Introduction
The epithelial-mesenchymal transition (EMT), an
important morphological event in which polarized epithelial cells
convert to contractile and motile mesenchymal cells, is recognized
as an important process during embryonic development and tissue
organization (1). EMT leads to the
generation of cancer cells with stem cell-like characteristics,
increasing their self-renewal and tumor-initiating capabilities,
and resistance to apoptosis and chemotherapy; collectively, these
properties promote tumor cell invasion and metastasis (2). Given the previously established
clinical importance of these EMT-associated processes, inhibition
of EMT is an attractive therapeutic approach that could significant
affect disease outcome, although it remains unclear which tumors
should be treated and at what state of progression (3). Dietary chemoprevention using natural
compounds such as herbal medicines has recently been highlighted as
a safe method for preventing or suppressing the development of
cancer, despite uncertainties about the molecular mechanisms of
such compounds.
Prunella vulgaris (PV), commonly known as
'Ha-gocho' in Korea, it is a perennial herb that is widely
distributed in East Asia and Europe. PV, which is effective in
preventing or treating symptoms such as hypertension, inflammation,
sore throat and fever, is commonly used as a dietary supplement
worldwide. Phytochemical studies have indicated that PV contains
oleanolic, betulinic, ursolic and rosmarinic acid; triterpenoids;
flavonoids; tannins; and the anionic polysaccharide prumelline
(4). Previous studies have reported
that PV extracts possess various biological activities, including
antimicrobial, anticancer and anti-inflammatory actions (5–8).
Additionally, the aqueous extract of PV (PVAE) suppresses tumor
cell invasion by regulating matrix metalloproteinase (MMP)
expression (9,10). However, an antimetastatic effect of
PVAE in relation to EMT has not yet been reported.
In the present study, we hypothesized that PVAE, a
typical medicine for decoction, may have a role as an inhibitor of
EMT in cancer progression and thus could serve as a dietary
chemopreventive agent against malignant tumors. We report that PVAE
significantly inhibited the invasion and migration of
lipopolysaccharide (LPS)-induced EMT in two metastatic cancer cell
lines through downregulation of the NF-κB/Snail signaling pathway.
We propose that PVAE is a good candidate for use as a dietary
chemopreventive agent with antimetastatic activity against
malignant tumors.
Materials and methods
Materials and reagents
The PV used in the present study was kindly supplied
by Professor Jung-Hye Choi (Kyung Hee University). Aqueous
extraction procedures were performed by boiling 100 g PV in 500 ml
distilled water for 30 min and then filtering through Whatman
filter paper No. 2 (Advantec, Tokyo, Japan). Subsquently, the
filtrates were combined and evaporated under a vacuum and then
lyophilized with a freeze dryer (Ilshine Lab, Suwon, Korea). The
dry residue was stored at −20°C. MDA-MB-231 and SKOV-3 cells were
maintained in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/stereptomycin antibiotics. The antibodies NF-κB p65
subunit and β-actin were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA), Snail was purchased from Cell Signalling
Technology (Beverly, MA, USA), vimentin and β-catenin were
purchased from Abcam (Cambridge, MA, USA), and N-cadherin were
purchased from BD Biosciences (San Jose, CA, USA).
Proliferation assay
All proliferation assays were based on the
3-[4,5-dimethythiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)
method. Cells were seeded in a 96-well plate, 1×104
cells/well. After overnight culture, PVAE was added to the cells
and further cultured for 24 h. The media was removed and DMSO was
added at MTT solubilization solution. Absorbance was measured at
550 nm.
Colony forming assay
Single-cell suspensions of 5×103 cells
were seeded into 6-well plate and allowed to attach for 24 h at
37°C in culture medium. Cells were then treated with 100 µM
or 1,000 µM PVAE. After 10 days, colonies were fixed with
100% methanol for 10 min at room temperature and stained with 0.1%
crystal violet. Plates were washed with PBS and were
photographed.
Immunofluorescence staining
MDA-MB-231 cells were grown in 4-chamber slides in
serum-free media, and were treated with LPS (5 µg/ml) or
co-treated with LPS (5 µg/ml) and PVAE (100 µM).
After 24-h incubation, cells were fixed with 4% paraformaldehyde at
4°C. Cells were washed with PBS containing 0.1% BSA and incubated
with anti-N-cadherin antibody for 1 h followed by 1-h incubation
with fluorescence-tagged secondary antibody, then counterstained
with DAPI for 5 min. Cell images were captured at ×200
magnification on a Leica fluorescence microscope.
