Introduction
Each year more than 350,000 new cases of bladder
cancer are diagnosed globally (1),
making bladder cancer the ninth most frequent cancer worldwide
(2). Epirubicin (EPI) and
pirarubicin (THP) are a new generation of anthracycline drugs,
widely used as anticancer chemotherapeutic agents in various types
of cancers including bladder cancer (2,3).
Furthermore, these anthracycline drugs are less cardiotoxic than
doxorubicin. However intrinsic and acquired resistance to
chemotherapeutic drugs are considered as major problems. Therefore,
it is important to develop new treatment strategies for bladder
cancer patients to prevent recurrence and reduce the risk of tumor
progression.
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) is a pro-apoptotic member of the
TNF super-family and a potentially effective anticancer agent.
TRAIL has been identified as a powerful activator of programmed
cell death or apoptosis in various tumor cells, yet is considered
relatively nontoxic against most normal cells (4–6). TRAIL
induces apoptosis by interacting with two death-inducing receptors
TRAIL-R1 (DR4) and TRAIL-R2 (DR5) following activation of the
caspase cascade that initiates both extrinsic and intrinsic
apoptotic pathways (4,7–9). In
addition, TRAIL binds to two other receptors, TRAIL-R3 (DcR1) and
TRAIL-R4 (DcR2), which lack a functional cytoplasmic death domain
(8–10). These receptors have been proposed to
inhibit TRAIL-induced apoptosis by acting as decoy receptors. Thus,
using a specific activator of TRAIL-R1 and TRAIL-R2 is preferable
to avoid interference from competition with decoy receptors.
It has been reported that monoclonal antibodies
(mAbs) to human TRAIL-R1 or TRAIL-R2 have antitumor activities
in vitro and in vivo (11,12).
These agonistic antibodies work in a similar manner to TRAIL by
activating TRAIL-mediated apoptotic pathways (12,13).
Several studies have shown that the human TRAIL-R1 mAb induced
apoptotic cell death in renal cell carcinoma (RCC) (14,15)
and bladder cancer cells (16). It
has also been reported that mapatumumab which is a fully human
agonistic mAb specific for TRAIL-R1, reduced the viability of
multiple types of tumor cells in vitro and inhibited tumor
growth in vivo (17).
The results of phase I and II studies with
mapatumumab showed good compatibility and only mild non-specific
toxicity in patients with solid malignancies (18,19).
Although these clinical trials are promising, it was recognized
that only 29.3% of patients had stable disease among the patients
who received mapatumumab therapy (20). Therefore, developing strategies to
optimize the effects of mapatumumab, particularly through
combinations with chemotheraputic drugs, is warranted.
In the present study, we examined whether the
cytotoxic effect of mapatumumab would be enhanced in combination
with chemotherapeutic drugs against bladder cancer cells. The
subtoxic concentrations of the EPI and mapatumumab combinations
exhibited synergistic cytotoxicity and induction of apoptosis
through upregulation of DR4.
Materials and methods
Reagents and cell lines
Mapatumumab, the human TRAIL-R1 mAb, was kindly
provided by Human Genome Sciences (Rockville, MD, USA). This
monoclonal antibody was isolated using phage display technology
(21). Enzyme-linked immunosorbent
assay (ELISA) and/or Biacore analysis determined that mapatumumab
is highly specific for binding to TRAIL-R1. EPI and THP were
obtained from Meiji Pharmaceutical and Kyowa Hakkou (Tokyo, Japan),
respectively.
The human bladder carcinoma cells T24, KU7, RT112,
253J and J82 were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). Cells were cultured in
RPMI-1640 medium supplemented with 1 mM HEPES buffer (both from
Sigma, St. Louis, MO, USA), penicillin and streptomycin mixture
(Nakalai, Kyoto, Japan) and 10% heat-inactivated fetal bovine serum
(Sigma).
Cytotoxicity assay
Cytotoxicity was assessed using a
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. A 100 μl suspension of 1.5×104 cells was seeded
into a 96-well flat-bottom microtiter plate. Cells were grown for
24 h at 37°C in a humidified 5% CO2 atmospheric
condition, after which cells were exposed to 100 μl of drug
solution or medium (control) to the plates in triplicate. Each
plate was incubated for 24 h. Following incubation, 10 μl MTT
labeling reagent (Roche, Mannheim, Germany) was added to each
culture well, and the cultures were incubated for an additional 4
h. After incubation, 100 μl solubilization solution containing 10%
SDS in 0.01 M HCl was placed into each well and incubated
overnight. The absorbance (A) of each well was measured by a
microculture plate reader at 540 nm. Percent cytotoxicity was
calculated by the following equation: Cytotoxicity = [1 - (A of
experimental wells/A of control wells)] × 100.
Apoptosis assay
Following incubation with mapatumumab and/or EPI for
6–24 h, both floating and adherent cells were harvested. A cell
death detection ELISA kit (Roche) was used to assess quantification
of DNA fragmentation according to the manufacturer’s instructions.
