Introduction
Cancer is the second leading cause of death
worldwide. It is estimated that 19.1 million new cancer cases are
reported annually and 13 million cancer-related deaths will occur
in 2030 (1). Due to the frequent
cancer recurrence and metastasis, there is an urgent requirement to
explore novel therapeutic agents and molecular mechanisms for
precision-targeted cancer therapy (2). While cancer pathogenesis and the
mechanisms of progression remain to be fully elucidated, it is
known that the proliferation, invasion, migration and metastasis of
cancer cells are associated with apoptosis, autophagy,
epithelial-mesenchymal transition (EMT) and immune tolerance of
cancer cells. Therefore, induction of apoptosis, suppression of
invasion and metastasis and enhancing autophagy and the tumor
microenvironment (TME) have been proposed as cancer treatment
approaches (3,4).
Conventional cancer treatments include surgery,
chemotherapy and radiation therapy. While these treatment options
may kill or eradicate tumor cells, they damage surrounding normal
cells, resulting in adverse effects on patients. Therefore, there
is a requirement to develop effective anticancer treatments with
fewer adverse effects on patients. Another treatment option is
immunotherapy, which has advantages due to its use of specific
antibodies or chimeric antigen receptor-engineered T-cells
(CART-cell) to specifically target tumor antigens on the cancer
cell surface. However, its use is limited to a small number of
cancers, including blood cancers such as lymphoma (5).
Traditional Chinese Medicine (TCM) has long been
used as an anticancer agent to inhibit tumor growth, regulate the
TME and cancer immunity, and improve the efficacy of
chemotherapeutic drugs such as cisplatin to reduce tumor recurrence
and metastasis (6,7). TCM has the advantages of multiple
targets and minimal side effects compared to pharmaceutical
treatments. TCM decoctions, single TCM herbs and monomer
TCM-derived compounds have exhibited anticancer effects in
experimental animal tumor models and clinical trials (8,9).
Numerous TCMs have been found to increase tumor cell apoptosis and
autophagy, and inhibit their proliferation, angiogenesis, EMT,
invasion, migration and metastasis (10,11).
Certain TCM decoctions or their components have been indicated to
improve the efficacy of cancer chemotherapy, radiation therapy or
immunotherapy (12).
Astragalus membranaceus Bunge has been used
in TCM for >2,000 years and it is growing mainly in Shanxi,
Inner Mongolia, Gansu and other Chinese provinces of China, Korea
and Mongolia. The dried root of A. membranaceus Bunge is
known by its Chinese name Huangqi and is used in TCM herbal
decoctions for treating various diseases. There are two botanical
sources of A. membranaceus Bunge: A. membranaceus
Bunge and A. membranaceus Var. mongholicus Bunge.
Further details of these two botanical sources may be found in
Plants of World Online (13).
While the composition of A. membranaceus Bunge varies by
region and country, its major components include astragalus
polysaccharides, saponins and flavonoids. It also contains other
constituents, such as amino acids, trace elements (e.g. Fe, Zn, Se,
Mn, Co and Cu) and active small molecules such as riboflavin,
chlorogenic acid, folic acid and sterols (14). These components have different
biological activities, such as anti-inflammatory, antioxidant,
antitumor, antiviral and improvement of cardiovascular functions
(15,16).
Studies have indicated that saponins are the primary
constituents responsible for the antitumor activity of A.
membranaceus Bunge (17,18).