Cell migration assay
Migration was assessed by a wound healing assay.
Cells were seeded at 2×104 MDA-MB-231 and SKOV-3
cells/well and were cultured for 24 h. After scraping the cell
monolayer with a sterile micropipette tip, the wells were washed
with PBS, and treated with LPS (5 µg/ml) or co-treated with
LPS (5 µg/ml) and PVAE (100 µM). The first image of
each scratch was acquired at time zero. At 24 h, each scratch was
examined and captured at the same location and the healed area was
measured.
Transwell invasion assay
The invasion of tumor cells was assessed in
Transwell chambers equipped with 8-µm pore size, 6.5 mm
diameter polyvinylpyrrolidone-free polycarbonated membranes that
were coated with 1 mg/ml fibronectin. The cells were seeded onto
the upper wells at a concentration of 1×105 and
MDA-MB-231 and SKOV-3 cells/well were cultured for 24 h following
treatment with LPS (5 µg/ml) or co-treatment with LPS (5
µg/ml) and PVAE (100 µM). The bottom chambers of the
Transwell were filled with conditioned medium. After incubation for
24 h, cells were fixed with 100% methanol for 10 min at room
temperature, stained with 0.1% crystal violet and counted under a
light microscope.
Western blotting
MDA-MB-231 and SKOV-3 cells were treated with with
LPS (5 µg/ml) or co-treated with LPS (5 µg/ml) and
PVAE (100 µM) for 24 h. After lysing cells with RIPA buffer,
ptoteins were resolved by SDS-PAGE and immunoblotted using primary
antibodies such as anti-N-cadherin, anti-β-catenin, anti-vimentin,
anti-NF-κB p65 subunit, anti-Snail and anti-β-actin antibody. After
treatment with appropriate secondary antibodies, the immunoreactive
bands were visualized by standard ECL method.
Statistical analysis
The results are presented as mean ± SE, and
statistical comparisons between groups were carried out using
one-way ANOVA followed by the Student's t-test.
Results
Effects of PVAE on the growth of human
cancer cells in vitro
We initially examined the effect of PVAE on the
proliferation of the human metastatic cancer cell lines MDA-MB-231
(breast cancer cells) and SKOV-3 (ovarian cancer cells). Cells were
treated for 24 h with different concentrations of PVAE, and cell
viability was measured by MTT assay. As shown in Fig. 1A, PVAE inhibited cell proliferation
in a concentration-dependent manner, although the IC50
(the drug concentration that causes 50% growth inhibition) was
different for MDA-MB-231 cells (1094±7 µg/ml) and SKOV-3
cells (225±4 µg/ml). The long-term effects of PVAE were
determined by culturing MDA-MB-231 and SKOV-3 cells with or without
PVAE for 10–15 days, and then performing colony-forming assays. At
a concentration of 100 µg/ml, PVAE did not show any
significant effect, whereas 1,000 µg/ml PVAE almost
completely inhibited colony formation (Fig. 1B). In light of the additional
experiments, 100 µg/ml PVAE was considered a suitable
concentration for use in subsequent experiments.
PVAE inhibits EMT-related increases in
cell migration and invasion induced by LPS
LPS may act as an independent factor to trigger the
EMT process, as has previously been reported by Chen and colleagues
(11,12). To determine whether PVAE inhibits
LPS-induced EMT, which is associated with enhanced cellular
progression, we monitored the effects of PVAE on LPS (5
µg/ml)-induced migration and invasion. Cancer cell lines
were treated with LPS alone, LPS plus PVAE, or PVAE alone, and
wound-healing and fibronectin-based Transwell invasion assays were
performed. LPS induced at least a 1.3-fold increase in migration,
as determined by wound-healing assays, whereas treatment with 100
µg/ml PVAE inhibited this LPS-induced migration by an
average of 60% (Fig. 2). Transwell
invasion assays showed that treatment with LPS alone significantly
increased the number of invasive cells compared with untreated
control cells by 1.5-fold or more. This LPS-induced increase in the
number of invasive cells was significantly reduced by treatment
with PVAE (Fig. 3). Collectively,
these data suggest that PVAE inhibits the EMT-related increase in
the invasiveness of human cancer cells induced by LPS.