The harvested cells were mixed thoroughly with 200 μl cell lysis
buffer and kept in ice for 30 min. Subsequently, centrifugation at
1,500 rpm for 10 min was carried out. Using the Bio-Rad DC protein
assay (Bio-Rad, Hercules, CA, USA), the protein concentration of
the supernatant was measured, and 20 μl cell lysate corresponding
to 50 μg protein was transferred into a streptavidin-coated
microplate, 80 μl immunoreagent was added to each well and
incubation for 2 h at room temperature was carried out. After
removing the immunoreagent and washing with incubation buffer, 100
μl ABTS solution and ABTS stop solution were added accordingly, and
the A at 405 nm was measured.
Real-time reverse transcription-PCR
The mRNA expression of DR4 was examined by reverse
transcriptase-polymerase chain reaction (RT-PCR) analysis. Total
RNA was extracted from the bladder carcinoma cells using TRIzol
(Life technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions, and complementary DNA (cDNA) was
synthesized from 1 μg of the total RNA with the First-Strand cDNA
Synthesis kit (Applied Biosystems, Foster City, CA, USA). The
primer set for DR4 (Sigma) was as follows: forward,
5′-CGATGTGGTCAGAGCTGGTACAGC and reverse,
5′-GGACACGGCAGAGCCTGTGCCATC. The real-time RT-PCR was carried out
using LightCycler FastStart DNA Master SYBR-Green 1 (Roche). The
protocol applied for TRAIL-R1 was 40 cycles at 95°C for 10 sec,
63°C for 1 sec, and 72°C for 4 sec. Quantitative analysis of the
data was carried out using LightCycler Software version 3.5
(Roche). Standard curves for templates of TRAIL-R1 and
glyceraldehyde-3-phosphate dehydrogenase were generated by serial
dilution of the PCR products.
Western blot analysis
The protein expression of DR4 was examined by
western blot analysis. Cells were plated in 100-mm dishes for 24 h
and then treated with the combination of mapatumumab (100 ng/ml)
and EPI (1 μg/ml) for 3–12 h in a cell culture incubator at 37°C.
After the indicated treatment, the cells were lysed for 30 min on
ice in lysis buffer with protease inhibitor cocktail (Sigma). The
protein concentration of the extracts was determined by Bio-Rad
colorimetric assay. Then 50 μg of protein was separated on an
SDS-PAGE and transferred to a PVDF membrane (GE Healthcare Life
Sciences, Buckinghamshire, UK). After blocking the nonspecific
binding sites for 1 h at room temperature with 5% skim milk in TBST
(TBS with 0.1% Tween-20), the membranes were incubated overnight at
4°C with primary antibodies: DR4 mouse monoclonal antibody (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and β-actin rabbit
polyclonal antibody (Cell Signaling Technology, USA). The membranes
were then washed 3 times with TBST and incubated further at room
temperature for 1 h with the respective secondary antibodies.
Membranes were washed 3 times with TBST and signals were detected
using West Pico Chemiluminescent Substrate kit (Thermo
Scientific).
Caspase activity assay
The activities of caspase-3, -8 and -9 were measured
using a quantitative colorimetric assay with caspase-3, -8 and -9
colorimetric protease assay kits (MBL, Nagoya, Japan). Control and
treated cells (6–12 h) were homogenized in 400 μl cell lysis
buffer, incubated on ice for 10 min and centrifuged at 10,000 rpm
for 5 min at 4°C. The supernatant was recovered and the protein
concentration was measured with the Bio-Rad DC protein assay. Then
50 μl cell lysate corresponding to 100 μg of total protein, 50 μl
of 2× reaction buffer containing 10 nM DTT and 5 μl substrate were
added to each well of a 96-well plate. The plate was incubated for
24 h at 37°C. The A value of each well was measured with a
microculture plate reader at 405 nm.
Statistical analysis
All measurements were conducted 3 times. The results
are expressed as the means ± SD of 3 experiments. Statistical
significance was determined by the Student’s t-test with P-value. A
P-value of <0.05 was considered to indicate a statistically
significant result.
Calculations of synergistic cytotoxicity were
determined by isobolograpic analysis as described by Berenbaum
(22,23). Whether any particular dose
combination is synergistic, additive or antagonistic is shown by
whether the point representing that combination lies below, on or
above, respectively, the straight line joining the doses of two
drugs that, when given alone, produce the same effect as that
combination in the isobolographic analysis.
