Introduction
Angiomyofibroblastoma (AMF) is a rare tumor that
predominantly occurs in the female genital tract, such as the
vulva, perineum, vagina and pelvis. In 1992, Fletcher et
al(1) first described a rare,
benign tumor that occurs in the reproductive system of middle-aged
women, known as AMF. Thereafter, in 1998, Laskin et al
reported 11 cases of similar entities in males and suggested the
term AMF-like tumor (also known as cellular angiofibroma) (2). In males, AMF-like tumors are extremely
rare, but are known to occur in regions such as the inguinal area,
scrotum and perineum. In addition to two previous small series of
AMF-like tumors in males reported by Laskin et al and Iwasa
and Fletcher (2,3), AMF-like tumors have been described
only in isolated case reports. Clinically, the tumor has
asymptomatic, well-circumscribed and slow growing characteristics.
The current case report presents a case of AMF-like tumor in the
scrotum. To the best of our knowledge, only one case of AMF-like
tumor of the perineum has been previously reported in the Chinese
population of a 54-year-old male by Hlaing and Tse in Hong Kong
(4). The purpose of the current
study was to expand the experience with AMF-like tumors in males by
describing the second case in the Chinese population of this rare
lesion with a long period of follow-up and review of the
literature. Written informed consent was obtained from the
patient.
Case report
A 37-year-old male visited the Sir Run-Run Shaw
Hospital (Hangzhou, China) due to a painless mass in the left
scrotum. The patient observed that the swelling had gradually
increased in size during recent months. On physical examination at
the time of admission, a hard, painless mass was palpated in the
left scrotum. The patient’s laboratory results were within normal
limits. Tumor markers, such as α-fetoprotein and human chorionic
gonadotropin, were normal. Scrotal ultrasonography showed a mass of
~4×5 cm in size in the left scrotum that was not clearly
differentiated from the testis (Fig.
1A) and vascularity was observed inside and around the mass
