Introduction
The recorded incidence of cancer is increasing
because of the increasing number of aging populations, as well as
increasing prevalence of risk factors such as smoking, overweight
and changing dietary patterns (1,2).
Smoking is the most common reason for cancer incidence and deaths.
In 2012, lung cancer patients due to smoking and other causes were
the leading cause of cancer death worldwide both in males and
females (3). According to this
tendency, there is a movement to reduce smoking in the world.
However, apart from a decrease in smoking, the number of patients
and death rate caused by lung cancer has not decreased. Recent
studies have shown that smoking does not simply induce an increase
in lung cancer patients. Other known risk factors for lung cancer
include exposure to outdoor pollution and this is the largest cause
of lung cancer patients in China and East Asia (4–6).
Many of the factors involved in outdoor pollution
induce cancerization through gene mutation, as well as induction of
overgrowth and abnormal-metastasis of cancer cells leading to
over-activity of surface proteins such as the growth factor
receptors (7,8). Over-activation of epidermal growth
factor receptor (EGFR) leads to not only abnormal proliferation by
inducing Erk and Akt activation, which is a common cancer factor
but increases abnormal metastasis of cancer cells through the
JAK-STAT signaling pathways (9–11). The
active EGFR directly translocates into the cancer cell nucleus and
induces expression of factors involved in cancer cells metastasis.
Recent studies have shown that EGFR-activated Stat3 induces
expression of the SNAIL family and induces abnormal metastasis of
cancer cells (12,13). Moreover, the EGFR co-binding with
Stat3 and EGFR dimers translocate into the cancer cell nucleus and
it leads to expression of factors related to cancer cell
proliferation and invasion (14–16).
Therefore, to inhibit abnormal growth and metastasis of cancer
cells, it is very important to find a target substance capable of
inhibiting the EGFR activation. Recently, we discovered a novel
substance that can inhibit the abnormal metastasis of MCF-7 breast
cancer cells by targeting EGFR (17).
We found that Torilis japonica extract (TJE)
major fraction substance not only inhibits the activity of EGFR but
regulate the expression of factors involved in cancer cell abnormal
metastasis. However, we found that the substance targeted EGFR, but
could not confirm that exact mechanism. In this study, we
investigated the mechanism by which TJE targets EGFR and inhibit
the abnormal metastasis in A549 lung cancer cells. In addition to
the inhibition of surface of EGFR, we also examined the mechanism
of expression suppression of abnormal metastasis-related factors
through translocation to the cancer cell nucleus.
Materials and methods
Plant material and preparation of
TJE
Dried whole fruit of TJE was purchased from Na-num
Pharmacy (Kyung-buk, Korea). Plant material (200 g) was extracted
two times with 95% ethanol (800 ml) at room temperature for 3 days
and it was subsequently filtered (raw compound). For the active
compound fraction, raw compound was mixed with compound:Methanol
(9:1), and 200 µl aliquots were injected into a series 1100 HPLC
system (Agilent Technologies, Inc., Santa Clara, CA, USA). A
symmetry C-18 column (4.6×2.5 cm; Waters, Milford, MA, USA) was
used. The mobile phase consisted of acetonitrile:water solution
(3:1, v/v) pumped at a rate of 1 ml/min. Major peaks was recorded
and same fractions were combined. The combined filtrate was
concentrated under vacuum at 60°C, and completely dried by freeze
drying. TJE powder was dissolved in DMSO and filtrated by 0.2-µm
pore size filter for in vitro and ex vivo
studies.
Reagent
Phalloidin, EGF were from Sigma-Aldrich (St. Louis,
MO, USA). Specific antibodies that recognized p-EGFR, p-Stat3,
Stat3, p-JAK2, JAK2, p-Akt, p-Erk, Akt, Erk, β-actin were obtained
from Cell Signaling Technology (Beverly, MA, USA) and EGFR,
E-cadherin, N-cadherin from Santa Cruz Biotechnology (Dallas, TX,
USA).
Cell culture
A549 and fibroblast cells were obtained from the
American Type Culture Collection (ATCC, Rockville, MD, USA). Cells
were grown in DMEM medium (HyClone, Waltham, MA, USA) containing
10% fetal bovine serum (HyClone) and 1% antibiotics (100 mg/l
streptomycin, 100 U/ml penicillin) at 37°C in a 5% CO2
atmosphere. Cells were suspended by Trypsin-EDTA (HyClone) and
separated 1.5×105/ml at each plate, every 48 h.