Results
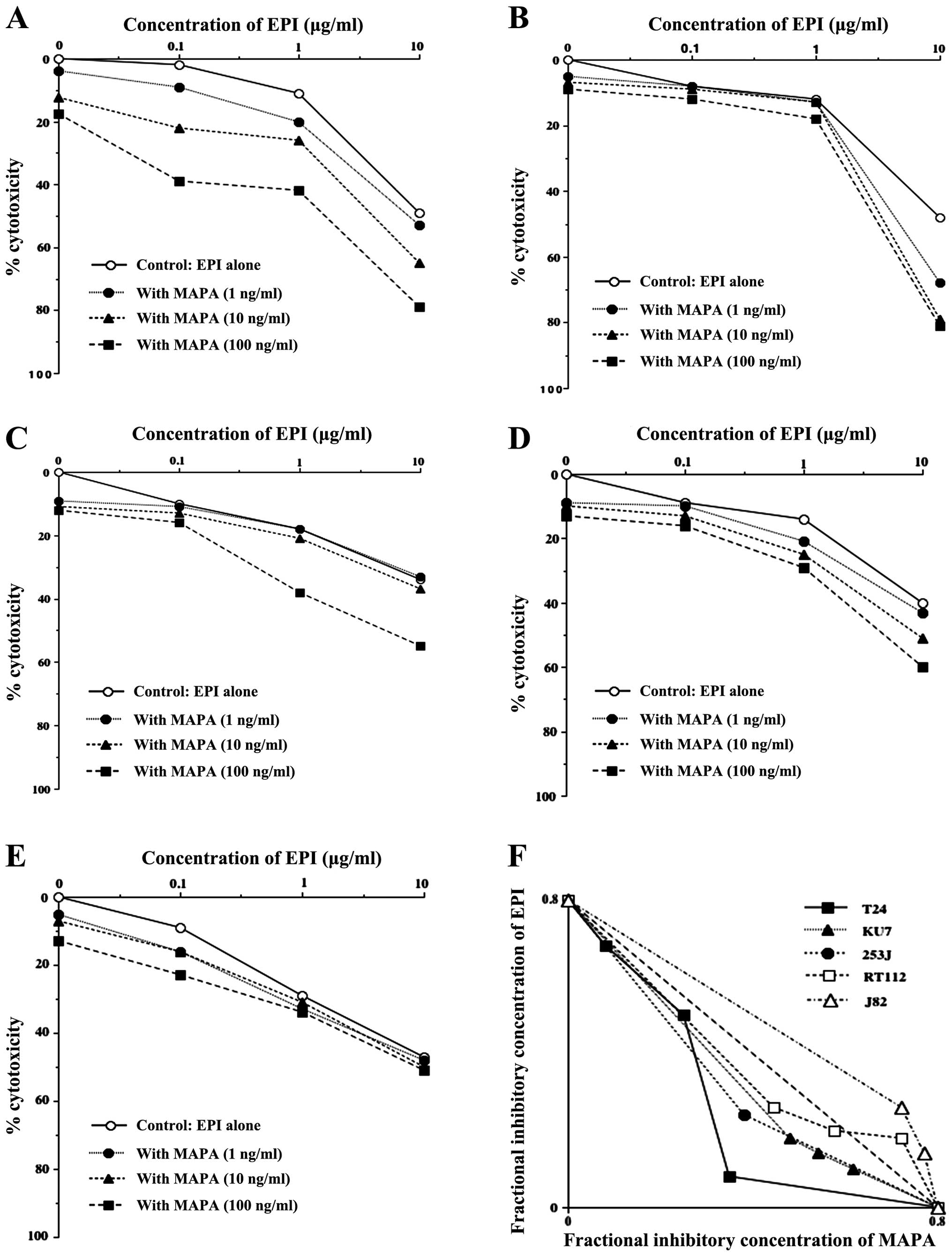
Cytotoxicity of a combination of
mapatumumab and EPI on bladder cancer cells
We examined whether the cytotoxic effect of
mapatumumab on bladder cancer cells would be enhanced by a
combination with EPI, mitomycin C, vinblastine or gemcitabine. When
T24 cells were treated with a combination of mapatumumab (1–100
ng/ml) and subtoxic concentrations of EPI (0.1–10 μg/ml) for 24 h,
a significant potentiation of cytotoxicity and synergy was achieved
(Fig. 1A and F), although there was
no synergistic effect of mapatumumab in combination with mitomycin
C, vinblastine or gemcitabine (0.1–10 μg/ml; data not shown). A
similar synergistic effect was also achieved in the KU7 (Fig. 1B and F) and 253J cells (Fig. 1C and F). The synergistic effect was
also observed with mapatumumab in combination with THP (data not
shown). In addition, an additive effect was observed in the RT112
cells (Fig. 1D and F). On the other
hand, an antagonize effect was achieved in the J82 cells (Fig. 1E and F). These studies showed that
treatment of most human bladder cancer cells with a combination of
mapatumumab and EPI/THP resulted in the potentiation of
cytotoxicity.
