Introduction
Breast cancer is the most frequent cause of
mortality in females in the developed world. Although early
detection, precise resection using wide margins and systematic
adjuvant therapy have improved survival, distant metastasis remains
the leading cause of breast cancer-related mortality (1). Circulating tumor cells (CTCs) are
isolated tumor cells that disseminate from the sites of metastatic
and/or primary cancer, including breast cancer, and are identified
and measured in peripheral blood (2). In a previous study, we observed that
the rate of detection and number of CTCs correlated with the
disease stage in breast cancer patients. Moreover, the assessment
of CTCs in metastatic breast cancer patients predicts the efficacy
of chemotherapy (1).
It is generally accepted that tumor cells become
damaged during circulatory transport. This circulatory trauma may
be due to humoral factors, including macrophages, natural killer
cells and antibody-mediated complement lysis, as well as physical
factors, including shear forces and mechanical trauma due to
passage through the microvasculature. During hematogenous
dissemination, CTCs undergo an extensive variety of interactions
with host cells before they establish a secondary metastatic colony
(3). The involvement of platelets
in hematogenous metastasis has long been recognized. A correlation
between venous thromboembolism and cancer was first observed by
Trousseau in 1865 (4), while more
recently, a study identified that the risk of cancer diagnosis is
elevated following primary deep vein thrombosis (DVT) or pulmonary
embolism (PE) (5). The ability of
malignant tumor cells to aggregate platelets via tumor cell-induced
platelet aggregation (TCIPA) (6,7)
confers a number of advantages for the successful metastasis of
cancer cells. When covered with a coat of platelets, a tumor cell
acquires the ability to evade the body’s immune system. Indeed,
platelets protect tumors from tumor necrosis factor α-mediated
cytotoxicity (8). TCIPA also
enables embolization of the large tumor-platelet aggregates at new
extravasation sites within the microvasculature (9). Additionally, platelets facilitate the
adhesion of tumor cells to the vascular endothelium (10) and release a number of growth factors
which stimulate tumor cell growth (11). Furthermore, platelets contribute to
tumor-induced angiogenesis by releasing angiogenic growth factors,
including vascular endothelial growth factor (VEGF) (12).
In the initiation phase of primary hemostasis,
interaction of the glycoprotein (GP) Ib/V/IX receptor complex with
von Willebrand factor (vWF) on the surface of platelets mediates
the adhesion of platelets. Subsequently, several
platelet-activating factors, including thromboxane A2 (TXA2) and
adenosine diphosphate (ADP), are secreted in an
auto-crine/paracrine fashion and activate or prime approaching
platelets. The release of ADP and TXA2 also leads to the conversion
of the GPIIb/IIIa receptor into an active form, which mediates
platelet aggregation. Tumor cells may use a variety of mechanisms
to interact with platelets and induce platelet aggregation. Tumor
cells express cell surface molecules, which induce tumor
cell-platelet interactions and subsequent platelet aggregation
(13), or induce platelet
aggregation by generating typical platelet agonists, including
thrombin (14) or ADP (15), which are considered as ‘soluble
stimulators’ of platelet aggregation.
Characterization of the interactions between
platelets and metastasizing tumor cells could potentially be used
to develop therapies to disrupt this correlation and prevent cancer
metastasis (16). Therefore, in the
present study, we investigated the activation of the GPIb-IX, TXA2,
ADP and GPIIb/IIIa pathways in platelets during TCIPA and
determined the effect of inhibiting these pathways on TCIPA.
Materials and methods
Cell culture
MCF-7 human breast cancer cells (American Type
Culture Collection, Manassas, VA, USA) were maintained in RPMI-1640
(Gibco, Grand Island, NY, USA) supplemented with 10% fetal calf
serum (FCS; Hyclone Laboratories Inc., Logan, UT, USA), 100 U/ml
penicillin and 100 mg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2 and passaged every 2–3 days to
maintain exponential growth. The study was approved by the Ethics
Committee of the First Affiliated Hospital of Soochow University,
Suzhou, China.