Invasion assay
Quantitative cell invasion assays were performed
using a modified Boyden chamber (Costar, Corning Inc., Corning, NY,
USA) with 8.0 µm pore polycarbonate membrane inserts with
Matrigel-coated 24-well plates as described previously. The lower
chamber was filled with the complete medium for control and
complete medium with TJE at the indicated dose and EGF (see
Figures). The A549 cells (5×104 cells/ml) in serum-free
medium were added into the upper chamber. The cells were allowed to
invade for 24 h at 37°C. The non-invasive cells were removed from
the upper surface of the membrane by scraping with a cotton swab,
and the invasive cells were stained with crystal violet and
photographed under a bright field microscope (Carl Zeiss, Thomwood,
NY, USA).
Immunofluorescence staining with wound
healing assay
Cells were seeded 2.5×106/ml in 12-well
plate with the cover glass, and incubated to 100% confluence. After
the incubation, the wound in the cell monolayer in the center of
the well was treated with TJE. After treatment, at the indicated
time and dose at 37°C in a 5% CO2 atmosphere, the cells
were fixed with 3.7% formaldehyde for 20 min and permeabilized with
0.2% Triton X-100 for 20 min for stress fiber staining. For
staining of specific proteins, cells were fixed and permeabilized
with 95% methyl alcohol for 15 min. Cells were washed with PBS
twice and reacted with specific antibody overnight at 4°C. Cells
were washed with PBS twice and reacted to secondary antibody and
stained with 0.1% Phalloidin-FITC for 40 min. Fluorescence was
detected by confocal microscopy (Carl Zeiss).
3D cell culture (organotypic cell
culture) for invasive cell detection
Human fibroblast cells were seeded on 0.3-µm pore
size cell culture insert plate and incubation with Matrigel and
type I collagen mixture. After cell mixture was detached from the
insert plate, 2×105/ml A549 cancer cells were placed in
the mixture and incubated for 1 week in the complete medium. After
incubation, the 3D cell formation medium was placed in the bottom
well and cultured for 3 weeks while changing the medium every 2
days. The appropriate amount of TJE and EGF was added to the new
medium, and place in the bottom well, and incubate for 1 week.
Metastatic cancer cells were identified using hematoxylin and eosin
(H&E) staining.
Egg preparation and cancer cell
inoculation
Fertilized chicken eggs were purchased from a local
hatchery and incubated for 8 days after breeding at 37°C with 45%
humidity. Eggs were cleaned with pre-warmed 70% ethanol and a small
hole was drilled into the eggshell where the air sac is located. A
2-cm window was carefully opened for inoculation. The hole was then
vacuumed to exclude air, thus creating space for the CAM. A total
of 1×106 cells re-suspended in 20 µl serum and
antibiotics-free DMEM medium with Matrigel was added on the CAM.
The window was then covered with medical film and the egg was
placed back into the incubator. After five-day inoculation, silicon
ring was implanted on micro-tumor tissue and TJE treated with or
without EGF. Seven more days after incubation, micro-tumors were
removed from CAMs, for metastasis study, specific tissue of
developing chickens was harvested and stored at −80°C before DNA
extraction.
Subcellular protein fraction
A subcellular protein fraction kit was used (Thermo
Fisher Scientific, Waltham, MA, USA). Cells were seeded at
1×106/ml in 100-mm dish and incubated for 24 h. After
the incubation, treated with the test compound for the indicated
times (see Figures) at 37°C in a 5% CO2 atmosphere.
After the incubation, subcellular proteins were separated according
to the manufacturer's instructions. A separate protein was analyzed
by western blotting.
Immunoprecipitation
We used sure-bead protein G magnetic beads kit
(Bio-Rad, Hercules, CA, USA). Cells were seeded at
1×106/ml in 100-mm plate and incubated for 24 h. After
incubation, cells were treated with the test compound for 24 h at
37°C in a 5% CO2 atmosphere. Whole lysate and nucleus
fraction proteins were incubated with specific antibody bound
magnetic beads. Beads were washed using a magnetizer and PBS.
Target proteins were eluted in 1X non-reducing sample buffer and
analyzed by western blotting.
Protein dimerization
We used a BS3 (Thermo Fisher Scientific) protein
crosslinker. Cells were seeded at 1×106/ml in 100-mm
dish and incubated for 24 h. After the incubation, treated with the
test compound for the indicated times at 37°C in a 5%
CO2 atmosphere. After the incubation, proteins were
extracted with non-denaturing lysis buffer, and reacted with BS3
according to manufacturer's instructions. Protein dimerization was
analyzed by western blotting.
