Introduction
Icotinib hydrochloride is a new, small-molecule,
selective epidermal growth factor receptor tyrosine kinase
inhibitor (EGFR-TKI) (1–4). A large randomized head-to-head phase
III clinical trial (ICOGEN) demonstrated that icotinib has a
similar efficacy to gefitinib in previously treated non-small cell
lung cancer (NSCLC), with less toxicity (4). The most frequent side effects of
icotinib include rash (40.0%) and diarrhea (18.5%) (4). Since August 2011, this drug has been
available on the Chinese market. The recommended dose is 125 mg TID
orally, which is same as that used in the ICOGEN trial.
Hand-foot syndrome (HFS) is a distinctive and
relatively common cutaneous toxicity of traditional
chemotherapeutic agents, including 5-fluorouracil, liposomal
doxorubicin, cytarabine, docetaxel and capecitabine (5), as well as certain oral targeted
therapies such as sunitinib and sorafenib (6). HFS induced by EGFR-TKI is uncommon and
has been only sporadically reported in the literature (7–9). We
report a case of HFS caused by high-dose icotinib, which, to the
best of our knowledge, has not been previously described.
Case report
A 65-year old female (non-smoker) with metastatic
lung adenocarcinoma received one cycle of intravenous chemotherapy
with pemetrexed (500 mg/m2, on day 1) plus cisplatin (25
mg/m2, on days 1–3). The patient experienced serious
vomiting (NCI-CTC 3.0, grade 3) and refused to continue. The
patient then participated in the dose-escalation study of the
safety and pharmacokinetics of icotinib after signing an informed
consent form. A baseline target and non-target lesions were
obtained through contrast-enhanced computed tomography (CT;
Fig. 1A).
The patient received icotinib 625 mg TID orally.
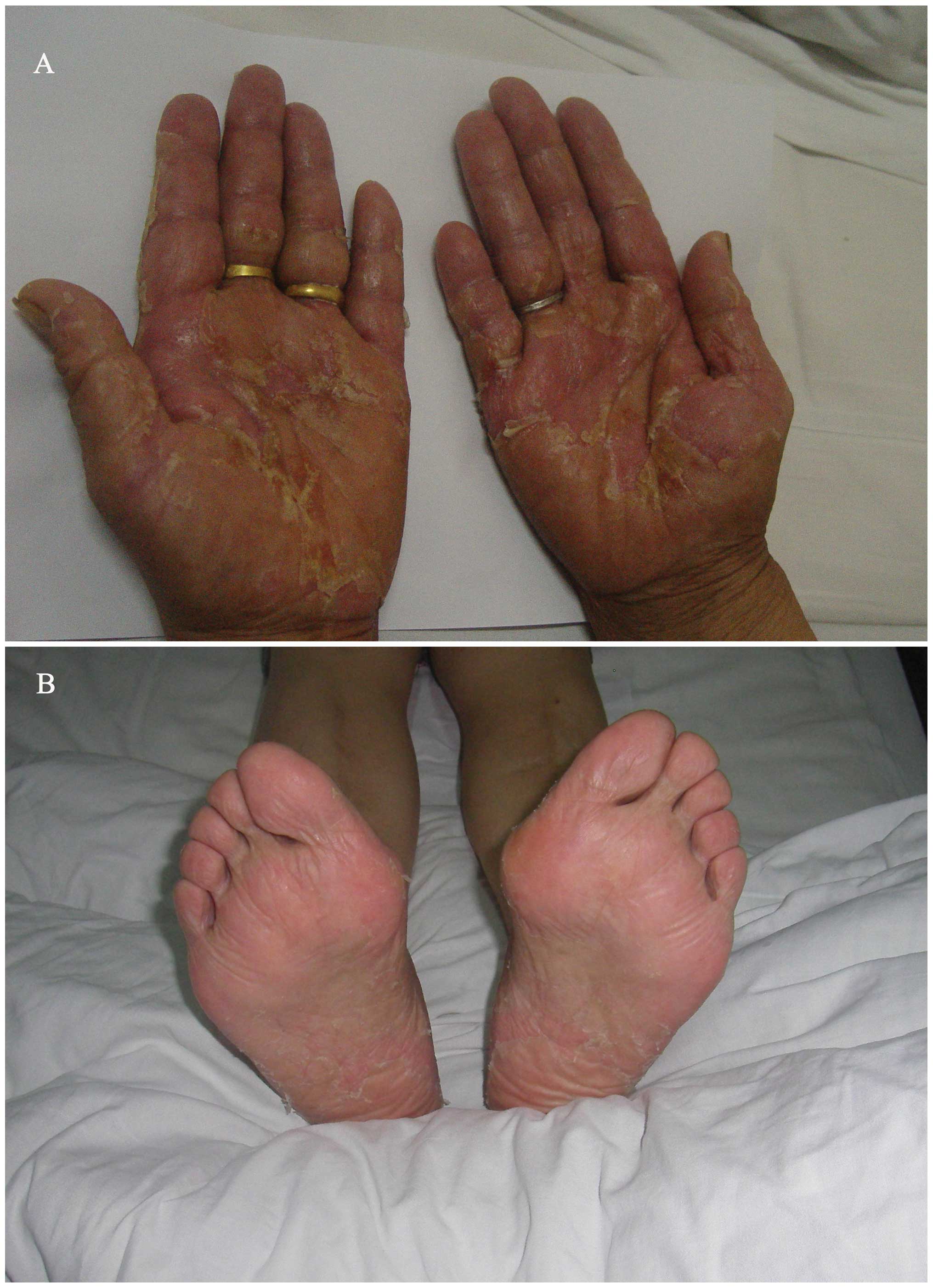
Five days after the initiation of icotinib, she noticed scattered
red, point-like rashes in the palms of the hands and soles of the
feet, with a tingling sensation. In the second week, the patient
progressively developed serious desquamation in the palms and less
in the soles of the feet, accompanied by pain and redness (Fig. 2). Grade 3 HFS was diagnosed. The
patient also experienced grade 2 diarrhea and grade 1 rash in the
trunk with itching. The dose of icotinib was reduced to 250 mg TID.
The patient was prescribed oral vitamin B6 and urea cream for HFS
and zyrtec for the trunk rash and pruritus. In the fourth week, the
HFS subsided to grade 1. The trunk rash persisted as grade 1. The
diarrhea resolved spontaneously. A repeated CT demonstrated that
the patient obtained a partial remission (PR) of the disease
(Fig. 1B). A subsequent CT in the