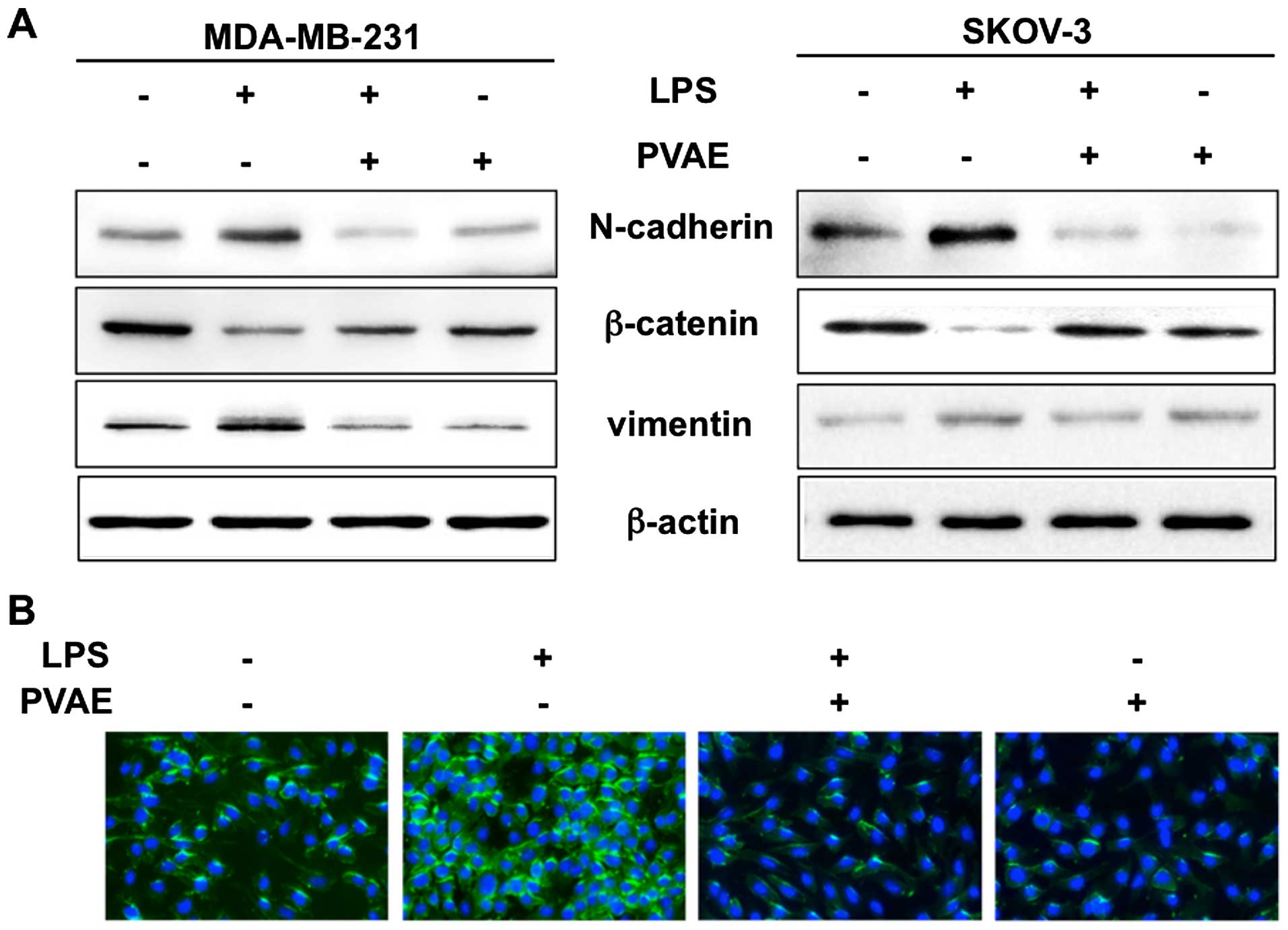
PVAE inhibits the expression of markers
of EMT in human cancer cells
To further investigate the effect of PVAE on
LPS-induced EMT, we monitored the expression of the EMT-related
proteins, N-cadherin, β-catenin and vimentin by western blotting
(Fig. 4A). β-catenin expression was
significantly downregulated in LPS-treated cancer cells compared
with controls, whereas N-cadherin and vimentin expression were
upregulated. Notably, PVAE attenuated both downregulation of
β-catenin and upregulation of N-cadherin/vimentin, suggesting that
it reversed LPS-induced EMT. We also examined N-cadherin expression
in cancer cells by immunofluorescence (Fig. 4B). Consistent with the western
blotting results, N-cadherin was highly expressed after LPS
treatment, but was significantly decreased by co-treatment with
PVAE. Taken together, western blotting and fluorescence imaging
results imply that PVAE has an inhibitory effect on EMT.