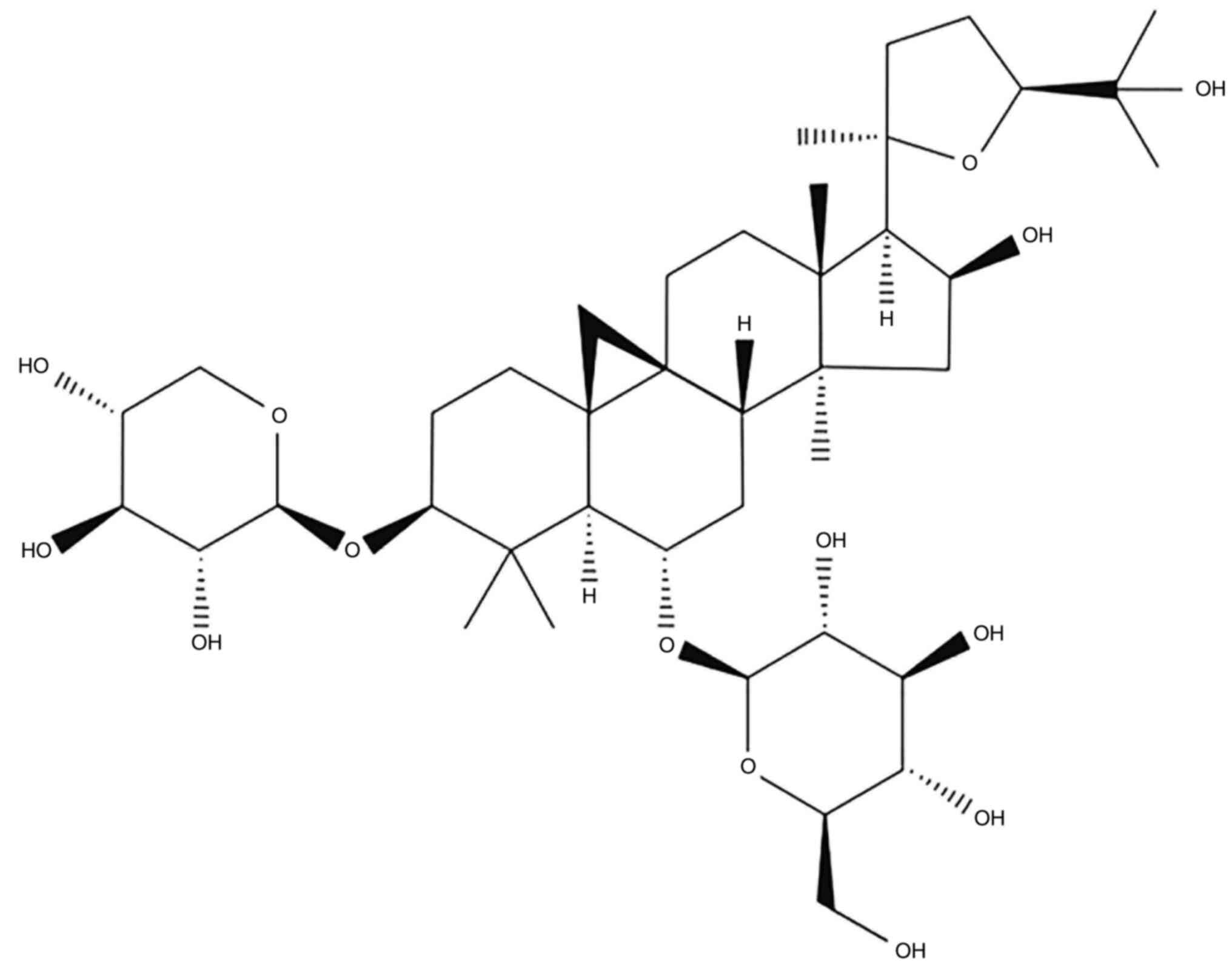
Astragaloside-IV (AS-IV;
3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl-cycloastragenol) is
one saponin extracted from A. membranaceus Bunge. As a major
active component of A. membranaceus Bunge, AS-IV has been
found to have multiple biological functions, including antitumor,
anti-oxidation, immune regulation and anti-inflammatory. The
chemical structure of AS-IV is presented in Fig. 1 (19–21).
The antitumor effects of A. membranaceus Bunge and its
component AS-IV have been assessed in different cancer types,
including lung, colon, liver, gastric and breast cancers, through
in vitro cultured cancer cell lines and in vivo
animal models with xenografted tumors (22,23).
Accumulating evidence suggests that the antitumor effects of AS-IV
are mainly conferred via mechanisms that increase apoptosis and
autophagy, inhibit cell proliferation, invasion, migration and
metastasis, and regulate the TME, including angiogenesis and innate
and acquired immunity (10,23).
The present review focuses on recent progress made regarding the
antitumor effects in various cancer types and the antitumor
mechanisms and related signaling pathways of AS-IV identified by
in vitro and in vivo studies, and proposes potential
applications of AS-IV in antitumor therapy.