eighth week confirmed the PR. The patient has been undergoing
icotinib treatment for 12 months, which continues, and was doing
well at the time of writing.
This study was approved by the institutional ethics
committee of The First Affiliated Hospital, School of Medicine,
Zhejiang University. Informed consent was obtained from the patient
and her family.
Discussion
HFS is also known as palmar-plantar
erythrodysesthesia or acral erythema and is characterized by
symmetrical erythema of the palms and soles, together with sores,
edema, paresthesia and blisters. In some instances, desquamation
and ulceration occur. HFS generally arises after 2–4 weeks of
treatment with oral targeted therapies (6,7,9). In
the present case, the patient suffered from evident desquamation
with pain, interfering with activities of daily living (ADL). The
clinical presentation was characteristic; however, the condition
was developed during the first week of treatment, which is earlier
than normal. We hypothesize that the early exhibition of HFS was
induced by high-dose icotinib.
When it occurs following treatment with cytotoxic
agents, HFS appears to be dose-dependant and its occurrence seems
to be decided by peak drug concentration and total cumulative dose
(10). Since 2006, our institute
has participated in phase I, II and III clinical trials of
icotinib. Learning from this experience, the incidence of HFS in
patients with long-term oral icotinib treatment is not elevated. In
the present case, the peak drug concentration appears to have been
an important factor in the occurrence of HFS. However, the exact
correlation remains to be further elucidated.
A wide variety of therapies have been suggested to
treat HFS: dose reduction and treatment interruption, COX-2
inhibitors, topical urea-containing creams, pyridoxine supplement,
emollient creams, regional cooling and oral corticosteroids. The
most definitive treatment is dose modification (5,6). We
treated the patient with oral vitamin B6 and topical urea cream. A
decision of treatment reduction was immediately made when the HFS
progressed to grade 3, since the dose-limiting toxicity of icotinib
was observed. It is postulated that the rapid regression of HFS is
mostly due to prompt dose reduction.
Icotinib and erlotinib share a common chemical
backbone structure but differ in their side chains (2); nonetheless, their side-effect profiles
are different (4,11). In the two reported patients with HFS
induced by erlotinib, dose reduction was ineffective (7,9). In
our case, the reduction of icotinib dose contributed most to the
regression of HFS symptoms.