Transient transfection with small
interfering RNA
Small interfering RNA (siRNA) was purchased by
Dharmacon (Chicago, IL, USA), and a Nucleofector (Lonza, Basel,
Switzerland) for transfection. For transient transfection,
2×106/ml cells were re-suspended in a transfection
reagent with targeting siRNA. The siRNA was inserted by electric
shock according to the manual provided by the manufacturer. After
incubation for 24 h, cells were treated with the compound.
Western blotting
Cells were seeded at 1×105/ml in 6-well
plate and incubated for 24 h, and after the incubation, treated
with the test compound for indicating times at 37°C in a 5%
CO2 atmosphere. Cells were rinsed twice with ice-cold
PBS and scraped with lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM
NaCl, 1% NP40, 0.5% sodium deoxycholate, 1 mM PMSF) and subjected
to the western blot analysis. The 1st antibody reaction was
overnight at 4°C and 2nd antibody for 75 min at room temperature
with slow agitation.
Quantitative polymerase chain reaction
(PCR)
Total RNA was extracted using RiboEx (GeneAll
Biotechnology, Seoul, Korea) according to the manufacturer's
instructions, and cDNA was generated using ReverseAids cDNA
synthesis kit (Thermo Fisher Scientific) according to the
manufacturer's instructions. RT-PCR was performed with the
following temperature profile: a pre-denaturation step of 10 min at
95°C, followed by 35 cycles of 95°C for 30 sec, annealing
temperature for 30 sec and 72°C for 30 sec and a final exposure at
72°C for 10 min. qPCR was performed using qPCR greenstar master-mix
(Bioneer, Seoul, Korea) and StepOne™ (Applied Biosystems, Foster
city, CA, USA) for amplification and detection. A pre-denaturation
step of 10 min at 95°C, followed by 42 cycles of 95°C for 20 sec,
annealing and extension for 60 sec. Specific primer sequence for
amplification was: forward, TAGATGCCCCCAAATCTCAG; reverse, GAGCT
GCTCCATCTGTAGGG.
Statistical analysis
Invasive cells, wound healing area and gene
expression were statistically analyzed using ANOVA test (Prism
version 7; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
TJE suppresses cell migration and
invasion in EGF-stimulated A549 cells
To confirm the abnormal metastasis inhibition effect
of TJE, the number of invasion cancer cells was counted after TJE
treatment in EGF-stimulated A549 lung cancer cells. The
concentration was increased, the number of metastatic cancer cells
was reduced (Fig. 1A and B).
Moreover, it was confirmed through 3D cell culture method that the
extent of cancer cells that were transferred to the normal cell
layer was reduced by the TJE treatment (Fig. 1E). Immunofluorescence analysis of
E-cadherin, N-cadherin and F-actin with or without TJE treatment in
EGF-stimulation. Despite the EGF-stimulation, TJE not only
upregulated expression of E-cadherin but reduced N-cadherin
expression. Moreover, EGF-induced cell migration activities, but
there was a decrease in the migration area in TJE co-treated group
(Fig. 1C and D).
TJE regulates expression of EMT marker
and activation of EGFR downstream signaling pathways
To confirm the EGFR signaling regulation by TJE, we
analyzed the activity of EGFR and its downstream signaling proteins
such as Akt, Erk, Stat3 and JAK2 in EGF was treated with time after
TJE treatment. Fig. 2A shows that
the activity of EGFR and its downstream proteins were decreased by
TJE treatment. Moreover, the expression of specific factors related
to cancer cell metastasis such as SNAIL family, N-cadherin and
vimentin was reduced while the expression of E-cadherin was found
to be normal in TJE treatment group (Fig. 2B). In the case of EGFR and Stat3,
which form a dimer in EGF-stimulation, dimer formation was
inhibited in TJE treatment group (Fig.
2C). We confirm that TJE inhibited the translocation to the
nucleus of EGFR and Stat3, and the co-binding of EGFR with Stat3
was also decreased (Fig. 2D and
E).