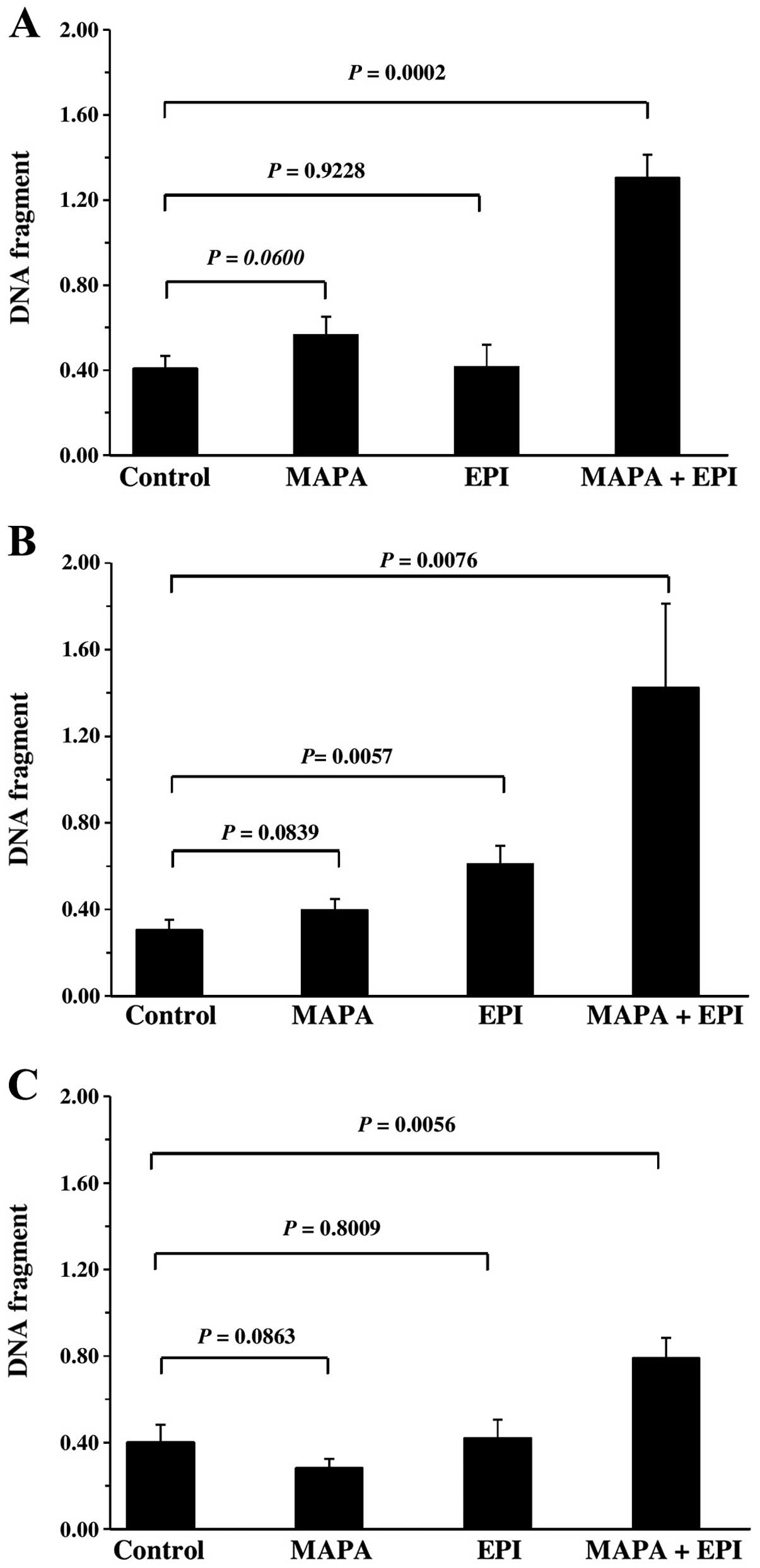
Induction of apoptosis
We assessed whether the enhanced cytotoxicity of
mapatumumab and EPI combination was mediated through apoptosis.
When mapatumumab and EPI were used alone neither mapatumumab nor
EPI caused DNA fragmentation. However, obvious DNA fragmentation
was observed (Fig. 2A), when T24
cells were incubated with these two agents simultaneously. A
similar effect was also observed in the 253J cells (Fig. 2B), yet the effect was not
significant in the case of J82 cells (Fig. 2C). These results indicate that the
combined cytotoxicity and the level of apoptosis were consistent in
the bladder cancer cells.
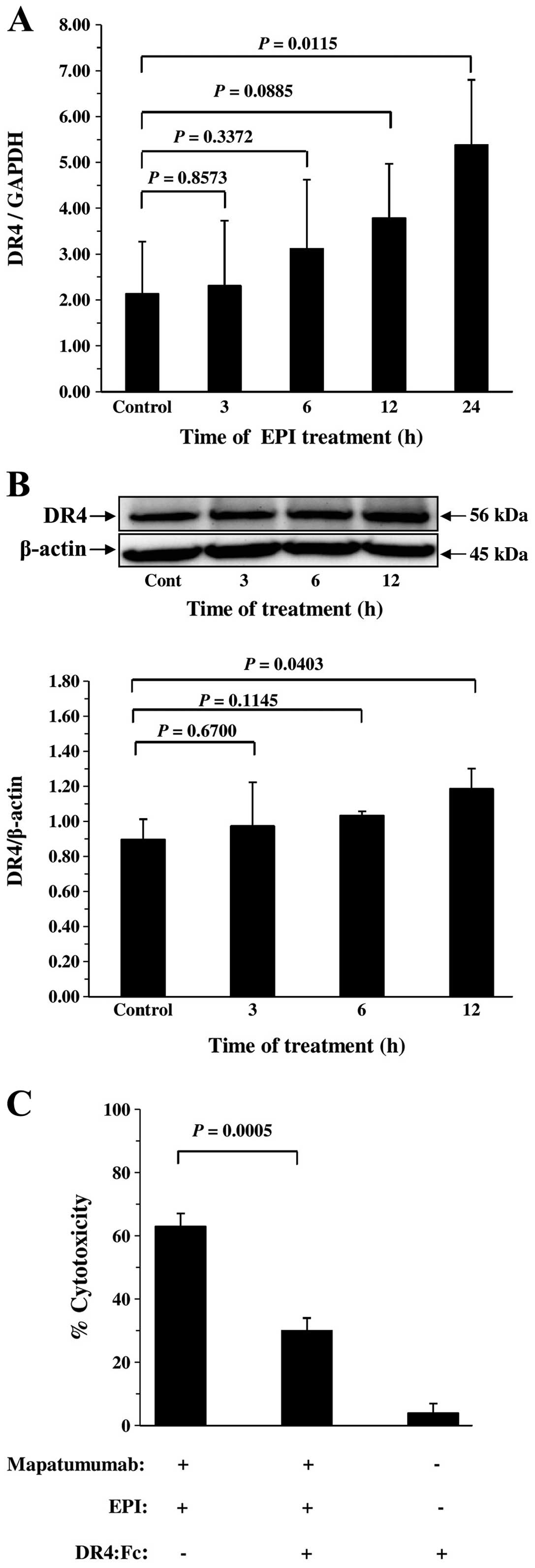
Synergistic cytotoxicity of mapatumumab
and EPI is DR4-dependent
It has been reported that cytotoxic drugs increase
the expression of the TRAIL receptor in cancer cells (24,25).