(Fig. 1B). An inguinal orchiectomy
was then performed on July 7, 2005. The mass was a
well-encapsulated, soft pink-tan tumor attached to the testis,
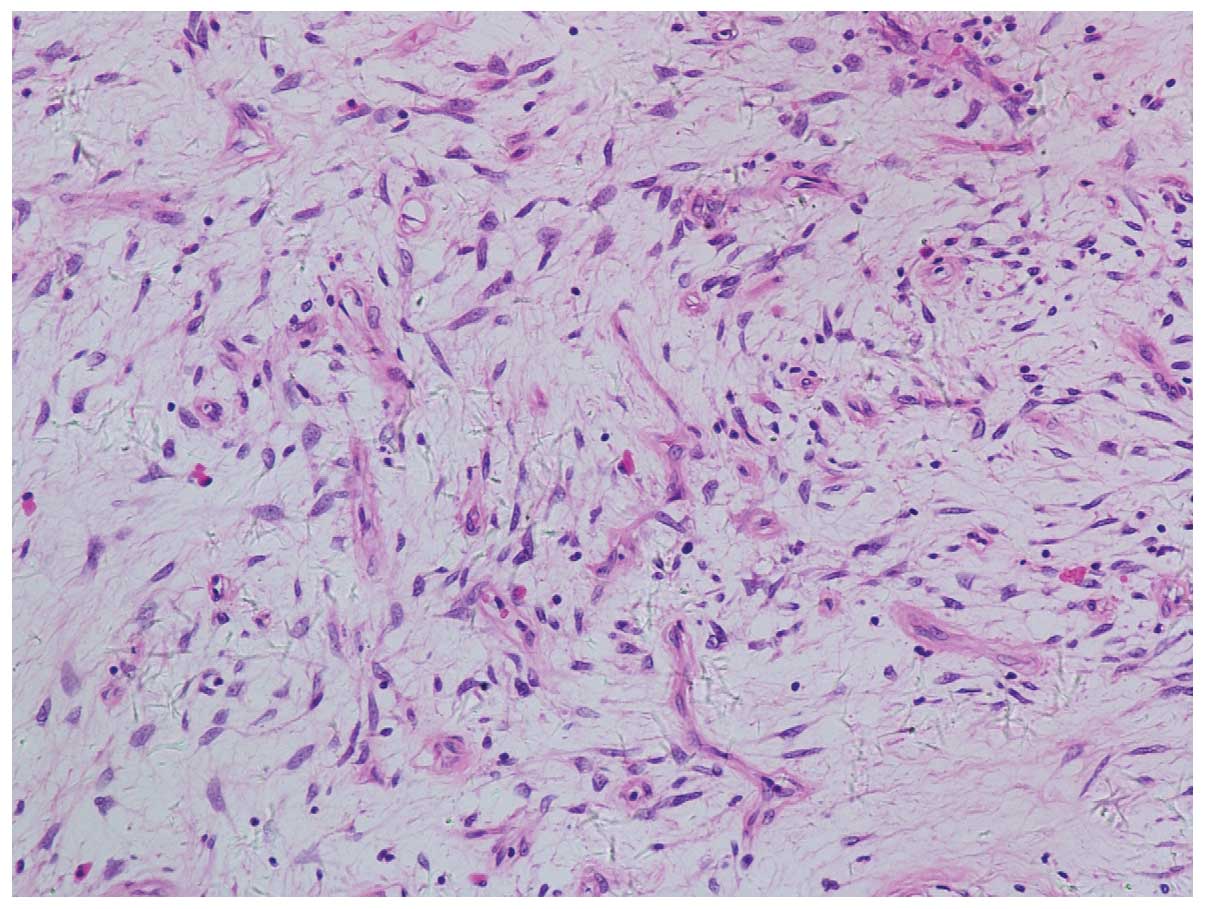
measuring 5.0×4.5×3.3 cm. Microscopically, the tumor was composed
of spindle-shaped cells and small vessels proliferating in the
edematous stroma. The tumor was scattered throughout with mature
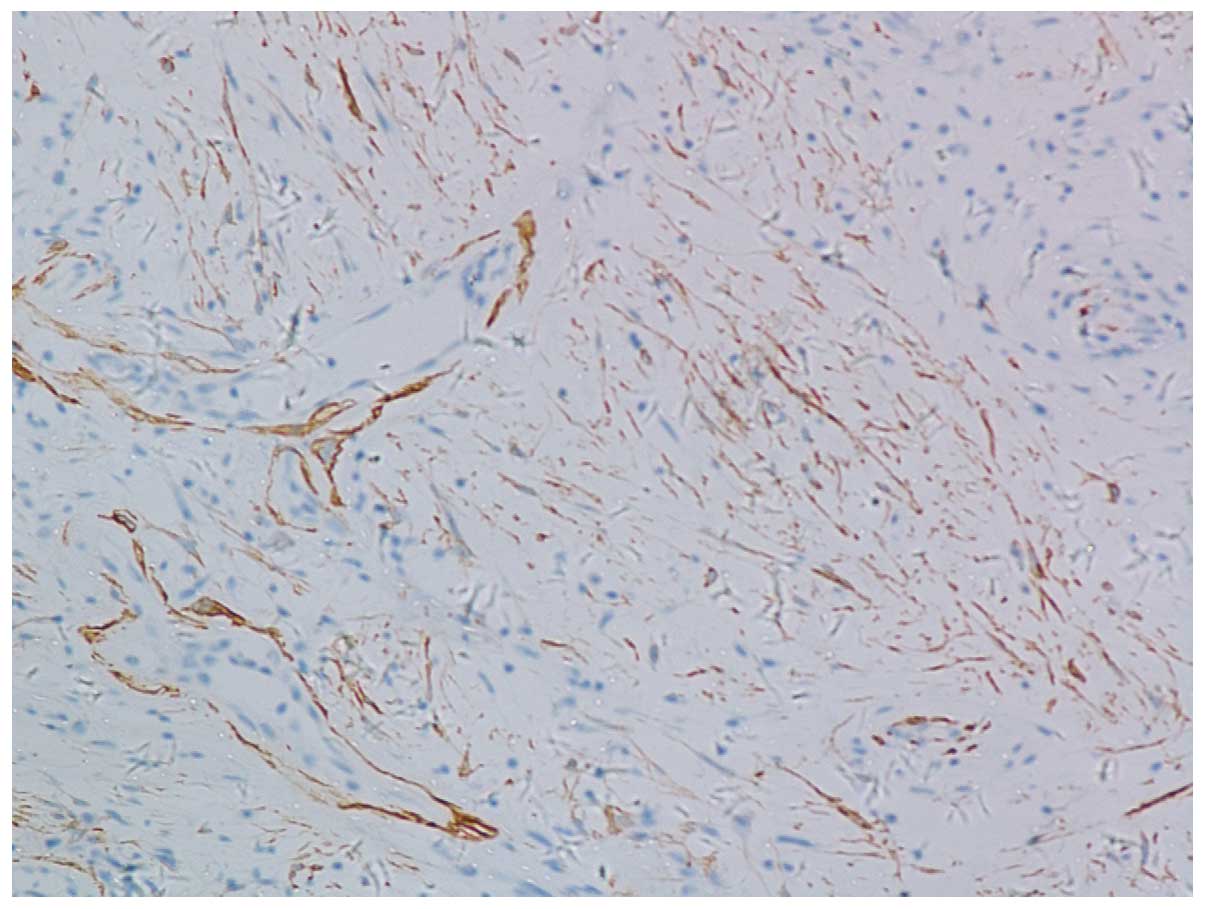
adipocytes and infiltrated with lymphocytes (Fig. 2). By immunostaining, the tumor cells
stained positive for smooth muscle actin (SMA) (Fig. 3) and negative for S-100 and actin.
CD34 was also negative in the tumor cells, but highlighted
endothelial cells in numerous vessels. Pathological diagnosis was
AMF-like tumor. Following seven years of follow-up, the patient was
asymptomatic and no tumor was found by physical examination or
pelvic computed tomography.
Discussion
Tumors occurring in the scrotum are diverse due to
various embryological origins of the scrotal contents. An accurate
diagnosis of tumors in the scrotum is not easily determined.
AMF-like tumor is a benign mesenchymal tumor with extremely low
incidence. The majority of AMFs are reported in the vulva of
premenopausal women. In male patients, only a few cases have been
reported, which predominantly occurred in the scrotal and inguinal
regions. To date, only 14 studies of AMF-like tumors in males have
been reported in the literature worldwide (2–14).
Table I summarizes the major
clinical and pathological features of the previously reported
cases.
 | Table IClinical and pathological features of
male AMF-like tumor in the literature. |
Table I
Clinical and pathological features of
male AMF-like tumor in the literature.
| Authors (year)
[ref] | Cases, n | Age, years
(median) | Sites (cases, n) | Tumor size, cm | Pathological
characteristics (cases, n) |
|---|
| Siddiqui et
al(1997) [5] | 1 | NA | Spermatic cord | NA | NA |
| Laskin et
al(1998) [2] | 11 | 39–88 (57) | Scrotum (6) and
inguinal region (5) | 2.5–14 (mean, 7) | Vimentin+
(7/7), CD34+ (4/8), desmin+ (3/8),
muscle−, specific actin+ (3/8),