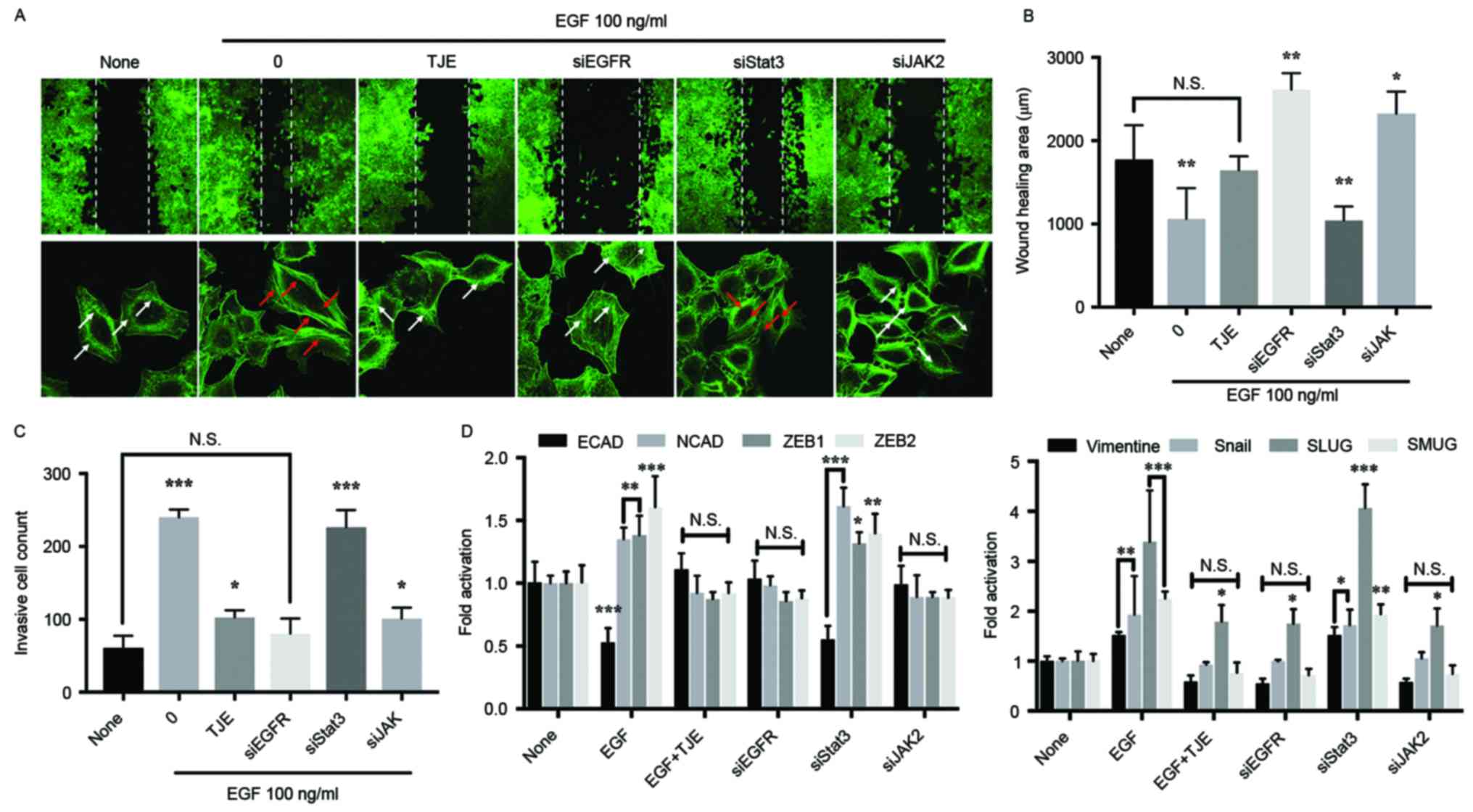
TJE suppresses abnormal metastasis
through the EGFR target pathways
As a result of comparing the inhibitory effect of
the TJE against cancer cell abnormal metastasis in the state where
a specific protein is knocked down using a siRNA transfection, the
inhibition of invasive cells, metastasis-related gene expression
and the change of wound healing area in TJE treatment group were
not different compared with EGFR knockdown group. There was no
difference in the JAK2 knockdown group. However, in the Stat3
knockdown group, the change was similar to that of the EGF only
treatment group (Fig. 3B-D). In
addition, the formation of stress fibers, which indicate the
movement of cancer cells, was not significantly different from that
of the EGFR knockdown group in the TJE treated group (Fig. 3A).