To ascertain the mechanism underlying the interaction between
mapatumumab and EPI, we examined whether EPI regulates the mRNA and
protein levels of DR4 in bladder cancer cells by quantitative
real-time RT-PCR and western blot analysis, respectively. The
examination revealed that EPI significantly increased the DR4
expression in the T24 cells at the mRNA (Fig. 3A) and protein levels (Fig. 3B) in a time-dependent manner.
We further analyzed the molecular mechanism of the
synergistic cytotoxicity of mapatumab and EPI using a human
recombinant DR4:Fc chimeric protein which has a dominant-negative
function against DR4. Cells were incubated with 100 ng/ml
mapatumumab plus 1 μg/ml EPI in the absence or presence of 1 μg/ml
DR4:Fc chimeric protein. As shown in Fig. 3C, the synergistic cytotoxicity was
blocked significantly by DR4:Fc chimeric protein in the T24 cells.
These results indicate that the synergistic cytotoxicity and levels
of apoptosis of mapatumumab and EPI were DR4-dependent.
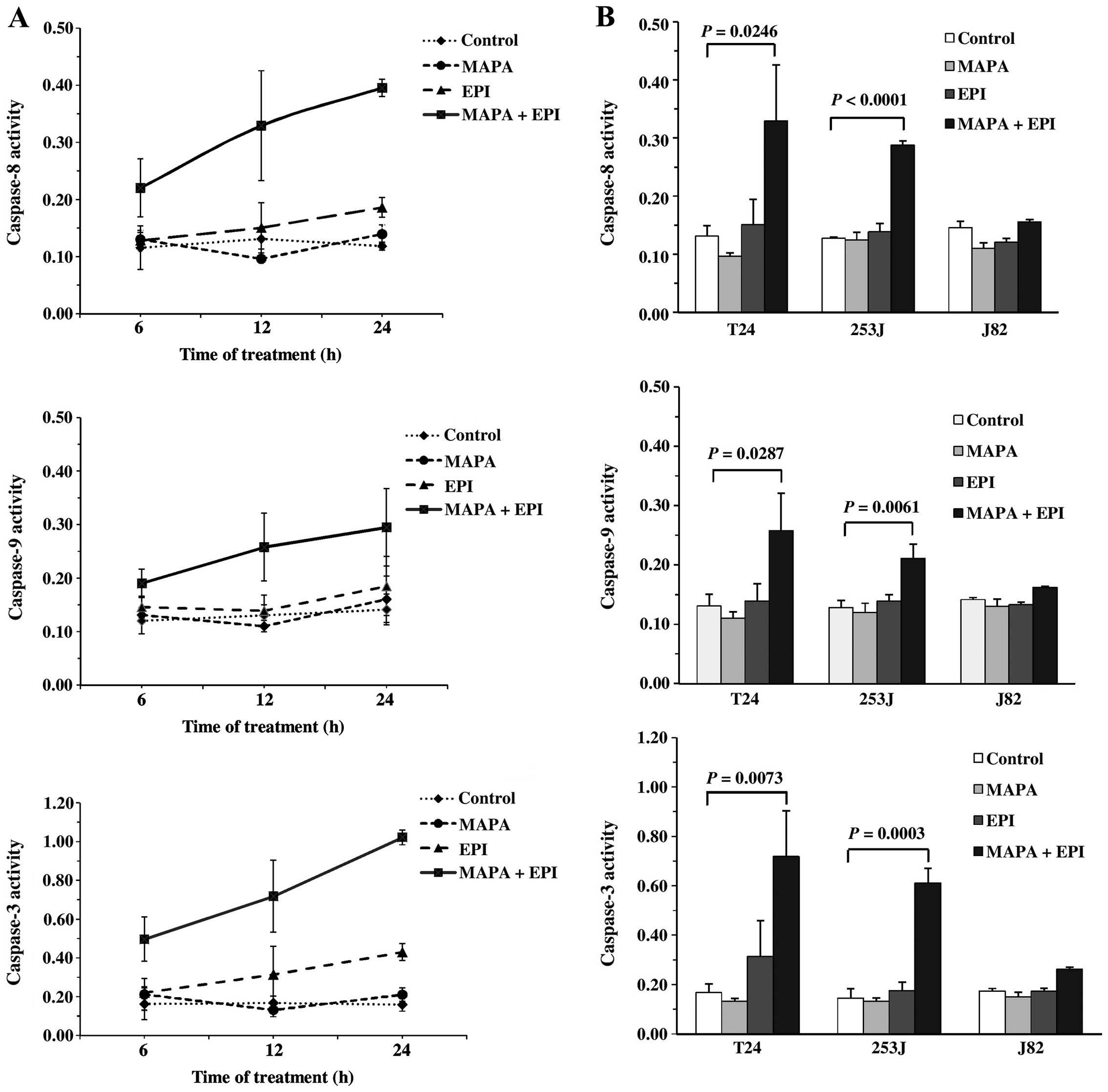
Functional activation of caspase-8, -9
and -3 following treatment with mapatumumab and EPI
In most biological systems, caspase activation plays
an important role in executing apoptosis (26). Activation of caspase-8 is initially
led by the TNF receptor family including TRAIL-R1, resulting in the
initiation of a cascade in which other caspases such as caspase-3
and -9 are activated. This ultimately causes the irreversible
commitment of cells to undergo apoptosis (11,13).
Treatment of T24 with the combination of mapatumumab and EPI
resulted in significant activation of caspase-8, -9 and -3,
although EPI alone slightly activated caspase-8 and -3 (Fig. 4A). In contrast, mapatumumab alone
did not activate casapse-8, -9 or -3. Caspase activation was
observed when the treatment time of T24 cells with mapatumumab and
EPI was shortened from 24 to 6 h (Fig.
4A). We also examined caspase activation using 253J and J82
cells, and observed that the 253J cells exhibited a similar effect,
whereas the J82 cells showed a poor effect (Fig. 4B).
Discussion
Although human agonistic antibodies to TRAIL
receptors are currently in clinical trials for the treatment of
cancer patients, resistance of cancer cells to apoptosis is one of
the major hurdles in the effective application of these molecular
target therapeutic drugs. Thus, agents that can overcome resistance
are urgently needed (18–20,27).
In the present study, we demonstrated that the anthracycline EPI,
in combination with mapatumumab, enhanced apoptosis-inducing
activity in human bladder cancer cells T24, KU7, 253J and RT112.
The observed activation of caspase-8, -9 and -3 in our experiments