NF-κB/Snail signaling is required for
PVAE-mediated reversal of LPS-induced EMT marker expression
Numerous studies have reported that many drugs may
inhibit the invasion and migration of cancer cells by suppressing
NF-κB activation and Snail induction, suggesting that the NF-κB
signaling pathway is critically involved in the acquisition of EMT
through its downstream target, the transcription factor Snail. To
determine whether the effects of PVAE on LPS-induced changes in EMT
marker expression involve inhibition of NF-κB/Snail activation, we
monitored expression of the NF-κB p65 subunit and Snail by western
blotting. As shown in Fig. 5, LPS
significantly upregulated the expression of NF-κB p65 and Snail
protein. These effects were blunted by PVAE, suggesting that PVAE
suppresses LPS-triggered EMT by counteracting NF-κB p65 activation
and Snail induction by LPS.
Discussion
Cancer cell metastasis is frequently associated with
activation of EMT, which results in a loss of the cells' epithelial
traits and acquisition of many properties of mesenchymal cells. The
profound changes in cytoskeletal architecture that occur during
EMT, together with a reduction in intercellular adhesion and an
increase in motility, are fundamental to the metastatic process,
enabling these cells to break through the basal membrane and
migrate over long distances (2,13). In
addition, it was recently reported that the EMT process is related
to the cancer stem cell-like properties and therapeutic resistance
of cancer cells (14–16). EMT is a complex, stepwise phenomenon
that is not only involved in embryonic development, but also in
other physiological and pathological conditions, playing a role in
enhancing the invasive and metastatic behavior of cancer cells.
Therefore, inhibition of EMT is an attractive therapeutic approach
that could have a significant effect on disease outcome.
As previously reported, LPS induces EMT in breast
cancer cells, increasing their invasion and migration and resulting
in enhanced lung metastasis (17).
In the present study, we showed that LPS induces these typical
features of EMT in various cancer cell types, and also promotes the
characteristic decreases in β-catenin expression, and increases in
N-cadherin and vimentin expression. PVAE inhibited LPS-induced EMT,
reversing the pattern of protein expression associated with
invasion and migration. Additionally, we found that NF-κB/Snail
signaling was required for LPS-induced EMT in various cancer cell
types, suggesting a mechanism by which PVAE may act to inhibit
cancer cell metastasis.
PV, a Labiate plant, is a traditional fever remedy,
and more recently has been used to treat tuberculosis, thyroid
gland swelling, jaundice, infectious hepatitis, bacillary
dysentery, pleuritis, hypertension and cancer (18). PV has been highlighted in the
dietary supplements research field as an anticancer agent that has
been reported to exert strong anti-tumor effects by promoting
apoptosis and regulating the cell cycle (19–21).
Although some researchers have reported that PV and rosmarinic acid
extracted from PV suppress cancer metastasis (9,22,23),
this action has not been linked to an EMT-dependent mechanism. To
the best of our knowledge, the present study is the first to
demonstrate that the antimetastatic effects of PV are associated
with the EMT process in cultured human cancer cells, suggesting a
new dietary chemopreventive role of PV in inhibiting the
progression of EMT-related cancer metastasis.
In the present study, we showed that PVAE inhibited
LPS-induced EMT, determined by monitoring changes in cell migration
and invasion, and expression of cell-cell adhesion proteins and
EMT-related proteins like N-cadherin. Cadherins are transmembrane
glycoproteins that mediate Ca2+-dependent cell-cell
adhesion (24). N-cadherin,
typically expressed by mesenchymal cells and overexpressed in some
cancer cells, is correlated with enhanced invasiveness (25). Thus, the gain of N-cadherin
expression in cancer cells have functional significance for cancer
progression and metastasis (26).
PVAE also regulated the expression of other EMT-related proteins,
including β-catenin and vimentin, resulting in inhibition of cell
migration and invasion. β-catenin, a transcription factor in the
Wnt signaling pathway involved in the regulation of cell adhesion,
is typically more abundant in epithelial-like cells (27). Vimentin is an intermediate filament
typically found in non-epithelial and mesenchymal cells (28).