Inhibitory effects of AS-IV on growth and
proliferation of cancer cells
Apoptosis induction and cell cycle
arrest in cancer cells
Cell proliferation and apoptosis are crucial
cellular processes for cancer pathogenesis. Apoptosis is a
continuous cascade to induce cell death that is usually regulated
by extrinsic and intrinsic signaling pathways and characterized by
morphological and molecular changes in the cancer cells (Fig. 2). In each cellular apoptotic
pathway, different caspases are ubiquitously activated to induce
apoptosis, of which caspase 3, 8 and 9 are the primary upregulated
caspases. In addition, certain anti-apoptotic proteins, such as
B-cell lymphoma 2 apoptosis regulator (BCL2) and B-cell
lymphoma-extra large (BCL-xl) are downregulated (24,25).
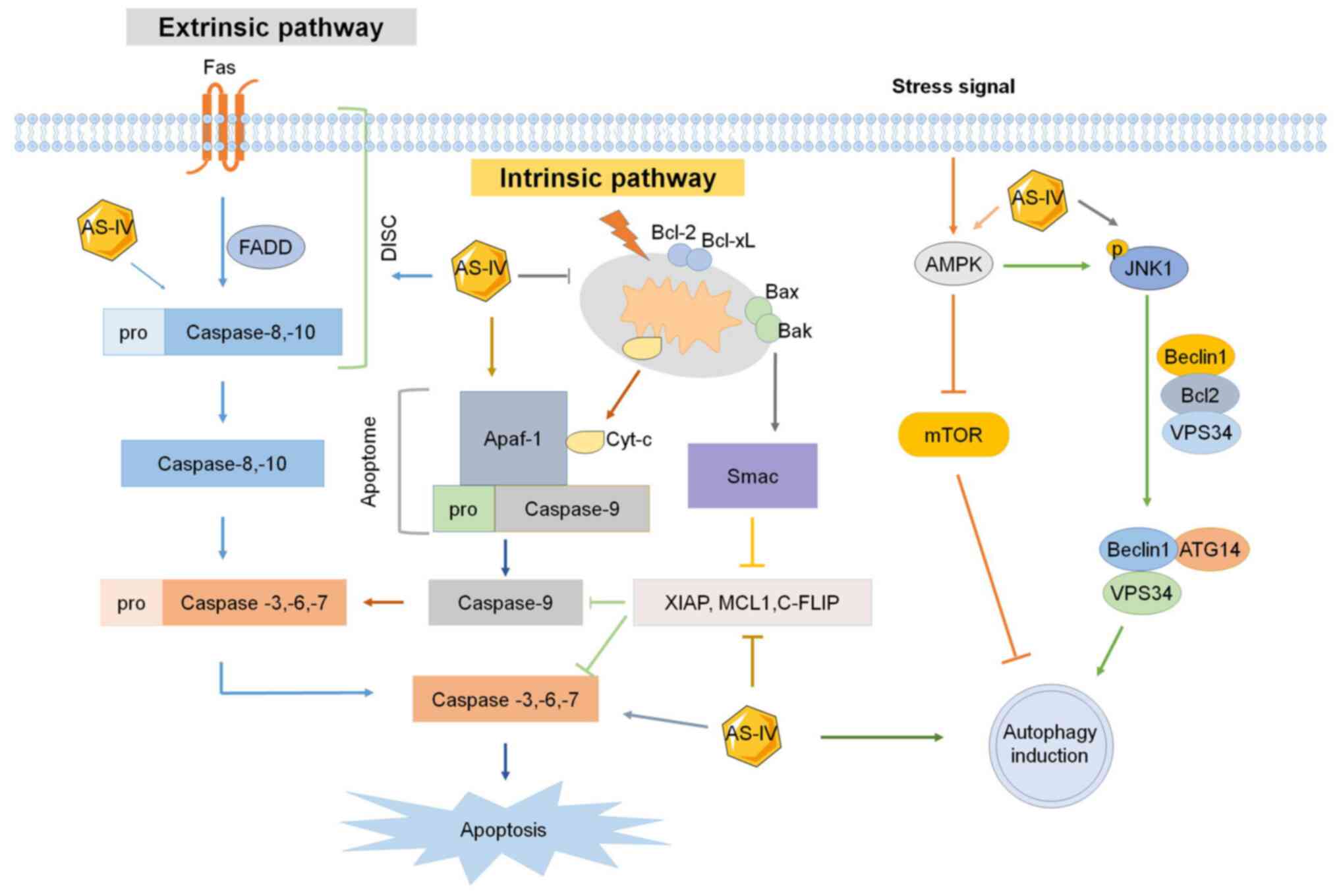 | Figure 2.Effect of AS-IV on apoptosis and
autophagy. Extrinsic and intrinsic signaling pathways of apoptosis
and autophagy are all affected by AS-IV. XIAP, X-linked mammalian
inhibitor of apoptosis; MCL1, myeloid cell leukemia 1; c-FLIP,
cellular FLICE-like inhibitory protein; SMAC, second
mitochondria-derived activator of caspases; AS-IV,
astragaloside-IV; Apaf-1, apoptotic peptidase activating factor 1;
JNK1, c-Jun N-terminal kinase 1; FADD, Fas-associated protein with
death domain; DISC, death-inducing signaling complex; BCL2, B-cell
lymphoma 2 apoptosis regulator; BCL-xl, B-cell lymphoma-extra
large; BAX, BCL2-associated X apoptosis regulator; Bak, BCL2
antagonist/killer 1; AMPK, adenosine monophosphate-activated
protein kinase; VPS34, vacuolar protein sorting 34; ATG14,
autophagy related 14. |
The extrinsic apoptotic pathway is activated via
dimerization of cell surface receptors such as Fas (CD95/Apo1),
tumor necrosis factor receptors (TNFRs) and TNF-related
apoptosis-inducing ligand receptors, which are bound by specific
ligands. When cells are stimulated by death signals, the
cytoplasmic domains of death receptors recruit death adaptor
proteins, such as Fas-associated protein with death domain (FADD)
and TNFR1-associated death domain, which then activate pro-caspase
8 and pro-caspase 10 and form death-inducing signaling complex
(DISC) to initiate apoptosis. The activated pro-caspase 8 and
pro-caspase 10 in the DISC are then directly cleaved to induce
cleavage of pro-caspase 3, pro-caspase 6 and pro-caspase 7 to form