suggests that the combination of mapatumumab and EPI potentiates
both the extrinsic and intrinsic pathways of apoptosis. A
relatively low concentration of each agent was required, thus
minimizing drug toxicity and maximizing the therapeutic potential
in vivo. In addition, a synergistic effect was also observed
with mapatumumab in combination with other anthracyline derivatives
such as THP. These data suggest that combination treatment using
mapatumumab and anthracyclines is promising from a clinical
perspective.
Cell surface expression of DR4 or DR5 is essential
for TRAIL-induced apoptosis, although tumor cells expressing these
death receptors are not always sensitive to TRAIL due to
intracellular mechanisms (28). It
was reported that the efficacy of TRAIL correlates with the cell
surface levels of DR4 and/or DR5 in leukemia cells (29). However, some studies showed that
TRAIL receptor expression does not correspond to the synergy of
TRAIL and chemotherapeutic drugs in certain cell lines (30–33).
The present study showed that EPI significantly upregulated DR4
expression in bladder cancer cells at both the mRNA and protein
levels. Furthermore, the synergistic cytotoxicity of mapatumumab
and EPI was significantly inhibited by DR4:Fc chimeric protein that
had a dominant-negative function against DR4. These findings imply
that mapatumumab and EPI synergistically induce cytotoxicity and
apoptosis in bladder cancer cells through upregulation of DR4.
Caspases are critical protease mediators of
apoptosis triggered by different stimuli including TRAIL (9,34,35).
In the present study, we found that in T24 and 253J cells treated
with the combination of mapatumumab and EPI, the initiative
caspases such as caspase-8, -9 and effective caspases including
caspase-3 were significantly activated. The strong activation of
caspase-8 in bladder cancer cells treated with the combination of
mapatumamab and EPI may be the result of increased death-inducing
signaling complex activity or secondary to activation by downstream
caspases. These findings revealed that the activation of the
extrinsic and intrinsic apoptotic pathways has an important role in
synergistic cytotoxicity of mapatumumab and EPI in bladder cancer
cells.
It was noted that the J82 cells exhibited no
significant enhanced cytotoxicity and apoptosis after treatment
using mapatumumab in combination with EPI, although the synergistic
effect was achieved in most bladder cancer cells. In addition,
neither caspase-8, -9 nor -3 was activated in the J82 cells treated
with mapatumumab and EPI. These findings further indicate that
caspases are critical in the enhancement of DR4-mediated apoptosis
in most bladder cancer cells following treatment with EPI.