Reagents
Aspirin, apyrase and the anti-GPIb-IX complex
monoclonal antibody, 7E3, were obtained from Sigma (St. Louis, MO,
USA). The anti-GPIb-IX complex monoclonal antibody, SZ-1, was
prepared according to our previously described methods (17). Fluorescein-isothiocyanate
(FITC)-conjugated monoclonal antibody against high-affinity
GPIIb/IIIa (PAC-1-FITC) was purchased from Becton Dickinson
Biosciences (Mississauga, ON, Canada). Recombinant-phycoerythrin
(PE)-conjugated monoclonal antibody against human platelet GPIb
(CD42b-PE) was purchased from Dako Diagnostics (Glostrup,
Denmark).
Preparation of washed platelets
Fresh blood obtained from healthy volunteers was
anticoagulated with a 1/7 volume of acid-citrate dextrose (ACD; 85
mM trisodium citrate, 110 mM dextrose, 78 mM citric acid) as
previously described (18), washed
twice with CGS buffer (0.12 M sodium chloride, 0.0129 M trisodium
citrate and 0.03 M D-glucose; pH 6.5), resuspended in freshly
prepared Tyrode’s buffer (18) and
allowed to rest for at least 1 h at 37°C before use.
Platelet aggregation
The interactions between platelets and tumor cells
were measured by light aggregometry using a whole-blood ionized
calcium lumi-aggregometer (Chrono-Log, Havertown, PA, USA).
Briefly, 200 μl platelets (200×106 cells/ml) were
placed in the aggregometer and incubated for 2 min at 37°C with
stirring at 900 rpm, prior to the addition of cancer cells. TCIPA
was initiated by the addition of 50 μl tumor cells
(0.05–50×106 cells/ml) and the reactions were monitored
and analyzed using the Aggro-link data processing system
(Chrono-Log) for up to 15 min. Platelet aggregation was expressed
as a percentage of the maximum aggregation rate. The structure of
the platelet-tumor cell aggregates were also observed using an
Olympus CKX41 phase-contrast microscope (Olympus, Melville, NY,
USA).
Flow cytometry analysis
The abundance of GPIb-IX and GPIIb/IIIa on the
surface of the platelets during TCIPA was measured by flow
cytometry. When TCIPA reached 50% maximal light transmission, the
reaction was terminated by 10-fold dilution with physiological
saline. The samples were then incubated with saturating
concentrations (10 μg/ml) of PE-anti GPIb-IX (CD42b-PE) or
FITC-anti GPIIb/IIIa (CD41-FITC) in the dark for 5 min at room
temperature and analyzed using a FC500 dual-laser five-color flow
cytometer (Beckman Coulter, Fullerton, CA, USA). The mean
fluorescence intensity was determined following correction for cell
autofluorescence.
Enzyme-linked immunosorbent assay
(ELISA)
As TXA2 quickly transforms into TXB2 in aqueous
solution, the concentration of TXB2 was measured using an enzyme
immunoassay kit (Amersham Pharmacia Biotech, Buckinghamshire, UK)
(18). When TCIPA reached 50%
maximal light transmission, the reaction was terminated by 10-fold
dilution with physiological saline, centrifuged and the
supernatants were assayed for the generation of TXB2 using
ELISA.
ADP assays
ADP secreted from dense granules in stimulated
platelets was measured using a whole blood ionized calcium
lumi-aggregometer as previously described (19). Briefly, platelets were incubated
with luciferin-luciferase reagent (440 U/ml luciferase and 4
μg/ml luciferin) for 2 min at 37°C to convert ADP to
adenosine triphosphate (ATP) and to generate chemiluminescence.
Following incubation, the agonist was added and luminescence was
monitored. To quantify the generation of ATP by platelets, standard
curves were constructed using standard ATP.
Statistical analysis
Each experiment was performed in triplicate, at
least. Results are expressed as mean ± standard deviation (SD).