caspase 3, caspase 6 and caspase 7, respectively, leading to cell
apoptosis.
The intrinsic apoptotic pathway is initiated by
inducing mitochondria to release cytochrome c into the cytoplasm.
The apoptotic peptidase activating factor 1 and pro-caspase 9 are
recruited to the cytoplasm to form the apoptosome with cytochrome
c, which then activates apoptosis effector proteins caspase 3, 6, 7
and 9, resulting in apoptosis (Fig.
2). The intrinsic apoptotic pathway is usually regulated by the
mitochondrial apoptotic proteins, including pro-apoptotic proteins
such as BCL2-associated X apoptosis regulator (BAX), BCL2
antagonist/killer 1, BID, BCL2-interacting killer, BCL2-like 11
(BCL2L11; also known as BIM) and
phorbol-12-myristate-13-acetate-induced protein 1 (also known as
NOXA), as well as anti-apoptotic proteins such as BCL2, BCL-xL,
BCL2L2 (also known as BCL-W), BCL2-related protein A1 (also known
as BFL1), Diablo IAP-binding mitochondrial protein (also known as
SMAC), X-linked inhibitor of apoptosis (XIAP), MCL1 apoptosis
regulator BCL2 family member (MCL1) and caspase 8 and FADD-like
apoptosis regulator (also known as CFLIP) (26,27).
Therefore, inducing apoptosis in cancer cells via drugs targeting
each apoptotic pathway is a promising approach in anticancer
therapy.
AS-IV was indicated to suppress cell proliferation
in three non-small cell lung cancer (NSCLC) cell lines, A549,
NCIH1299 and HCC827. The expression of anti-apoptotic BCL2 was
decreased in AS-IV-treated cells, while the expression of BAX and
caspase 3 was increased, indicating that AS-IV induced apoptosis of
NSCLC and osteosarcoma cells via the intrinsic pathway (28,29).
In addition, when colorectal cancer (CRC) SW620 and HCT116 cell
lines were treated with AS-IV, their growth was significantly
inhibited. Flow cytometry indicated that these cells mainly
exhibited G0/G1 phase arrest after being treated with AS-IV. The
mRNA and protein levels of cyclin D1 and cyclin-dependent kinase 4
(CDK4) were markedly decreased during G0/G1 arrest (30). Another study determined that AS-IV
caused cell cycle arrest in G0 phase with increased expression of
the tumor suppressor CDK inhibitor 1A (also known as P21). An in
vivo study suggested that AS-IV inhibited CRC cell
proliferation and tumor growth in a xenograft CRC mouse model.