The chemotherapeutic drug resistance of bladder
cancer cells remains a major obstacle to successful treatment, and
thus more effective therapy is needed. The present study clearly
demonstrated that mapatumumab and EPI had a synergistic cytotoxic
effect in most bladder cancer cells at a relatively low
concentration of EPI compared to a regular dose. The synergistic
cytotoxicity of mapatumumab and EPI was realized through induction
of apoptosis upon upregulating DR4 expression and activating the
caspase cascade. These findings suggest that the treatment of
bladder cancer using mapatumumab combined with EPI is promising in
a potential clinical setting.
References
|
1
|
Griffiths TR; Action on Bladder Cancer.
Current perspectives in bladder cancer management. Int J Clin
Pract. 67:435–448. 2013. View Article : Google Scholar
|
|
2
|
Li Y, Jin X, Li J, Yu J, Sun X, Chu Y, Xu
C, Li X, Wang X, Kakehi Y and Wu X: Expression of TRAIL, DR4, and
DR5 in bladder cancer: correlation with response to adjuvant
therapy and implications of prognosis. Urology. 79:968.e7–968.e15.
2012. View Article : Google Scholar
|
|
3
|
Chen SY, Du LD and Zhang YH: Pilot study
of intravesical instillation of two new generation anthracycline
antibiotics in prevention of superficial bladder cancer recurrence.
Chin Med J. 23:3422–3426. 2010.
|
|
4
|
Wu XX and Kakehi Y: Enhancement of
lexatumumab-induced apoptosis in human solid cancer cells by
cisplatin in caspase-dependent manner. Clin Cancer Res.
15:2039–2047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C,
Smolak P, Goodwin RG, Rauch CT, Schuh JC and Lynch DH: Tumoricidal
activity of tumor necrosis factor-related apoptosis-inducing ligand
in vivo. Nat Med. 5:157–163. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hao C, Song JH, Hsi B, Lewis J, Song DK,
Petruk KC, Tyrrell DL and Kneteman NM: TRAIL inhibits tumor growth
but is nontoxic to human hepatocytes in chimeric mice. Cancer Res.
64:8502–8506. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R,
Ebner R, Ni J and Dixit VM: The receptor for the cytotoxic ligand
TRAIL. Science. 276:111–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan G, Ni J, Wei YF, Yu G, Gentz R and
Dixit VM: An antagonist decoy receptor and a death
domain-containing receptor for TRAIL. Science. 277:815–818. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashkenazi A and Dixit VM: Death receptors:
signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimada O, Wu X, Jin X, Nouh MA, Fiscella
M, Albert V, Matsuda T and Kakehi Y: Human agonistic antibody to
tumor necrosis factor-related apoptosis-inducing ligand receptor 2
induces cytotoxicity and apoptosis in prostate cancer and bladder
cancer cells. Urology. 69:395–401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chuntharapai A, Dodge K, Grimmer K,
Schroeder K, Marsters SA, Koeppen H, Ashkenazi A and Kim KJ:
Isotype-dependent inhibition of tumor growth in vivo by monoclonal
antibodies to death receptor 4. J Immunol. 166:4891–4898. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ichikawa K, Liu W, Zhao L, Wang Z, Liu D,
Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP and Zhou T:
Tumoricidal activity of a novel anti-human DR5 monoclonal antibody
without hepatocyte cytotoxicity. Nat Med. 7:954–960. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohtsuka T, Buchsbaum D, Oliver P, Makhija
S, Kimberly R and Zhou T: Synergistic induction of tumor cell
apoptosis by death receptor antibody and chemotherapy agent through
JNK/p38 and mitochondrial death pathway. Oncogene. 22:2034–2044.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin X, Wu XX, Abdel-Muneem Nouh MA and
Kakehi Y: Enhancement of death receptor 4 mediated apoptosis and
cytotoxicity in renal cell carcinoma cells by subtoxic
concentrations of doxorubicin. J Urol. 177:1894–1899. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng Y, Wu XX, Fiscella M, Shimada O,
Humphreys R, Albert V and Kakehi Y: Monoclonal antibody to tumor
necrosis factor-related apoptosis-inducing ligand receptor 2
(TRAIL-R2) induces apoptosis in primary renal cell carcinoma cells
in vitro and inhibits tumor growth in vivo. Int J Oncol.
28:421–430. 2006.PubMed/NCBI
|
|
16
|
Szliszka E, Mazur B, Zydowicz G, Czuba ZP
and Krol W: TRAIL-induced apoptosis and expression of death
receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia
Histochem Cytobiol. 47:579–585. 2009.PubMed/NCBI
|
|
17
|
Pukac L, Kanakaraj P, Humphreys R,
Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M,
Mahoney A, Carrell J, Boyd E, Yao XT, Zhang L, Zhong L, von Kerczek
A, Shepard L, Vaughan T, Edwards B, Dobson C, Salcedo T and Albert
V: HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody,
induces cell death in multiple tumour types in vitro and in vivo.