Our data also demonstrated that the mechanism of
action of PV may involve suppression of NF-κB/Snail signaling.
NF-κB is a structurally conserved family of dimeric transcription
factors that plays pivotal roles in maintaining an invasive
phenotype as well as promoting carcinogenesis (29,30).
It also plays a central role in EMT through direct activation of
the transcription of Snail, which has been established as a
critical mediator of EMT (31).
Consistent with the role of NF-κB as an upstream regulator of Snail
expression, inhibition of NF-κB reverses the induction of Snail
mRNA during EMT (32). Our results
support these previous findings and provide a mechanistic basis for
the inhibition of tumor progression by PV as well as PVAE.
In conclusion, we demonstrated that PVAE inhibition
of tumor invasion and migration is associated with the EMT process
during tumor progression, and is possibly mediated by suppressing
NF-κB/Snail signaling and regulating the expression of N-cadherin,
β-catenin and vimentin-important downstream EMT markers. Although
further in vivo studies are needed to establish the
potential of PVAE as an anticancer therapeutic agent, we suggest
that PVAE is an effective dietary chemopreventive agent with
antimetastatic activity against malignant tumors.
Acknowledgments
The present study was supported by the Traditional
Korean Medicine R&D program funded by the Ministry of Health
& Welfare through the Korea Health Industry Development
Institute (HI14C05830000) and by the Basic Science Research Program
through the National Research Foundation of Korea (NRF) funded by
the Ministry of Education (2014R1A1A2057861).
References
|
1
|
Kong D, Li Y, Wang Z and Sarkar FH: Cancer
stem cells and epithelial-to-mesenchymal transition
(EMT)-phenotypic cells: Are They Cousins or Twins? Cancers (Basel).
3:716–729. 2011. View Article : Google Scholar
|
|
2
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu HX, Lee SH, Lee SF, White RL and Blay
J: Isolation and characterization of an anti-HSV polysaccharide
from Prunella vulgaris. Antiviral Res. 44:43–54. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang YJ, Lee EJ, Kim HR and Hwang KA: In
vitro antioxidant and anticancer effects of solvent fractions from
Prunella vulgaris var. lilacina. BMC Complement Altern Med.
13:3102013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park SH, Koo HJ, Sung YY and Kim HK: The
protective effect of Prunella vulgaris ethanol extract against
vascular inflammation in TNF-α-stimulated human aortic smooth
muscle cells. BMB Rep. 46:352–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Psotová J, Kolár M, Sousek J, Svagera Z,
Vicar J and Ulrichová J: Biological activities of Prunella vulgaris
extract. Phytother Res. 17:1082–1087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoon MY, Choi GJ, Choi YH, Jang KS, Park
MS, Cha B and Kim JC: Effect of polyacetylenic acids from Prunella
vulgaris on various plant pathogens. Lett Appl Microbiol.
51:511–517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi JH, Han EH, Hwang YP, Choi JM, Choi
CY, Chung YC, Seo JK and Jeong HG: Suppression of PMA-induced tumor
cell invasion and metastasis by aqueous extract isolated from
Prunella vulgaris via the inhibition of NF-kappaB-dependent MMP-9
expression. Food Chem Toxicol. 48:564–571. 2010. View Article : Google Scholar
|
|
10
|
Kim SH, Huang CY, Tsai CY, Lu SY, Chiu CC
and Fang K: The aqueous extract of Prunella vulgaris suppresses
cell invasion and migration in human liver cancer cells by
attenuating matrix metalloproteinases. Am J Chin Med. 40:643–656.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC
and Lee CH: Resveratrol inhibits LPS-induced epithelial-mesenchymal
transition in mouse melanoma model. Innate Immun. 18:685–693. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang T, Chen Z and Fang L: Curcumin
inhibits LPS-induced EMT through downregulation of NF-κB-Snail
signaling in breast cancer cells. Oncol Rep. 29:117–124. 2013.
|
|
13
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herschkowitz JI, Zhao W, Zhang M, Usary J,
Murrow G, Edwards D, Knezevic J, Greene SB, Darr D, Troester MA, et
al: Comparative oncogenomics identifies breast tumors enriched in
functional tumor-initiating cells. Proc Natl Acad Sci USA.