Cytochrome c and HtrA serine peptidase 2 (also known as OMI) were
released from mitochondria into the cytoplasm, promoted by AS-IV,
suggesting that AS-IV has antitumor effects via the intrinsic
apoptosis pathway. Further analysis indicated that the BAX/BCL2
ratio and expression levels of caspase 3 and caspase 9 were
upregulated by AS-IV (Fig. 2)
(23).
A recent study found the viability and proliferation
of SK-Hep1 and Hep3B cells of hepatocellular carcinoma (HCC) cells
to be significantly reduced after treatment with different doses of
AS-IV. Western blots indicated that AS-IV activated the extrinsic
and intrinsic apoptotic pathway proteins caspase 3, 8 and 9. In
addition, the levels of the anti-apoptotic proteins XIAP, MCL1,
CFLIP and baculoviral IAP repeat containing 5 (BIRC5/Survivin) were
all reduced by AS-IV in both SK-Hep1 and Hep3B cells. This study
suggested that AS-IV suppresses HCC cell proliferation and growth
via both the extrinsic and intrinsic apoptotic pathways (Fig. 2) (31).
Suppressing tumor cell growth by
activation of autophagy
Autophagy is the self-phagocytosis of cytoplasmic
organelles to degrade misfolded proteins, damaged organelles or
cancer cells. Autophagy frequently occurs through the fusion of the
endoplasmic reticulum (ER) and mitochondria, and its activation is
mainly through the adenosine monophosphate (AMP)-activated protein
kinase (AMPK)-mammalian target of rapamycin (mTOR) pathway and
mitogen/activated protein kinase 8 (MAPK8; also known as
JNK1)-phosphatidylinositol 3-kinase (PI3K) catalytic subunit type 3
(PIK3C3; also known as VPS34) pathways (32,33).
Several molecular complexes are involved in autophagic processes,
including the JNK1, autophagy-related 13 like (ATG13L), ATG101,
VPS34 complex-PI3K regulatory subunit 4 (PIK3R4; also known as
VPS15), PIK3C3/VPS34, Beclin 1 (BECN1), ATG14 and
ubiquitin-conjugation proteins-ATG5, ATG12 and ATG16L1 (Fig. 2) (34). Certain autophagy-related proteins
such as sequestosome 1 (also known as P62), microtubule-associated
protein 1 light chain 3 (LC3) unlipidated (LC3-I) and lipidated
(LC3-II) forms, and BECN1 are usually affected in cancer cells. P62
is responsible for recognizing ubiquitin-labeled substrates
targeted for autophagy. LC3 is a major effector of autophagy and
its conversion from LC3-I to LC3-II may activate autophagy
(35).
Bevacizumab inhibits tumor growth of multiple cancer
types, including lung adenocarcinoma, but its therapeutic efficacy
is markedly decreased by drug resistance. A recent study suggested
that AS-IV was able to improve Bevacizumab's antitumor efficacy in
treating lung adenocarcinoma. They observed that the levels of the
autophagy-related proteins P62, LC3I and LC3II in cells treated
with AS-IV + Bevacizumab were significantly higher than those in
cells only treated with Bevacizumab, indicating that AS-IV reduced
drug resistance and improved the antitumor efficacy of Bevacizumab
(Fig. 2) (36). Another study combined AS-IV with
α-solanine, neferine and 2,3,5,6-tetramethylpyrazine (named as
SANT) to significantly inhibit cancer cell proliferation and
migration and enhance autophagy flux in the cultured breast cancer
cell line MDA-MB-231. SANT increased the levels of the autophagy
proteins ATG16L1 and ATG4D and ATG9B to promote autophagic activity
(37).
Cisplatin is a classical antitumor drug widely used
to clinically treat cancer patients. It was found that cisplatin
leads to upregulation of autophagy-related proteins, such as BECN1
and LC3-II/I, and the ER stress-related proteins heat shock protein
family A member 5 (also known as GRP78) and eukaryotic translation
initiation factor 2α kinase 3 (also known as PERK), indicating that
cisplatin increases autophagy and ER stress in NSCLC cells. AS-IV
was reported to significantly increase chemosensitivity to
cisplatin in the NSCLC cell lines A549, HCC827 and NCI-H1299.