Br J Cancer. 92:1430–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tolcher AW, Mita M, Meropol NJ, von Mehren
M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern
W, Corey A and Cohen RB: Phase I pharmacokinetic and biologic
correlative study of mapatumumab, a fully human monoclonal antibody
with agonist activity to tumor necrosis factor-related
apoptosis-inducing ligand receptor-1. J Clin Oncol. 25:1390–1395.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greco FA, Bonomi P, Crawford J, Kelly K,
Oh Y, Halpern W, Lo L, Gallant G and Klein J: Phase 2 study of
mapatumumab, a fully human agonistic monoclonal antibody which
targets and activates the TRAIL receptor-1, in patients with
advanced non-small cell lung cancer. Lung Cancer. 61:82–90. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hotte SJ, Hirte HW, Chen EX, Siu LL, Le
LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL and Oza AM: A
phase 1 study of mapatumumab (fully human monoclonal antibody to
TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer
Res. 14:3450–3455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berenbaum MC: Synergy, additivism and
antagonism in immunosuppression. A critical review. Clin Exp
Immunol. 28:1–18. 1977.PubMed/NCBI
|
|
23
|
Berenbaum MC: A method for testing for
synergy with any number of agents. J Infect Dis. 137:122–130. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gibson SB, Oyer R, Spalding AC, Anderson
SM and Johnson GL: Increased expression of death receptors 4 and 5
synergizes the apoptosis response to combined treatment with
etoposide and TRAIL. Mol Cell Biol. 20:205–212. 2000. View Article : Google Scholar
|
|
25
|
Wu XX, Jin XH, Zeng Y, El Hamed AM and
Kakehi Y: Low concentrations of doxorubicin sensitizes human solid
cancer cells to tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL)-receptor (R) 2-mediated apoptosis by inducing
TRAIL-R2 expression. Cancer Sci. 98:1969–1976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Green DR and Evan GI: A matter of life and
death. Cancer Cell. 1:19–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
von Pawel J, Harvey JH, Spigel DR, Dediu
M, Reck M, Cebotaru CL, Humphreys RC, Gribbin MJ, Fox NL and
Camidge DR: Phase II trial of mapatumumab, a fully human agonist
monoclonal antibody to tumor necrosis factor-related
apoptosis-inducing ligand receptor 1 (TRAIL-R1), in combination
with paclitaxel and carboplatin in patients with advanced
non-small-cell lung cancer. Clin Lung Cancer. 15:188.e2–196.e2.
2014. View Article : Google Scholar
|
|
28
|
Amantana A, London CA, Iversen PL and Devi
GR: X-linked inhibitor of apoptosis protein inhibition induces
apoptosis and enhances chemotherapy sensitivity in human prostate
cancer cells. Mol Cancer Ther. 3:699–707. 2004.PubMed/NCBI
|
|
29
|
Liu Q, Hilsenbeck S and Gazitt Y: Arsenic
trioxide-induced apoptosis in myeloma cells: p53-dependent
G1 or G2/M cell cycle arrest, activation of
caspase-8 or caspase-9, and synergy with APO2/TRAIL. Blood.
101:4078–4087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galligan L, Longley DB, McEwan M, Wilson
TR, McLaughlin K and Johnston PG: Chemotherapy and TRAIL-mediated
colon cancer cell death: the roles of p53, TRAIL receptors, and
c-FLIP. Mol Cancer Ther. 4:2026–2036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lacour S, Hammann A, Wotawa A, Corcos L,
Solary E and Dimanche-Boitrel MT: Anticancer agents sensitize tumor
cells to tumor necrosis factor-related apoptosis-inducing
ligand-mediated caspase-8 activation and apoptosis. Cancer Res.
61:1645–1651. 2001.PubMed/NCBI
|
|
32
|
Liu W, Bodle E, Chen JY, Gao M, Rosen GD
and Broaddus VC: Tumor necrosis factor-related apoptosis-inducing
ligand and chemotherapy cooperate to induce apoptosis in
mesothelioma cell lines. Am J Respir Cell Mol Biol. 25:111–118.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsai WS, Yeow WS, Chua A, Reddy RM and
Nguyen DM, Schrump DS and Nguyen DM: Enhancement of
Apo2L/TRAIL-mediated cytotoxicity in esophageal cancer cells by
cisplatin. Mol Cancer Ther. 5:2977–2990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Derosier LC, Vickers SM, Zinn KR, Huang Z,
Wang W, Grizzle WE, Sellers J, Stockard CR Jr, Zhou T, Oliver PG,
Arnoletti P, Lobuglio AF and Buchsbaum DJ: TRA-8 anti-DR5
monoclonal antibody and gemcitabine induce apoptosis and inhibit
radiologically validated orthotopic pancreatic tumor growth. Mol
Cancer Ther. 6:3198–3207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O’Kane HF, Watson CJ, Johnston SR, Petak
I, Watson RW and Williamson KE: Targeting death receptors in
bladder, prostate and renal cancer. J Urol. 175:432–438. 2006.
View Article : Google Scholar : PubMed/NCBI
|