβ and HIP/PAP were less expressed in CRCs
with than without venous invasion. The positive rates of REG Iα and
HIP/PAP were significantly higher in CRCs without than with lymph
node metastasis. A positive relationship was found between REG Iα,
Iβ, III and HIP/PAP expression. Survival analysis indicated that
the REG Iβ or HIP/PAP expression was positively correlated with
favorable prognosis of carcinoma patients. To the best of our
knowledge, the receptors and biological functions of REG proteins
remain to be clarified (34,35).
11. Lauren's classification
Lauren's classification has been widely applied in
clinical practice and is useful in clarifying the histopathogenesis
with epidemiological priority. Histologically, the intestinal types
principally include papillary, well-differentiated,
moderately-differentiated or mucinous adenocarcinoma without SCR
cells, whereas the diffuse-type mainly consisted of the
poorly-differentiated adenocarcinoma, SRC carcinoma and
undifferentiated adenocarcinoma of the World Health Organization
(WHO) classification. Although approximately 15% of gastric
carcinomas are characterized as unclassified or mixed type, an
intermediate type of gastric carcinoma may show a few special
changes, reflecting polycolonal histogenesis and aggressiveness.
Our group found that intestinal-type carcinoma frequently occurred
in older males, while comparatively, the diffuse-type occurred in
young women. The latter was more inclined to invasion into
muscularis propria, lymphatic invasion and lymph node metastasis,
and belonged to higher TNM staging, compared with intestinal-type
counterparts. Mixed-type (MT) carcinomas exhibited large size, deep
invasion, frequent local invasion and lymph node metastasis in
comparison with intestinal- and diffuse-type carcinoma. Nuclear
ING5, cortactin, MUC-1, MUC-6, parafibromin, Pim-3, p53, FHIT,
maspin, VEGF, GSK3β-ser9, MUC-2, MUC-4, CD44,
E-cadherin, membrane β-catenin and EMMPRIN showed a higher
expression in intestinal-than diffuse-type carcinomas. The
expression of maspin, EMMPRIN, VEGF, MUC-4 and membrane E-cadherin
was stronger in MT intestinal than diffuse components.
Immunoreactivities to EMMPRIN and VEGF were weaker in intestinal
carcinoma than in the MT intestinal portion, whereas the opposite
was true for CD44, MUC-2 and −6. The MT diffuse component exhibited
a higher expression of FHIT, VEGF and P-GSK3β-ser9 than
diffuse-type carcinoma. These findings suggested that MT carcinomas
were also shown to be more aggressive and that different components
of mixed-type carcinoma may originate from common stem cells, but
follow a distinct histogenic pathway, as the significant difference
in the proliferation, apoptosis, angiogenesis, mucin secretion and
cell adhesion between the intestinal- and diffuse-type carcinomas
becomes smaller between intestinal and diffuse components of the MT
carcinoma (36,37).
12. WHO classification
The WHO classification classifies adenocarcinoma
into papillary, well-, moderately-, or poorly-differentiated,
mucinous adenocarcinoma, SRC and undifferentiated carcinoma. WHO
classification is performed according to histomorphological
features and it is easy to establish its relationship with other
grouping approaches, including Lauren's, Nakamura and Goseki's
classification. The majority of cases were well-, poorly-, or
moderately-differentiated subtypes, whereas the minority were
papillary or SRC. Patients with poorly-differentiated or SRC
carcinoma were predominantly young and female.
Poorly-differentiated and mucinous carcinomas were larger, with
deeper invasion, more venous or lymphatic invasion, frequent lymph
node involvement and peritoneal dissemination, or higher staging.
The SRC group exhibited a weaker expression of caspase-3, p53,
parafibromin, GRP78, GRP94, P-GSK3β-ser9, VEGF or
cortactin. The moderately-differentiated subtype exhibited a lower
expression of FHIT and Arp3 positivity. The poorly-differentiated
group showed a weaker expression of caspase-3, EMMPRIN, MUC-2,
MUC-5AC and MUC-6. Mucinous carcinoma more frequently expressed REG
IV protein than well-, and moderately-differentiated carcinomas.
Survival analysis indicated that the patients with
poorly-differentiated or mucinous subtypes had a lower cumulative
survival rate than those with papillary, well-,
moderately-differentiated or SRC carcinomas (34,38).