Combined treatment with cisplatin and AS-IV increased cell
apoptosis and suppressed cell proliferation in A549 and H1299
cells, suggesting that AS-IV acts synergistically with cisplatin to
produce stronger antitumor effects in NSCLCs. AS-IV may also
enhance chemosensitivity to cisplatin in HCC. After co-treatment of
HepG2 cells with cisplatin and AS-IV, the antitumor effects of
cisplatin on cell proliferation and tumor growth were significantly
increased in HepG2 cells and H22 tumor-bearing mice, respectively
(38–40).
Inhibiting cancer cell invasion,
migration and metastasis
Cancer cells not only proliferate rapidly, but also
invade surrounding cells or tissues in the TME or migrate to near
and distant organs via direct invasion or the circulatory system.
In a previous study, flow cytometry was used to analyze cell growth
and migration of NSCLC A459 cells treated with AS-IV, indicating
that, in addition to inhibiting cell proliferation and promoting
apoptosis, AS-IV reduced cell migration (28). The inhibitory effect of AS-IV on
cancer cell invasion was also observed in SiHa cervical cancer
cells using the Transwell migration assay. Western blot analysis
suggested that the protein levels of transforming growth factor β1
(TGF-β1), N-cadherin (also known as CDH2) and vimentin (VIM) were
significantly decreased, but E-cadherin (also known as CDH1)
protein levels were increased in AS-IV-treated cells compared to
untreated cells. These results indicated that AS-IV suppressed SiHa
cell invasion and migration by downregulating TGF-β1, CDH2 and VIM
expression and upregulating CDH1 expression. Tumor growth and
metastasis were also lower in AS-IV-treated cells than those in the
untreated control cells (22).
In addition, a transwell invasion assay suggested
that numbers of invading SK-Hep1 and Hep3B cells were significantly
reduced by AS-IV (31). Assessment
of the invasion of cervical cancer cells treated with AS-IV
indicated that it significantly reduced cancer cell invasion of
HeLa and SiHa cells. Furthermore, an in vivo study in which
mouse models with xenografted human cervical cancer were treated
with AS-IV suggested that after 35 days of treatment, tumor volumes
in the AS-IV-treatment group (25 mg/kg/day) were significantly
lower compared with those in the PBS-control group. These findings
indicated that AS-IV was able to inhibit invasion of cultured
cancer cells and in in vivo animals with cancer xenografts
(41).
Suppressing EMT in cancer
pathogenesis
EMT is one mechanism for transforming normal
epithelial cells into mesenchymal cells and then malignant cancer
cells. In tumorigenesis, EMT can be initiated by growth factors
such as TGF-β1 or epidermal growth factor receptor (EGFR) (42). Therefore, drugs inhibiting EMT have
therapeutic potential in cancer treatment. The glycogen synthase
kinase 3β (GSK-3β) inhibitor 1-Az was found to attenuate the
decrease in cell migration, invasion and EMT, indicating that
GSK-3β activity is required for promoting migration, invasion and
EMT in GBM cells via AKT/GSK-3β signaling (43–45).
AS-IV was found to inhibit the EMT process of gastric cells and
glioma U251 cells by inhibiting the TGF-β1-induced PI3K/AKT/nuclear
factor κB (NF-κB) pathway, indicating that AS-IV may have
therapeutic potential for treating gastric cancer (GC) (46,47).
AS-IV was also found to suppress phosphorylation of P38, MAPK,
PI3K, AKT and mTOR in cultured SiHa cervical cancer cells. Further
analysis indicated that AS-IV suppressed the invasion and
metastasis of cervical cancer by interfering with the EMT (Fig. 3) (22).