Statistical analysis was performed using unpaired Student’s
t-tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
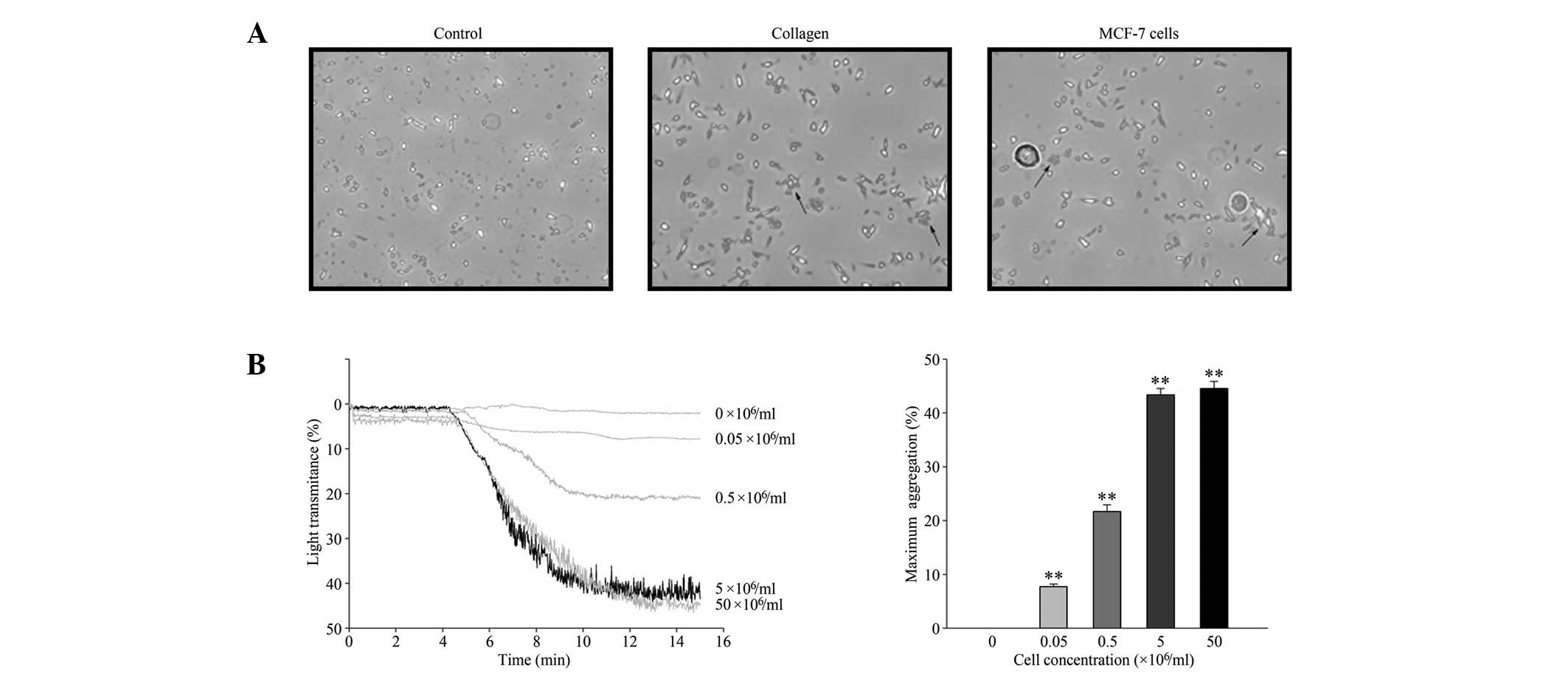
MCF-7 cells induce platelet
aggregation
The TCIPA effect of MCF-7 cells was observed using
phase-contrast microscopy and quantified by light aggregometry.
MCF-7 cells induced platelet aggregation in a similar manner to
collagen, a classic inducer of platelet aggregation (Fig. 1A). MCF-7 cells induced platelet
aggregation in a concentration-dependent manner (Fig. 1B), up to a maximal concentration of
5×106 cells/ml. Therefore, 5×106 cells/ml was
selected as the standard cell concentration for all further
experiments.
Activation of the GPIb-IX, TXA2, ADP and
GPIIb/IIIa pathways during TCIPA
Activation of GPIb-IX and GPIIb/IIIa were evaluated
by quantifying the abundance of GPIb-IX and GPIIb/IIIa on the
surface of platelets using flow cytometry. As shown in Fig. 2A and B, GPIb-IX and GPIIb/IIIa were
upregulated on the surface of platelets during TCIPA. These effects
were repressed by pretreatment of the platelets with SZ-1 (10
μg/ml) or 7E3 (20 μg/ml), respectively.
TXA2 release was measured as the level of the stable
TXA2 metabolite, TXB2, using ELISA (19). As shown in Fig. 2C, the increased levels of TXB2
observed during TCIPA were attenuated by pretreatment of the
platelets with 50 μg/ml aspirin.
ADP was measured as the level of ATP generated
(20). As presented in Fig. 2D, the increased quantity of ADP
released during TCIPA was inhibited by pretreatment of the
platelets with 250 μg/ml apyrase.