SMA+ (2/8) and S-100− |
| Hisaoka et
al(1998) [6] | 2 | 78 and 55 | Inguinal region | 3×2 and 4×4.3×2 | Vimentin and
α-SMA+ |
| Ito M et
al(2000) [7] | 1 | 27 | Inguinal region | 6.5×3.5×3.5 | Vimentin+,
desmin+, CD34+ and α-SMA− |
| Hlaing et
al(2000) [4] | 1 | 54 | Perineum | 3 | Vimentin+,
desmin−, actin−, S100− and
CD34− |
| Shintaku et
al(2002) [8] | 1 | 45 | Inguinal region | 3.9 | Vimentin+,
CD34+ and α-SMA− |
| Iwasa et
al(2004) [3] | 25 | 43–78 (52) | Inguinal region
(9/25), scrotum (4/25), spermatic cord (3/25), testis (2/25) and
others (7/25) | 0.6–25 (median,
6.7) | CD34+
(18/24), SMA+(6/24), desmin (2/24) and S-100−
(24/24) |
| Hara et
al(2005) [9] | 1 | 72 | Inguinal region | 4 | Vimentin+,
muscle specific actin+, desmin−,
α-SMA−, S-100−, CD34− and
CD31− |
| Canales et
al(2006) [19] | 2 | 34 and 64 | Scrotum | 7×4×3 and
13×10×3 | Vimentin+
(2/2), CD99+ (1/2), factor VIII-related
antigen+ (1/2), cytokeratin− (1/2),
desmin− (2/2), actin−, S-100−
(2/2), CD34− (1/2), CD34+ (1/2),
SMA− (1/2) and myogenin− (1/2) |
| Miyajima et
al(2007) [10] | 1 | 50 | Inguinal region | 5.6×2.3×6.0 | CD34+,
desmin+, muscle specific actin− and
α-SMA− |
| de Souza et
al(2009) [11] | 1 | 19 | Inguinal region | 2.8 | Smooth muscle
vimentin+, desmin+ and actin+ |
| Lee et
al(2010) [12] | 1 | 71 | Scrotum | 13×10×6 | Vimentin+,
desmin−, S-100− and CD34− |
| Tzanakis et
al(2010) [13] | 1 | 36 | Spermatic cord | 4.5 | Vimentin+,
CD34+, desmin+ and SMA+ |
| Flucke et
al(2011) [14] | 8 | 32–83 (67) | Inguinal region
(4/8), scrotum (1/8), perianal region (1/8), knee (1/8) and upper
eyelid (1/8) | 1–9 (mean,
4.1) | CD34+,
desmin-, SMA+ and CD99+ |
According to these previously documented cases,
AMF-like tumors in males share a number of immunopathological
features with their female counterparts. Similar to female AMFs,
male AMFs are superficial and well-marginated masses.
Histologically, male and female AMFs consist of spindle-shaped or
epithelioid cells and small- to medium-sized vessels accompanied by
the clear presence of myofibroblastic differentiation. Some of the