TJE inhibits cancer abnormal
metastasis in CAM
To confirm the suppressed abnormal metastasis
activity by TJE in an ex vivo model, we made a cancer cell
implantation model in CAM. As shown in Fig. 4, EGF treatment group formed blood
vessels in the cellular matrix with cancer cells. However, TJE
co-treatment group did not form blood vessel or contract the
cellular matrix. From quantification PCR using Chick's organ
tissue, the EGF treatment group cancer cells were detected with
human-specific genes especially the brain and heart tissue. Despite
the EGF stimulation in cellular matrix, TJE co-treatment group had
reduced detection of human-specific genes and it is not detected
similarly to that in normal Chick's organ tissue.
Discussion
The number of patients who die from lung cancer is
steadily increasing worldwide. Previous studies have shown that
smoking is the leading cause of lung cancer, and smoking cessation
has become active worldwide (1–3).
However, recent research found that lung cancer incidence can not
be suppressed simply by quitting, and they found that another
reason for lung cancer is outdoor pollution. It was found that many
factors in the air induce over-activation of surface proteins such
as growth factor receptors, and induce cancerization (4–6). In
particularly, the over-activation of EGFR has been shown to induce
abnormal proliferation and metastasis to normal organ resulting in
the death of the patient. Thus, finding a substance that can
inhibit the EGFR activation has become a very important research
topic. In this study, we investigated the mechanism of inhibiting
the cancer cell abnormal metastasis by EGFR inactivation through
the TJE treatment. First of all, we examined the inhibitory effect
of the TJE in the abnormal metastasis of cancer cells. We confirmed
that the number of the metastatic cells was reduced
concentration-dependently. In addition, the range of the wound
healing area and metastasis-related protein expression were not
different in the TJE treatment group when compared with the normal
condition (Fig. 1). The activity of
EGFR and its downstream proteins was also reduced when compared
with the EGF-stimulated group (Fig.
2A).
Recent studies have shown that dimer formation of
EGFR and Stat3 play a crucial role in nucleus translocation
(18–20). We showed that the formation of dimer
by EGF-stimulation was inhibited by the TJE treatment. We confirmed
that the intranuclear translocation of EGFR and the co-binding with
Stat3 were inhibited by TJE treatment in EGF-stimulated A549 lung
cancer cells (Fig. 2C and D). We
examined the expression of cancer cell abnormal metastasis and
metastasis-related factor compared with EGFR, Stat3 and JAK2
knockdown group using a siRNA transfection. The cancer cell
metastasis and expression of related factors was decreased in EGFR
knockdown group and TJE treated group when compared with
EGF-stimulated group (Fig. 3).
Moreover, we confirm that the formation of stress fiber, which is a
cell metastasis marker, decreased. However, in the knockdown group
of Stat3, the inhibition of cancer metastasis and the expression of
related factors did not appear. This indicates that EGFR can induce
cancer metastasis without going through Stat3. Previous studies
have found that the activity of EGFR can induce cancer cell
metastasis and proliferation through its own dimer formation
without co-binding with Stat3 (18,21,22).
Base on the above results, it was confirmed that the TJE that we
secured not only regulates the EGFR signaling pathway but inhibits
the cancer cell metastasis due to the EGFR-exclusive activity. In
addition, the inhibitory effect of the TJE on cancer cell
metastasis was confirmed by CAM assay. As a result, it was
confirmed that human cancer cells were invasive to CAM organs in
the EGF-stimulated group, whereas it did not appear in the TJE
treated group even by EGF-stimulation (Fig. 4).
In conclusion, we suggest that the inhibitory effect
of the TJE major fraction substance in the cancer abnormal
metastasis is indicated by regulation of EGFR signaling pathway and
suppression of its own activity through the targeting of EGFR.