Thus, the GPIb-IX, TXA2, ADP and GPIIb/IIIa pathways
were all activated during MCF-7-induced TCIPA and the activation of
each pathway during TCIPA was repressed by pretreatment of the
platelets with the appropriate inhibitors.
Repression of TCIPA using a combination
of GPIb-IX, ADP and/or GPIIb/IIIa pathway inhibitors
To investigate whether activation of the GPIb-IX,
TXA2, ADP and GPIIb/IIIa pathways participated in MCF-7-induced
TCIPA, platelets were pretreated with the inhibitors of each
pathway. As shown in Fig. 3A and B,
aspirin did not exert any significant effect on MCF-7-induced
TCIPA. However, SZ-1, 7E3 and apyrase inhibited TCIPA, suggesting
that MCF-7-induced TCIPA was executed through the GPIb-IX, ADP and
GPIIb/IIIa pathways, but not the TXA2 pathway.
The combined effects of these platelet-aggregation
inhibitors on MCF-7-induced TCIPA were investigated further. As
shown in Fig. 3, paired
pretreatment of platelets with SZ-1, 7E3 and/or apyrase lead to a
slightly greater inhibition of MCF-7 cell-induced TCIPA and the
combination of all three inhibitors significantly inhibited
MCF-7-induced TCIPA, compared to the single inhibitors alone.
Discussion
Metastasis is the major cause of mortality in breast
cancer patients, yet there is no effective strategy to prevent
tumor metastasis. Fewer than 0.01% of the cells that enter the
bloodstream result in metastases (21). During hematogenous dissemination,
the ability of circulating tumor cells to interact with platelets
via TCIPA is believed to promote tumor cell survival within the
circulation (22) and increase the
arrest of tumor cell emboli within the microcirculation (21), thereby facilitating metastasis.
TCIPA is currently gaining acceptance as a key intermediate step in
the process of blood-borne metastasis (23). Pre-clinical animal models have
demonstrated that pharmacologically- or genetically-induced
thrombocytopenia (6,24) and platelet function defects are
associated with reduced metastasis (24–26).
These observations have prompted the use of antiplatelet and
anticoagulation agents to prevent metastasis in experimental models
(7,26) and human cancer patients (27). Thus, in the present study, we
investigated the mechanisms involved in MCF-7 breast cancer
cell-induced TCIPA and the effect of antiplatelet strategies on
MCF-7-induced TCIPA.
In the present study, we observed that the GPIb-IX
and GPIIb/IIIa pathways were activated during MCF-7-induced TCIPA
and inhibition of the GPIb-IX and GPIIb/IIIa pathways repressed
MCF-7-induced TCIPA. GPIb-IX and GPIIb/IIIa are the major platelet
surface transmembrane receptors implicated in TCIPA (28). GPIb-IX mainly mediates platelet
adhesion (29), while GPIIb/IIIa
plays an important role in platelet aggregation (30). A previous study supported the
hypothesis that the functions of GPIb-IX in platelets, which
support normal hemostasis or pathological thrombosis also
contribute to tumor malignancy (25). A functional absence of GPIb-IX
correlated with a 15-fold reduction in the number of lung
metastatic foci in an animal model using B16F10 melanoma cells,
demonstrates that platelet GPIb-IX contributes to experimental
metastasis (25). Competitive
inhibition of platelet GPIIb/IIIa, either pharmacologically or by
using antibodies against GPIIIa (31,32)
and knockout of the GPIIIa subunit in mice also diminished the
formation of metastases (33).
TXA2 (34) and ADP
(35,36) are considered to be ‘soluble
stimulators’ of platelet aggregation. In this study, we observed
that the TXA2 and ADP pathways are activated during MCF-7
cell-induced TCIPA. Several lines of evidence support the
hypothesis that TXA2 plays an important role in tumor metastasis.
Firstly, TXA2 is a potent stimulator of platelet aggregation
(37), which promotes the binding
of tumor cell-platelet aggregates to endothelial cells (26). Secondly, several types of tumor
cells release large amounts of TXA2, compared to normal tissues,
which potentiates tumor growth in vitro(38). Thirdly, TXA2 has been demonstrated
to increase endothelial cell migration and angiogenesis in several
in vitro and in vivo models (39). Fourthly, TXA2 potentiates tumor cell
growth in culture and increases metastasis in animals (34). Finally, the use of TXA2 inhibitors
has been shown to reduce metastasis in animals (26,40).