tumors exhibit abundant mature adipose cells, as presented in the
current case. Immunologically, tumors exhibit marked vimentin
expression and varied expression of desmin, muscle-specific actin
and CD34, but are negative for S-100 protein (3,14,15).
In the present case, tumor cells were positive for SMA staining,
but no immunoreactivity was observed for CD34, desmin or S-100.
However, the AMF-like tumors that occur in male
patients demonstrate notably different clinicopathological features
from those occurring in females. Firstly, tumors present in older
male patients than female patients. Secondly, male AMF-like tumors
are composed primarily of spindle- rather than epithelioid-like
mesenchymal cells. The cellular matrix of the AMF-like tumors is
denser and abundant with collagen. Thirdly, although the male
neoplasms are consistent with myofibroblastic differentiation, the
immunoprofile is slightly different from that of female AMFs.
Desmin expression by the neoplastic cells has been found in the
majority of female AMFs, but has only been expressed in one-third
of the male tumors (2,3). The majority of male AMF-like tumors
express muscle-specific actin, while few female AMFs are positive
for muscle-specific actin staining (3).
In the current case, the tumor consisted of a mature
adipocytic component, which has also been identified in certain
previous cases. Therefore, differential diagnosis from spindle cell
lipoma must be determined (14,15).
Although spindle cell lipoma is a benign tumor most commonly
occurring in the subcutis of the neck, shoulder and back (16), occasional cases may occur in the
male genital tract (17). The
majority of spindle cell lipomas are more cellular than AMF-like
tumors. The stromal collagen of spindle cell lipoma is more
brightly eosinophilic and ropey collagen is a characteristic
observation in spindle cell lipoma compared with wispy collagen
fibers in AMF-like tumors. In addition, the blood vessels in
spindle cell lipoma are usually capillary-sized and thin-walled,
while AMF-like tumors are composed of small- to medium-sized
thick-walled vessels (3,14).