Razis et al (8) reported three cases of HFS associated
with gefitinib, which the authors considered to be ‘recall
reactions’ to previous exposure to liposomal doxorubicin. In our
case, the patient underwent one cycle of chemotherapy with
pemetrexed plus cisplatin one month before icotinib treatment.
Cisplatin-induced acral erythema has been reported in the
literature (12,13). It is unlikely that the HFS was
induced by cisplatin in our case. First, the incidence of acral
erythema in patients receiving cisplatin is extremely rare, having
been reported in only four cases (13). Second, the HFS in the present case
began in the first week of icotinib treatment. Furthermore, the
symptoms soon regressed when the icotinib dose was reduced.
However, excluding the possibility of a ‘recall reaction’ to
previous exposure to cisplatin was difficult in this patient.
In conclusion, EGFR-TKI-associated HFS is unusual.
Icotinib, a EGFR-TKI, may induce the condition, especially when it
is administered at a high dose. The syndrome is painful and
distressing to patients; in some instances it interferes with ADL
and results in dose interruption. Clinicians should consider the
syndrome when a patient commences icotinib treatment.
References
|
1.
|
Q ZhaoJ ShentuN XuPhase I study of
icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase
inhibitor, in patients with advanced NSCLC and other solid
tumorsLung
Cancer73195202201110.1016/j.lungcan.2010.11.00721144613
|
|
2.
|
Z GuanX ChenY WangD ZhongMetabolite
identification of a new antitumor agent icotinib in rats using
liquid chromatography/tandem mass spectrometryRapid Commun Mass
Spectrom2221762184200810.1002/rcm.359918536068
|
|
3.
|
D LiuJ JiangP HuF TanY WangQuantitative
determination of icotinib in human plasma and urine using liquid
chromatography coupled to tandem mass spectrometryJ Chromatogr B
Analyt Technol Biomed Life
Sci87737813786200910.1016/j.jchromb.2009.08.055
|
|
4.
|
Y SunY ShiL ZhangX LiuC ZhouL ZhangD WangQ
LiS ZhangS QinA randomized, double-blind phase III study of
icotinib versus gefitinib in patients with advanced non-small cell
lung cancer (NSCLC) previously treated with chemotherapy (ICOGEN)J
Clin Oncol29Supplabstr 7522201123948351
|
|
5.
|
JD Webster-GandyC HowK
HarroldPalmar-plantar erythrodysesthesia (PPE): a literature review
with commentary on experience in a cancer centreEur J Oncol
Nurs11238246200710.1016/j.ejon.2006.10.00417350337
|
|
6.
|
ME LacoutureS WuC RobertEvolving
strategies for the management of hand-foot skin reaction associated
with the multitargeted kinase inhibitors sorafenib and
sunitinibOncologist1310011011200810.1634/theoncologist.2008-013118779536
|
|
7.
|
S BenomarS BoutayebY AfifiHand-foot
syndrome and seborrheic dermatitis-like eruption induced by
erlotinibDermatol Online J152200919951638
|
|
8.
|
E RazisM KarinaS KaranastassiG
FountzilasThree case reports of hand-foot syndrome with
gefitinibCancer
Invest24514516200610.1080/0735790060081484716939960
|
|
9.
|
AM RouxelAM RoguedasR DescourtL
MiseryHand-foot syndrome: A new side effect of erlotinibAnn
Dermatol Venereol1357627642008(In French)
|
|
10.
|
E NagoreA InsaO SanmartinAntineoplastic
therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’)
syndrome. Incidence, recognition and managementAm J Clin
Dermatol12252342000
|
|
11.
|
FA ShepherdJ Rodrigues PereiraT
CiuleanuNational Cancer Institute of Canada Clinical Trials Group:
Erlotinib in previously treated non-small-cell lung cancerN Engl J
Med353123132200510.1056/NEJMoa05075316014882
|
|
12.
|
D VakalisD IoannidesE LazaridouG
Mattheou-VakaliA TeknetzisAcral erythema induced by chemotherapy
with cisplatinBr J
Dermatol139750751199810.1046/j.1365-2133.1998.02487.x9892931
|
|
13.
|
S FukamachiM NakamuraY
TokuraCisplatin-induced acral erythemaEur J
Dermatol19171172200919153065
|