However, aspirin, an inhibitor of the TXA2-mediated pathway exerted
no significant effect on MCF-7 cell-induced TCIPA, suggesting that
the TXA2 aggregation pathway is not required during MCF-7-induced
aggregation, in agreement with previous studies (5,24,41).
ADP is a potent pro-aggregatory agent, which is
released during TCIPA induced by fibrosarcoma, breast carcinoma and
neuroblastoma cells (36,42,43),
whereas the ADP scavenger apyrase (35,42),
ADP receptor antagonist ticlopidine (36) and ADP receptor inhibitor
2-methylthio-AMP (43) inhibit
TCIPA. These findings are in agreement with the results of this
study, in which MCF-7-induced TCIPA leads to activation of the ADP
pathway and MCF-7-induced TCIPA is inhibited by the ADP scavenger,
apyrase. ADP-mediated platelet activation is associated with a net
increase in the release of VEGF in healthy individuals, with no
effect on the release of endostatin. VEGF release in response to
ADP-mediated platelet activation is abolished by selective
inhibition of the P2Y12 receptor (44). Moreover, ADP depletion is associated
with reduced formation of metastases in vivo(45) and improved biochemical control in
prostate cancer patients receiving radiotherapy with curative
intent (46).
We observed that combination of the GPIb-IX,
GPIIb/IIIa and ADP pathway inhibitors exhibited a significant
repression of TCIPA when compared with inhibition of a single
pathway alone. Further studies are required to investigate the
interactions between the GPIb-IX, GPIIb/IIIa and ADP pathways
during TCIPA. The findings of the present study may be useful for
the development of new clinical strategies to arrest TCIPA and
prevent or reduce the formation of metastases.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (Nos.
81101867 and 81272542), the China International Medical Foundation
(CIMF-F-H001-057) and the Science and Education for Health
Foundation of Suzhou for Youth (SWKQ1003).
References
|
1.
|
Tao M, Ma D, Li Y, Zhou C, Zhang Y, Duan
W, Xu X, Wang R, Wu L and Liu H: Clinical significance of
circulating tumor cells in breast cancer patients. Breast Cancer
Res Treat. 129:247–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Swaby RF and Cristofanilli M: Circulating
tumor cells in breast cancer: a tool whose time has come of age.
BMC Med. 9:432011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Honn KV, Tang DG and Crissman JD:
Platelets and cancer metastasis: a causal relationship? Cancer
Metastasis Rev. 11:325–351. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sørensen HT, Mellemkjaer L, Steffensen FH,
Olsen JH and Nielsen GL: The risk of a diagnosis of cancer after
primary deep venous thrombosis or pulmonary embolism. N Engl J Med.
338:1169–1173. 1998.
|
|
5.
|
Bazou D, Santos-Martinez MJ, Medina C and
Radomski MW: Elucidation of flow-mediated tumour cell-induced
platelet aggregation using an ultrasound standing wave trap. Br J
Pharmacol. 162:1577–1589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gasic GJ, Gasic TB and Stewart CC:
Antimetastatic effects associated with platelet reduction. Proc
Natl Acad Sci USA. 61:46–52. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gasic GJ, Gasic TB, Galanti N, Johnson T
and Murphy S: Platelet-tumor-cell interactions in mice. The role of
platelets in the spread of malignant disease. Int J Cancer.
11:704–718. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Philippe C, Philippe B, Fouqueray B, Perez
J, Lebret M and Baud L: Protection from tumor necrosis
factor-mediated cytolysis by platelets. Am J Pathol. 143:1713–1723.
1993.PubMed/NCBI
|
|
9.
|
Malik AB: Pulmonary microembolism. Physiol
Rev. 63:1114–1207. 1983.PubMed/NCBI
|
|
10.
|
Mehta P: Potential role of platelets in
the pathogenesis of tumor metastasis. Blood. 63:55–63.
1984.PubMed/NCBI
|
|
11.
|
Honn KV, Tang DG and Chen YQ: Platelets
and cancer metastasis: more than an epiphenomenon. Semin Thromb
Hemost. 18:392–415. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Verheul HM, Hoekman K, Lupu F, Broxterman
HJ, van der Valk P, Kakkar AK and Pinedo HM: Platelet and
coagulation activation with vascular endothelial growth factor
generation in soft tissue sarcomas. Clin Cancer Res. 6:166–171.