13. Conclusions
The aberrant and cell-specific expression of
molecules such as tumor suppressor and metastasis suppressor genes,
angiogenesis, cell adhesion, cell mobility, ER stress, mucin
production, threonine protein kinase and REG family proteins is
essential in the malignant transformation of gastrointestinal
epithelium and subsequent cancer development. This expression also
underlies the histogenic mechanisms of gastric carcinoma according
to Lauren's and WHO classification. In MT carcinomas, the different
components of mixed-type carcinoma may originate from common stem
cells, but follow distinct histogenic pathways. Protein-protein or
protein-DNA interaction may be combined with phenotype analysis to
establish the pathways of cytoplasmic or nuclear proteins in GIAs.
Additionally, morphological examination of certain markers may
generate some novel questions. For instance, the in vivo
effects of certain molecules are occasionally opposite to their
in vitro functions, which requires further investigation.
Additionally, lamellipodia and invadopodia formation of gastric or
colorectal carcinoma cells has not been fully investigated, with
the effects of these mobility-related proteins on invasion and
migration remaining to be clarified, which has blocked the
anti-metastatic therapy of advanced GIAs. According to histological
classification, SRC is of note, posing the question of whether
there is a possible relationship between cell adhesion and mucin
synthesis in its histogenesis. In this review, we only observed the
in situ expression of well-known genes or proteins, which is
a limitation of the review. Additionally, the sensitivity and
specificity of the IHC and ISH are dependent on the antibody or
probe, and are also determined by the tissue contents of the
protein and mRNA. The combination of TMA, IHC and ISH may therefore
be widely applied to screen molecular markers in GIA.
Acknowledgements
This study was supported by the Shenyang Outstanding
Talent Foundation of China; Shenyang Science and Technology Grant
(1091175-1-00; F11-264-1-10); Liaoning Science and Technology
Grant; Natural Scientific Foundation of China (No. 81001093; No.
81101885; No. 81101886; No. 81172371); Grant-in-aid for Scientific
Research from the Ministry of Education, Culture, Sports and
Technology of Japan (23659958).
References
|
1
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society. Colorectal cancer
facts and figures. 2005
|
|
3
|
Moch H, Schraml P, Bubendorf L, Mirlacher
M, Kononen J, Gasser T, Mihatsch MJ, Kallioniemi OP and Sauter G:
High-throughput tissue microarray analysis to evaluate genes
uncovered by cDNA microarray screening in renal cell carcinoma. Am
J Pathol. 154:981–986. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumada T, Tsuneyama K, Hatta H, Ishizawa S
and Takano Y: Improved 1-h rapid immunostaining method using
intermittent microwave irradiation: practicability based on 5 years
application in Toyama Medical and Pharmaceutical University
Hospital. Mod Pathol. 17:1141–1149. 2004.
|
|
5
|
Zheng H, Tsuneyama K, Cheng C, Takahashi
H, Cui Z, Murai Y, Nomoto K and Takano Y: An immunohistochemical
study of P53 and Ki-67 in gastrointestinal adenoma and
adenocarcinoma using tissue microarray. Anticancer Res.
26:2353–2360. 2006.PubMed/NCBI
|
|
6
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Tsuneyama K and Takano Y: Low expression of FHIT and PTEN
correlates with malignancy of gastric carcinomas: tissue-array
findings. Appl Immunohistochem Mol Morphol. 15:432–440. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li XH, Zheng HC, Takahashi H, Masuda S,
Yang XH and Takano Y: PTEN expression and mutation in colorectal
carcinomas. Oncol Rep. 22:757–764. 2009.PubMed/NCBI
|
|
8
|
Xing YN, Yang X, Xu XY, Zheng Y, Xu HM,
Takano Y and Zheng HC: The altered expression of ING5 protein is
involved in gastric carcinogenesis and subsequent progression. Hum
Pathol. 42:25–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng HC, Xia P, Xu XY, Takahashi H and
Takano Y: The nuclear to cytoplasmic shift of ING5 protein during
colorectal carcinogenesis with their distinct links to pathologic
behaviors of carcinomas. Hum Pathol. 42:424–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng HC, Takahashi H, Li XH, Hara T,
Masuda S, Guan YF and Takano Y: Downregulated parafibromin
expression is a promising marker for pathogenesis, invasion,
metastasis and prognosis of gastric carcinomas. Virchows Arch.