Therefore, we demonstrated that TJE has potential as an anticancer
metastasis agent and may provide a substitute for chemotherapeutic
drugs.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mathers CD, Fat DM, Inoue M, Rao C and
Lopez AD: Counting the dead and what they died from: An assessment
of the global status of cause of death data. Bull World Health
Organ. 83:171–177. 2005.PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global Cancer Statistics. CA Cancer
J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamra GB, Guha N, Cohen A, Laden F,
Raaschou-Nielsen O, Samet JM, Vineis P, Forastiere F, Saldiva P,
Yorifuji T, et al: Outdoor particulate matter exposure and lung
cancer: A systematic review and meta-analysis. Environ Health
Perspect. 122:906–911. 2014.PubMed/NCBI
|
|
5
|
Straif K, Cohen A and Samet J: Air
Pollution and Cancer. IARC Scientific Publication No. 161 Lyon:
IARC Press; https://www.iarc.fr/en/publications/books/sp161/AirPollutionandCancer161.pdf
|
|
6
|
International Agency for Research on
Cancer: Personal Habits and Indoor Combustions. IARC Monographs on
the Evaluation of Carcinogenic Risks to Humans. 100E. Lyon: IARC
Press; http://monographs.iarc.fr/ENG/Monographs/vol100E/mono100E.pdf
|
|
7
|
Líbalová H, Krčková S, Uhlířová K, Kléma
J, Ciganek M, Rössner P Jr, Šrám RJ, Vondráček J, Machala M and
Topinka J: Analysis of gene expression changes in A549 cells
induced by organic compounds from respirable air particles. Mutat
Res. 770:94–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krishnan VG, Ebert PJ, Ting JC, Lim E,
Wong SS, Teo AS, Yue YG, Chua HH, Ma X, Loh GS, et al: Whole-genome
sequencing of Asian lung cancers: Second-hand smoke unlikely to be
responsible for higher incidence of lung cancer among Asian
never-smokers. Cancer Res. 74:6071–6081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alper O, Bergmann-Leitner ES, Bennett TA,
Hacker NF, Stromberg K and Stetler-Stevenson WG: Epidermal growth
factor receptor signaling and the invasive phenotype of ovarian
carcinoma cells. J Natl Cancer Inst. 93:1375–1384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmed N, Maines-Bandiera S, Quinn MA,
Unger WG, Dedhar S and Auersperg N: Molecular pathways regulating
EGF-induced epithelio-mesenchymal transition in human ovarian
surface epithelium. Am J Physiol Cell Physiol. 290:C1532–C1542.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siegelin MD and Borczuk AC: Epidermal
growth factor receptor mutations in lung adenocarcinoma. Lab
Invest. 94:129–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeom SY, Nam DH and Park C: RRAD promotes
EGFR-mediated STAT3 activation and induces temozolomide resistance
of malignant glioblastoma. Mol Cancer Ther. 13:3049–3061. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hipp S, Walch A, Schuster T, Losko S, Laux
H, Bolton T, Höfler H and Becker KF: Activation of epidermal growth
factor receptor results in snail protein but not mRNA
overexpression in endometrial cancer. J Cell Mol Med. 13:3858–3867.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anupama EG, Oliver B and Khatri L: Nuclear
signaling of EGFR and EGFRvIII in glioblastoma. Molecular Targets
of CNS tumors. Garami M: Croatia: InTech, Rijeka; 2011, https://www.scribd.com/document/112167777/Molecular-Targets-of-CNS-Tumors
|
|
15
|
Gong C, Zhang Y, Shankaran H and Resat H:
Integrated analysis reveals that STAT3 is central to the crosstalk
between HER/ErbB receptor signaling pathways in human mammary
epithelial cells. Mol Biosyst. 11:146–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia
W, Wei Y, Bartholomeusz G, Shih JY and Hung MC: Nuclear interaction
of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer
Cell. 7:575–589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim GT, Lee SH and Kim YM: Torilis
japonica extract, a new potential EMT suppressor agent by
regulation of EGFR signaling pathways. Int J Oncol. 45:1673–1679.
2014.PubMed/NCBI
|
|
18
|
Brand TM, Iida M, Luthar N, Starr MM,
Huppert EJ and Wheeler DL: Nuclear EGFR as a molecular target in
cancer. Radiother Oncol. 108:370–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, McBride KM and Reich NC: STAT3
nuclear import is independent of tyrosine phosphorylation and
mediated by importin-alpha3. Proc Natl Acad Sci USA. 102:8150–8155.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michael V, Tamas D, Dzina K, Swen L, Anne
S, Valeria P, Walter R and Gerhard MN: The role of the N-terminal
domain in dimerization and nucleocytoplasmic shutting of latent
STAT3. J Cell Sci. 124:900–909. 2010.
|
|
21
|
Wang SC and Hung MC: Nuclear translocation
of the epidermal growth factor receptor family membrane tyrosine
kinase receptors. Clin Cancer Res. 15:6484–6489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lo HW, Hsu SC and Hung MC: EGFR signaling
pathway in breast cancers: From traditional signal transduction to
direct nuclear translocalization. Breast Cancer Res Treat.
95:211–218. 2006. View Article : Google Scholar : PubMed/NCBI
|