2000.PubMed/NCBI
|
|
13.
|
Katagiri Y, Hayashi Y, Baba I, Suzuki H,
Tanoue K and Yamazaki H: Characterization of platelet aggregation
induced by the human melanoma cell line HMV-I: roles of heparin,
plasma adhesive proteins, and tumor cell membrane proteins. Cancer
Res. 51:1286–1293. 1991.PubMed/NCBI
|
|
14.
|
Tohgo A, Tanaka NG and Ogawa H:
Platelet-aggregating activities of metastasizing tumor cells. III
Platelet aggregation as resulting from thrombin generation by tumor
cells. Invasion Metastasis. 5:96–105. 1985.PubMed/NCBI
|
|
15.
|
Ugen KE, Mahalingam M, Klein PA and Kao
KJ: Inhibition of tumor cell-induced platelet aggregation and
experimental tumor metastasis by the synthetic Gly-Arg-Gly-Asp-Ser
peptide. J Natl Cancer Inst. 80:1461–1466. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nash GF, Turner LF, Scully MF and Kakkar
AK: Platelets and cancer. Lancet Oncol. 3:425–430. 2002. View Article : Google Scholar
|
|
17.
|
Du X, Beutler L, Ruan C, Castaldi PA and
Berndt MC: Glycoprotein Ib and glycoprotein IX are fully complexed
in the intact platelet membrane. Blood. 69:1524–1527.
1987.PubMed/NCBI
|
|
18.
|
Zhang G, Han J, Welch EJ, Ye RD,
Voyno-Yasenetskaya TA, Malik AB, Du X and Li Z: Lipopolysaccharide
stimulates platelet secretion and potentiates platelet aggregation
via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J
Immunol. 182:7997–8004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sakai H, Suzuki T, Murota M, Takahashi Y
and Takeguchi N: Nitric oxide-induced Cl- secretion in isolated rat
colon is mediated by the release of thromboxane A2. J Physiol.
543(Pt 1): 261–271. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Chung AW, Jurasz P, Hollenberg MD and
Radomski MW: Mechanisms of action of proteinase-activated receptor
agonists on human platelets. Br J Pharmacol. 135:1123–1132. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tsuruo T and Fujita N: Platelet
aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser
B Phys Biol Sci. 84:189–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Egan K, Crowley D, Smyth P, O’Toole S,
Spillane C, Martin C, Gallagher M, Canney A, Norris L, Conlon N, et
al: Platelet adhesion and degranulation induce pro-survival and
pro-angiogenic signalling in ovarian cancer cells. PloS One.
6:e261252011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Swaim MW, Chiang HS and Huang TF:
Characterisation of platelet aggregation induced by PC-3 human
prostate adenocarcinoma cells and inhibited by venom peptides,
trigramin and rhodostomin. Eur J Cancer. 32A:715–721. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Camerer E, Qazi AA, Duong DN, Cornelissen
I, Advincula R and Coughlin SR: Platelets, protease-activated
receptors, and fibrinogen in hematogenous metastasis. Blood.
104:397–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Jain S, Zuka M, Liu J, Russell S, Dent J,
Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann B
and Ware J: Platelet glycoprotein Ib alpha supports experimental
lung metastasis. Proc Natl Acad Sci USA. 104:9024–9028. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Honn KV: Inhibition of tumor cell
metastasis by modulation of the vascular prostacyclin/thromboxane
A2 system. Clin Exp Metastasis. 1:103–114. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zacharski LR, Henderson WG, Rickles FR,
Forman WB, Cornell CJ Jr, Forcier RJ, Harrower HW and Johnson RO:
Rationale and experimental design for the VA Cooperative Study of
Anticoagulation (Warfarin) in the Treatment of Cancer. Cancer.
44:732–741. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Santos-Martinez MJ, Medina C, Jurasz P and
Radomski MW: Role of metalloproteinases in platelet function.
Thromb Res. 121:535–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Andrews RK and Berndt MC: Platelet
physiology and thrombosis. Thromb Res. 114:447–453. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Shattil SJ, Kashiwagi H and Pampori N:
Integrin signaling: the platelet paradigm. Blood. 91:2645–2657.