AMF-like tumors must also be distinguished from
solitary fibrous tumor due to the spindle-shape and bland
appearance of the tumor cells, as well as positivity for CD34.
Solitary fibrous tumors are typically composed of hypocellular and
hypercellular areas with abundant keloid-type collagen and
hemangiopericytoma-like vessels, whereas, AMF-like tumors often
show evenly distributed spindle cells with short bundles of
collagen (18). Furthermore,
AMF-like tumors lack the numerous small- and medium-sized vessels
identified in solitary fibrous tumors (19).
Generally, AMF-like tumors in males exhibit a benign
clinical course with the exception of one invasive case previously
reported by Garcia Mediero et al(20) and one locally recurrent case
reported by Laskin et al(2),
which suggested sarcomatous degeneration. Therefore, it is
important to distinguish AMF-like tumors from aggressive
angiomyxoma. Aggressive angiomyxoma has been previously described
in the scrotum, perineum and inguinal region of males and is
associated with high risk of recurrence when incompletely resected,
although, it is also an extremely rare tumor (21–25).
Aggressive angiomyxoma is an aggressive neoplasm that usually shows
an infiltrative growth pattern and invasive borders in contrast to
the well-circumscribed lesions of AMF-like tumors. Short spindle
tumor cells with minimal atypia in a myxoid stroma surrounded by
small clusters of smooth muscle cells are a characteristic feature
of aggressive angiomyxoma. Furthermore, aggressive angiomyxoma
exhibits more numerous blood vessels with large and thick walls
compared with AMF-like tumors (1,26–28).
The treatment for AMF-like tumor is wide excision
with tumor-free margins and orchiectomy is also recommended if
clinically indicated. As abovementioned, occasional cases of
recurrence have been previously reported and long-term follow-up is
necessary (2,20).
References
|
1
|
Fletcher CD, Tsang WY, Fisher C, Lee KC
and Chan JK: Angiomyofibroblastoma of the vulva. A benign neoplasm
distinct from aggressive angiomyxoma. Am J Surg Pathol. 16:373–382.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laskin WB, Fetsch JF and Mostofi FK:
Angiomyofibroblastomalike tumor of the male genital tract: analysis
of 11 cases with comparison to female angiomyofibroblastoma and
spindle cell lipoma. Am J Surg Pathol. 22:6–16. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwasa Y and Fletcher CD: Cellular
angiofibroma: clinicopathologic and immunohistochemical analysis of
51 cases. Am J Surg Pathol. 28:1426–1435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hlaing T and Tse G: Angiomyofibroblastoma
of the male perineum: an unusual location for a rare lesion. Int J
Surg Pathol. 8:79–82. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siddiqui MT, Kovarik P and Chejfec G:
Angiomyofibroblastoma of the spermatic cord. Br J Urol. 79:475–476.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hisaoka M, Hashiomoto H and Daimaru Y:
Intranodal palisaded myofibroblastoma with so-called amianthoid
fibers: a report of two cases with a review of the literature.
Pathol Int. 48:307–312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito M, Yamaoka H, Sano K and Hotchi M:
Angiomyofibroblastoma of the male inguinal region. Arch Pathol Lab
Med. 124:1679–1681. 2000.PubMed/NCBI
|
|
8
|
Shintaku M, Naitou M and Nakashima Y:
Angiomyofibroblastoma-like tumor (lipomatous variant) of the
inguinal region of a male patient. Pathol Int. 52:619–622. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hara N, Kawaguchi M, Koike H, Nishiyama T
and Takahashi K: Angiomyxoid tumor with an intermediate feature
between cellular angiofibroma and angiomyofibroblastoma in the male
inguinal region. Int J Urol. 12:768–772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyajima K, Hasegawa S, Oda Y, et al:
Angiomyofibroblastoma-like tumor (cellular angiofibroma) in the
male inguinal region. Radiat Med. 25:173–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Souza LR, Filho EC, Braga WP, Martins
PT and De Nicola H: Angiomyofibroblastoma-like tumor of the
inguinal canal. J Ultrasound Med. 28:1269–1272. 2009.PubMed/NCBI
|
|
12
|
Lee SH, Yang JW, Do JM, et al:
Angiomyofibroblastoma-like tumor of the scrotum. Korean J Urol.