 | Figure 3.Molecular mechanisms and signaling
pathways of AS-IV's antitumor effects. AS-IV increases apoptosis
and autophagy and inhibits cell proliferation, invasion, migration,
metastasis and EMT via signaling pathways including
WNT/AKT/GSK-3β/CTNNB1, TGF-β/PI3K/AKT/mTOR, PI3K/MAPK/mTOR,
PI3K/AKT/NF-κB, RAC1/RAS/MAPK/ERK, TNF-α/PKC/ERK1-2/NF-κB,
IL-13/AMPK/TLR4 and Tregs/IL-11/STAT3. AS-IV, astragaloside-IV;
Tregs, regulatory T cells; CTLS, cytotoxic T lymphocytes; TLR4,
Toll-like receptor 4; EMT, epithelial-mesenchymal transition; GSK,
glycogen synthase kinase; PKC, protein kinase C; AMPK, adenosine
monophosphate-activated protein kinase; RAC1, Rac family small
GTPase 1. |
Inhibiting tumor growth by improving the
TME, tumor immunity and immunotherapy and decreasing tumor
angiogenesis
Improving the TME to suppress tumor
growth
The TME contributes to tumor immunity, angiogenesis,
tumor-associated macrophages (TAMs) with an M2 phenotype,
fibroblast growth and microRNAs (miRNAs) that surround tumor cells.
Macrophages are the most abundant immune cells in the TME and their
polarization has a key role in suppressing tumorigenesis. A recent
study indicated that AS-IV has inhibitory effects on interleukin
(IL)-4/IL-13-induced macrophage M2 polarization. AS-IV decreased M2
macrophages to inhibit cell proliferation, invasion and migration
in ovarian cancer cells. Further analysis suggested that AS-IV
attenuated the expression of high-mobility group box 1 (HMGB1) and
Toll-like receptor 4 (TLR4) in macrophages co-cultured with ovarian
cancer cells and decreased the expression of M2 markers of TGF-β,
matrix metallopeptidase 9 (MMP-9) and IL-10. Of note, exogenous
expression of HMGB1 reduced the inhibitory efficacy of AS-IV
against the macrophage M2 polarization-induced malignant potential
in ovarian cancer cells. Therefore, AS-IV may alleviate ovarian
cancer progression by regulating macrophage M2 polarization against
ovarian cancer cells within the TME (48). Tumor fibroblast cells typically
have roles in increasing tumor growth. In one study GC-associated
fibroblasts (GCAF) were isolated and treated with different AS-IV
concentrations. They indicated that AS-IV treatment markedly
inhibited GCAF-induced proliferation, migration and invasion of
cancer cells. Further analysis suggested that AS-IV treatment
significantly upregulated miR-214 expression and downregulated
miR-301a expression in GCAFs. These findings indicated that AS-IV
is able to inhibit the pathological functions of GCAFs, suggesting
that regulating the TME is a potential therapeutic approach for
cancer treatment (49).
Inducing the transformation of
M2-macrophages into M1-macrophages
Innate and adaptive immunities in the TME have a
modulatory role in recognizing and killing tumor cells. Therefore,
immune escape of cancer cells may promote tumor progression,
invasion and metastasis. One of the key innate immune cells is the
macrophage, which may promote or inhibit the development and
progression of tumor cells. Macrophages are usually divided into M1
and M2 subgroups, which exert different functions. Activated M1
macrophages mainly fight invading pathogens and inhibit tumor
growth, while activated M2 macrophages promote tumor progression
(50).
Since the M2 macrophages are found in most tumors,
decreasing their numbers or transforming them into M1 macrophages
is an important immunotherapeutic strategy for cancer treatment.
Certain studies have used small molecules to inhibit different
receptors, tyrosine kinases or other important transduction
pathways in TAMs to induce the M2 to M1 transformation and suppress
tumor growth (51–53). Another study used IL-1 and IL-4 to
transform monocytes into M2 macrophages, collected the conditioned
medium from M2 macrophages (M2-CM) and applied it to A549 and H1299
lung cancer cell lines treated with AS-IV. They observed that AS-IV
significantly inhibited IL-13- and IL-4-induced production of M2
macrophages by reducing the expression of cluster of
differentiation 206 (CD206) in M2 macrophages. AS-IV was also
indicated to suppress the M2-CM-induced proliferation and invasion
of A549 and H1299 cells (54). One
study investigated the effect of AS-IV on CRC by subcutaneously
injecting the CRC cell line CT26 into the mice to create a
xenografted CRC mouse model. They found that after AS-IV was
administered to mice with tumors, the proliferation of CT26
cell-derived tumors in vivo was suppressed by reducing M2
macrophages and increasing M1 macrophages. AS-IV was observed to
induce apoptosis of CT26 cells in vitro and inhibit
transplanted tumor growth and cell proliferation by inducing M2
macrophage transformation into M1 macrophages. AS-IV also decreased
the production of anti-inflammatory factors such as TGF-β, IL-10
and vascular endothelial growth factor A (VEGF-A) and increased the
production of pro-inflammatory factors such as interferon (IFN)-γ,
IL-12 and TNF-α to exert its antitumor activity via related
signaling pathways (Fig. 3)
(55).