1998.PubMed/NCBI
|
|
31.
|
Amirkhosravi A, Mousa SA, Amaya M, Blaydes
S, Desai H, Meyer T and Francis JL: Inhibition of tumor
cell-induced platelet aggregation and lung metastasis by the oral
GPIIb/IIIa antagonist XV454. Thromb Haemost. 90:549–554.
2003.PubMed/NCBI
|
|
32.
|
Trikha M, Zhou Z, Timar J, Raso E, Kennel
M, Emmell E and Nakada MT: Multiple roles for platelet GPIIb/IIIa
and alphavbeta3 integrins in tumor growth, angiogenesis, and
metastasis. Cancer Res. 62:2824–2833. 2002.PubMed/NCBI
|
|
33.
|
Bakewell SJ, Nestor P, Prasad S, Tomasson
MH, et al: Platelet and osteoclast beta3 integrins are critical for
bone metastasis. Proc Natl Acad Sci USA. 100:14205–14210. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
de Leval X, Benoit V, Delarge J, Julémont
F, Masereel B, Pirotte B, Merville MP, David JL and Dogné JM:
Pharmacological evaluation of the novel thromboxane modulator
BM-567 (II/II). Effects of BM-567 on osteogenic
sarcoma-cell-induced platelet aggregation. Prostaglandins Leukot
Essent Fatty Acids. 68:55–59. 2003.PubMed/NCBI
|
|
35.
|
Boukerche H, Berthier-Vergnes O, Penin F,
Tabone E, Lizard G, Bailly M and McGregor JL: Human melanoma cell
lines differ in their capacity to release ADP and aggregate
platelets. Br J Haematol. 87:763–772. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Bastida E, Escolar G, Almirall L and
Ordinas A: Platelet activation induced by a human neuroblastoma
tumor cell line is reduced by prior administration of ticlopidine.
Thromb Haemost. 55:333–337. 1986.PubMed/NCBI
|
|
37.
|
Dogné JM, de Leval X, Delarge J, David JL
and Masereel B: New trends in thromboxane and prostacyclin
modulators. Curr Med Chem. 7:609–628. 2000.
|
|
38.
|
Ogletree ML: Overview of physiological and
pathophysiological effects of thromboxane A2. Fed Proc. 46:133–138.
1987.PubMed/NCBI
|
|
39.
|
Nie D, Lamberti M, Zacharek A, Li L,
Szekeres K, Tang K, Chen Y and Honn KV: Thromboxane A(2) regulation
of endothelial cell migration, angiogenesis, and tumor metastasis.
Biochem Biophys Res Commun. 267:245–251. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Yokoyama I, Hayashi S, Kobayashi T, Negita
M, Yasutomi M, Uchida K and Takagi H: Prevention of experimental
hepatic metastasis with thromboxane synthase inhibitor. Res Exp Med
(Berl). 195:209–215. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Medina C, Jurasz P, Santos-Martinez MJ,
Jeong SS, Mitsky T, Chen R and Radomski MW: Platelet
aggregation-induced by caco-2 cells: regulation by matrix
metalloproteinase-2 and adenosine diphosphate. J Pharmacol Exp
Ther. 317:739–745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Jurasz P, Sawicki G, Duszyk M, Sawicka J,
Miranda C, Mayers I and Radomski MW: Matrix metalloproteinase 2 in
tumor cell-induced platelet aggregation: regulation by nitric
oxide. Cancer Res. 61:376–382. 2001.PubMed/NCBI
|
|
43.
|
Alonso-Escolano D, Strongin AY, Chung AW,
Deryugina EI and Radomski MW: Membrane type-1 matrix
metalloproteinase stimulates tumour cell-induced platelet
aggregation: role of receptor glycoproteins. Br J Pharmacol.
141:241–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Bambace NM, Levis JE and Holmes CE: The
effect of P2Y-mediated platelet activation on the release of VEGF
and endostatin from platelets. Platelets. 21:85–93. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Uluckan O, Eagleton MC, Floyd DH, Morgan
EA, et al: APT102, a novel adpase, cooperates with aspirin to
disrupt bone metastasis in mice. J Cell Biochem. 104:1311–1323.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Choe KS, Correa D, Jani AB and Liauw SL:
The use of anticoagulants improves biochemical control of localized
prostate cancer treated with radiotherapy. Cancer. 116:1820–1826.
2010. View Article : Google Scholar : PubMed/NCBI
|