452:147–155. 2008. View Article : Google Scholar
|
|
11
|
Zheng HC, Wei ZL, Xu XY, Nie XC, Yang X,
Takahashi H and Takano Y: Parafibromin expression is an independent
prognostic factor for colorectal carcinomas. Hum Pathol.
8:1089–1102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JH, Park SR, Chay KO, Seo YW, Kook H,
Ahn KY, Kim YJ and Kim KK: KAI1 COOH-terminal interacting
tetraspanin (KITENIN), a member of the tetraspanin family,
interacts with KAI1, a tumour metastasis suppressor, and enhances
metastasis of cancer. Cancer Res. 64:4235–4243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schoenfeld N, Bauer MK and Grimm S: The
metastasis suppressor gene C33/CD82/KAI1 induces apoptosis through
reactive oxygen intermediates. FASEB J. 18:158–160. 2004.PubMed/NCBI
|
|
14
|
Zheng H, Tsuneyama K, Cheng C, Takahashi
H, Cui Z, Nomoto K, Murai Y and Takano Y: Expression of KAI1 and
tenascin, and microvessel density are closely correlated with liver
metastasis of gastrointestinal adenocarcinoma. J Clin Pathol.
60:50–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maass N, Hojo T, Ueding M, Luttges J,
Kloppel G, Jonat W and Nagasaki K: Expression of the tumor
suppressor gene Maspin in human pancreatic cancers. Clin Cancer
Res. 7:812–817. 2001.PubMed/NCBI
|
|
16
|
Sood AK, Fletcher MS, Gruman LM, Coffin
JE, Jabbari S, Khalkhali-Ellis Z, Arbour N, Seftor EA and Hendrix
MJ: The paradoxical expression of maspin in ovarian carcinoma. Clin
Cancer Res. 8:2924–2932. 2002.PubMed/NCBI
|
|
17
|
Hirai K, Koizumi K, Haraguchi S, Hirata T,
Mikami I, Fukushima M, Yamagishi S, Kawashima T, Okada D, Shimizu K
and Kawamoto M: Prognostic significance of the tumor suppressor
gene maspin in non-small cell lung cancer. Ann Thorac Surg.
79:248–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito Y, Yoshida H, Tomoda C, Uruno T,
Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Matsuura N, Kuma K
and Miyauchi A: Maspin expression is directly associated with
biological aggressiveness of thyroid carcinoma. Thyroid. 14:13–18.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugimoto S, Maass N, Takimoto Y, Sato K,
Minei S, Zhang M, Hoshikawa Y, Junemann KP, Jonat W and Nagasaki K:
Expression and regulation of tumor suppressor gene maspin in human
bladder cancer. Cancer Lett. 203:209–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu M, Zheng H, Tsuneyama K, Takahashi H,
Nomoto K, Xu H and Takano Y: Paradoxical expression of maspin in
gastric carcinomas: correlation with carcinogenesis and
progression. Hum Pathol. 38:1248–1255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng H, Tsuneyama K, Cheng C, Takahashi
H, Cui Z, Murai Y, Nomoto K and Takano Y: Maspin expression was
involved in colorectal adenoma-adenocarcinoma sequence and liver
metastasis of tumors. Anticancer Res. 27:259–265. 2007.PubMed/NCBI
|
|
22
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Niwa H, Tsuneyama K and Takano Y: Expressions of MMP-2,
MMP-9 and VEGF are closely linked to growth, invasion, metastasis
and angiogenesis of gastric carcinoma. Anticancer Res.
26:3579–3583. 2006.PubMed/NCBI
|
|
23
|
Zheng HC, Sun JM, Li XH, Yang XF, Zhang YC
and Xin Y: Role of PTEN and MMP-7 expression in growth, invasion,
metastasis and angiogenesis of gastric carcinoma. Pathol Int.
53:659–666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng HC, Wang W, Xu XY, Xia P, Yu M,
Sugiyama T and Takano Y: Up-regulated EMMPRIN/CD147 protein
expression might play a role in colorectal carcinogenesis and its
subsequent progression without an alteration of its glycosylation
and mRNA level. J Cancer Res Clin Oncol. 137:585–596. 2011.