51:365–367. 2010. View Article : Google Scholar
|
|
13
|
Tzanakis NE, Giannopoulos GA, Efstathiou
SP, Rallis GE and Nikiteas NI: Angiomyofibroblastoma of the
spermatic cord: a case report. J Med Case Rep. 4:792010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flucke U, van Krieken JH and Mentzel T:
Cellular angiofibroma: analysis of 25 cases emphasizing its
relationship to spindle cell lipoma and mammary-type
myofibroblastoma. Mod Pathol. 24:82–89. 2011. View Article : Google Scholar
|
|
15
|
Nucci MR, Granter SR and Fletcher CD:
Cellular angiofibroma: a benign neoplasm distinct from
angiomyofibroblastoma and spindle cell lipoma. Am J Surg Pathol.
21:636–644. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fletcher CD and Martin-Bates E: Spindle
cell lipoma: a clinicopathological study with some original
observations. Histopathology. 11:803–817. 1987. View Article : Google Scholar
|
|
17
|
Kaneko G, Nishimoto K, Ogata K and Uchida
A: A case of lipomatous tumor arising from the paratesticular
lesion. Hinyokika Kiyo. 55:725–727. 2009.(In Japanese).
|
|
18
|
Nielsen GP, O’Connell JX, Dickersin GR and
Rosenberg AE: Solitary fibrous tumor of soft tissue: a report of 15
cases, including 5 malignant examples with light microscopic,
immunohistochemical, and ultrastructural data. Mod Pathol.
10:1028–1037. 1997.
|
|
19
|
Canales BK, Weiland D, Hoffman N, et al:
Angiomyofibroblastoma-like tumors (cellular angiofibroma). Int J
Urol. 13:177–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garcia Mediero JM, Alonso Dorrego JM,
Núñez Mora C, et al: Scrotal invasive angiomyofibroblastoma. First
reported case. Arch Esp Urol. 53:827–829. 2000.(In Spanish).
|
|
21
|
Tsang WY, Chan JK, Lee KC, Fisher C and
Fletcher CD: Aggressive angiomyxoma. A report of four cases
occurring in men. Am J Surg Pathol. 16:1059–1065. 1992.PubMed/NCBI
|
|
22
|
Clatch RJ, Drake WK and Gonzalez JG:
Aggressive angiomyxoma in men. A report of two cases associated
with inguinal hernias. Arch Pathol Lab Med. 117:911–913.
1993.PubMed/NCBI
|
|
23
|
Iezzoni JC, Fechner RE, Wong LS and Rosai
J: Aggressive angiomyxoma in males. A report of four cases. Am J
Clin Pathol. 104:391–396. 1995.PubMed/NCBI
|
|
24
|
Khelifi S, Ben Ali A, Tagougui W, et al:
Perineal recurrence of an aggressive angiomyxoma: Is an incomplete
resection useful? J Chir (Paris). 146:416–418. 2009.(In
French).
|
|
25
|
Korrect GS, Kesler MV and Strup SE:
Aggressive angiomyxoma presenting as urinary retention in a male: a
case report and literature review. Can J Urol. 18:5908–5910.
2011.PubMed/NCBI
|
|
26
|
Fetsch JF, Laskin WB, Lefkowitz M,
Kindblom LG and Meis-Kindblom JM: Aggressive angiomyxoma: a
clinicopathologic study of 29 female patients. Cancer. 78:79–90.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ockner DM, Sayadi H, Swanson PE, Ritter JH
and Wick MR: Genital angiomyofibroblastoma. Comparison with
aggressive angiomyxoma and other myxoid neoplasms of skin and soft
tissue. Am J Clin Pathol. 107:36–44. 1997.
|
|
28
|
Mentzel T and Katenkamp D: Myofibroblastic
tumors. Brief review of clinical aspects, diagnosis and
differential diagnosis. Pathologe. 19:176–186. 1998.(In
German).
|