109:2778–2783. 2012. View Article : Google Scholar :
|
|
15
|
Iliopoulos D, Lindahl-Allen M, Polytarchou
C, Hirsch HA, Tsichlis PN and Struhl K: Loss of miR-200 inhibition
of Suz12 leads to polycomb-mediated repression required for the
formation and maintenance of cancer stem cells. Mol Cell.
39:761–772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li QQ, Xu JD, Wang WJ, Cao XX, Chen Q,
Tang F, Chen ZQ, Liu XP and Xu ZD: Twist1-mediated
adriamycin-induced epithelial-mesenchymal transition relates to
multidrug resistance and invasive potential in breast cancer cells.
Clin Cancer Res. 15:2657–2665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HI, Quan FS, Kim JE, Lee NR, Kim HJ,
Jo SJ, Lee CM, Jang DS and Inn KS: Inhibition of estrogen signaling
through depletion of estrogen receptor alpha by ursolic acid and
betulinic acid from Prunella vulgaris var. lilacina. Biochem
Biophys Res Commun. 451:282–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blinman P, Alam M, Duric V, McLachlan SA
and Stockler MR: Patients' preferences for chemotherapy in
non-small-cell lung cancer: A systematic review. Lung Cancer.
69:141–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng L, Jia X, Zhu M, Chen Y and Shi F:
Chemoprevention by Prunella vulgaris L. extract of non-small cell
lung cancer via promoting apoptosis and regulating the cell cycle.
Asian Pac J Cancer Prev. 11:1355–1358. 2010.
|
|
21
|
Feng L, Jia XB, Jiang J, Zhu MM, Chen Y,
Tan XB and Shi F: Combination of active components enhances the
efficacy of Prunella in prevention and treatment of lung cancer.
Molecules. 15:7893–7906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Y, Jiang Z, Ji G and Liu J: Inhibition
of bone metastasis from breast carcinoma by rosmarinic acid. Planta
Med. 76:956–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Xu G, Liu L, Xu D and Liu J:
Anti-invasion effect of rosmarinic acid via the extracellular
signal-regulated kinase and oxidation-reduction pathway in Ls174-T
cells. J Cell Biochem. 111:370–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nguyen PT, Kudo Y, Yoshida M, Kamata N,
Ogawa I and Takata T: N-cadherin expression is involved in
malignant behavior of head and neck cancer in relation to
epithelial-mesenchymal transition. Histol Histopathol. 26:147–156.
2011.
|
|
26
|
Araki K, Shimura T, Suzuki H, Tsutsumi S,
Wada W, Yajima T, Kobayahi T, Kubo N and Kuwano H: E/N-cadherin
switch mediates cancer progression via TGF-β-induced
epithelial-to-mesenchymal transition in extrahepatic
cholangiocarcinoma. Br J Cancer. 105:1885–1893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang J, Xiao D, Li G, Ma J, Chen P, Yuan
W, Hou F, Ge J, Zhong M, Tang Y, et al: EphA2 promotes
epithelial-mesenchymal transition through the Wnt/β-catenin pathway
in gastric cancer cells. Oncogene. 33:2737–2747. 2014. View Article : Google Scholar
|
|
28
|
Liu Z, Chen L, Zhang X, Xu X, Xing H,
Zhang Y, Li W, Yu H, Zeng J and Jia J: RUNX3 regulates vimentin
expression via miR-30a during epithelial-mesenchymal transition in
gastric cancer cells. J Cell Mol Med. 18:610–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ennen M, Klotz R, Touche N, Pinel S,
Barbieux C, Besancenot V, Brunner E, Thiebaut D, Jung AC,
Ledrappier S, et al: DDB2: A novel regulator of NF-κB and breast
tumor invasion. Cancer Res. 73:5040–5052. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu K, Zeng J, Li L, Fan J, Zhang D, Xue Y,
Zhu G, Yang L, Wang X and He D: Silibinin reverses
epithelial-to-mesenchymal transition in metastatic prostate cancer
cells by targeting transcription factors. Oncol Rep. 23:1545–1552.
2010.PubMed/NCBI
|
|
31
|
Chua HL, Bhat-Nakshatri P, Clare SE,
Morimiya A, Badve S and Nakshatri H: NF-kappaB represses E-cadherin
expression and enhances epithelial to mesenchymal transition of
mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2.
Oncogene. 26:711–724. 2007. View Article : Google Scholar
|
|
32
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|