View Article : Google Scholar
|
|
25
|
Zheng HC, Takahashi H, Murai Y, Cui ZG,
Nomoto K, Miwa S, Tsuneyama K and Takano Y: Upregulated
EMMPRIN/CD147 might contribute to growth and angiogenesis of
gastric carcinoma: a good marker for local invasion and prognosis.
Br J Cancer. 95:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Zheng H, Hara T, Takahashi H, Masuda
S, Wang Z, Yang X, Guan Y and Takano Y: Aberrant expression of
cortactin and fascin are effective markers for pathogenesis,
invasion, metastasis and prognosis of gastric carcinomas. Int J
Oncol. 33:69–79. 2008.PubMed/NCBI
|
|
27
|
Zheng HC, Zheng YS, Li XH, Takahashi H,
Hara T, Masuda S, Yang XH, Guan YF and Takano Y: Arp2/3
overexpression contributed to pathogenesis, growth and invasion of
gastric carcinoma. Anticancer Res. 28:2225–2232. 2008.PubMed/NCBI
|
|
28
|
Takahashi H, Wang JP, Zheng HC, Masuda S
and Takano Y: Overexpression of GRP78 and GRP94 is involved in
colorectal carcinogenesis. Histol Histopathol. 26:663–671.
2011.PubMed/NCBI
|
|
29
|
Zheng HC, Takahashi H, Li XH, Hara T,
Masuda S, Guan YF and Takano Y: Overexpression of GRP78 and GRP94
are markers for aggressive behavior and poor prognosis in gastric
carcinomas. Hum Pathol. 39:1042–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng HC, Xu XY, Xia P, Yu M, Takahashi H
and Takano Y: Involvement of inactive GSK3beta overexpression in
tumorigenesis and progression of gastric carcinomas. Hum Pathol.
41:1255–1264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng HC, Tsuneyama K, Takahashi H, Miwa
S, Sugiyama T, Popivanova BK, Fujii C, Nomoto K, Mukaida N and
Takano Y: Aberrant Pim-3 expression is involved in gastric adenoma
adenocarcinoma sequence and cancer progression. J Cancer Res Clin
Oncol. 134:481–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li XH, Zheng HC, Wang ZG, Takahashi H,
Yang XH, Guan YF and Takano Y: The clinicopathological and
prognostic significance of MUC-1 expression in Japanese gastric
carcinomas: an immunohistochemical study of tissue microarrays.
Anticancer Res. 28:1061–1067. 2008.PubMed/NCBI
|
|
33
|
Zheng H, Takahashi H, Nakajima T, Murai Y,
Cui Z, Nomoto K, Tsuneyama K and Takano Y: MUC6 down-regulation
correlates with gastric carcinoma progression and a poor prognosis:
an immunohistochemical study with tissue microarrays. J Cancer Res
Clin Oncol. 132:817–823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng HC, Xu XY, Yu M, Takahashi H, Masuda
S and Takano Y: The role of Reg IV gene and its encoding product in
gastric carcinogenesis. Hum Pathol. 41:59–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng HC, Sugawara A, Okamoto H, Takasawa
S, Takahashi H, Masuda S and Takano Y: Expression profile of the
REG gene family in colorectal carcinoma. J Histochem Cytochem.
59:106–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Miwa S, Tsuneyama K and Takano Y: Pathobiological
characteristics of intestinal and diffuse-type gastric carcinoma in
Japan: an immunostaining study on the tissue microarray. J Clin
Pathol. 60:273–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng HC, Li XH, Hara T, Masuda S, Yang
XH, Guan YF and Takano Y: Mixed-type gastric carcinomas exhibit
more aggressive features and indicate the histogenesis of
carcinomas. Virchows Arch. 452:525–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng HC, Zheng YS, Xia P, Xu XY, Xing YN,
Takahashi H, Guan YF and Takano Y: The pathobiological behaviors
and prognosis associated with Japanese gastric adenocarcinomas of
pure WHO histological subtypes. Histol Histopathol. 25:445–452.
2010.PubMed/NCBI
|