Activating cytotoxic T lymphocytes
(CTLs)
The adaptive antitumor immunity is mainly mediated
by CTLs and regulatory T cells (Tregs). Tregs are characterized by
the expression of surface markers CD4 and CD25 and forkhead box P3
and have been proposed as a key component of acquired tolerance to
tumors. CD8+ CTLs are activated by Tregs and
immune-suppressive cytokines to kill tumor cells (56). It has been indicated that AS-IV is
able to induce antitumor effects by regulating the activities of
immune cells. A tryptophan-catabolizing enzyme, indoleamine
2,3-dioxygenase (IDO), was found to induce immune tolerance and be
involved in tumor escape from host immune surveillance (57). Another study established a
xenograft lung cancer model by implanting IDO-overexpressing murine
lung carcinoma cells into C57BL/6 mice, which were treated with
AS-IV, and their Tregs and CTLs in splenetic mononuclear cells were
isolated for analysis. They found that AS-IV was able to
downregulate Tregs and upregulate CD8+ CD28+
CTLs in vivo and in vitro by blocking IDO-induced
immune escape and inhibiting tumor growth (58) (Fig.
3).
Improving immunotherapy by decreasing
expression of programmed death-ligand 1 (PD-L1) and
pro-inflammatory factors IFN-γ, IL-12 and TNF-α
AS-IV was found to improve immunotherapy and
suppress angiogenesis. A study investigated the anticancer effects
of AS-IV by assessing the proliferation, EMT and angiogenesis of
the GC cell lines SGC7901 and MGC803 treated with AS-IV,
transfected with an miR-195-5p inhibitor or a pcDNA3.1 vector
expressing PD-L1. They found that AS-IV inhibited EMT and
angiogenesis in GC cells and that PD-L1 was a potential target of
miR-195-5p. Downregulation of miR-195-5p or upregulation of PD-L1
eliminated the inhibitory effect of AS-IV on EMT and angiogenesis
on GC cells. Therefore, AS-IV inhibited EMT and angiogenesis in GC
cells via upregulation of miR-195-5p and reduction of PD-L1
expression, indicating the therapeutic potential of AS-IV on GC
cells via miR-195-5p-mediated regulation of PD-L1. AS-IV treatment
was also observed to increase the chemosensitivity of CRC cells to
cisplatin (12,59–61).
One study explored the therapeutic mechanisms of AS-IV in CRC by
injecting CT26 cancer cells into mice and assessing the effects of
AS-IV on immunological functions based on macrophage markers,
inflammatory factors and cytokines in tumors. They found that AS-IV
induced apoptosis and decreased proliferation of CT26 cells in
vitro and significantly decreased the transformation of M1
macrophages into M2 macrophages. In a mouse tumor model, AS-IV
suppressed tumor growth and decreased anti-inflammatory factors
such as TGF-β, IL-10 and VEGF-A, but promoted the infiltration of
pro-inflammatory factors such as IFN-γ, IL-12 and TNF-α into
transplanted tumor tissues. Of note, combined treatment of AS-IV
and PD-1 produced a synergistic antitumor effect by inhibiting
tumor growth and increasing T-cell infiltration. Therefore, in
combination with immune checkpoint inhibitors, AS-IV may achieve
improved antitumor effects in several cancer types (55).
Suppressing tumor angiogenesis to
prevent tumor growth
Tumor angiogenesis is a growth abnormality in
malignant tumors to maintain vascular supply and provide essential
nutrients and oxygen to proliferating cancer cells. Due to the
low-oxygen TME, angiogenesis is triggered by hypoxia to release
multiple growth factors via hypoxia-induced factors by cancer
cells, fibroblasts and macrophages, which are recruited to tumors
(62). Therefore, blocking
vascular supply or inhibiting the function of blood vessel
receptors with pharmacological drugs has been proposed to promote
tumor starvation and induce cell death in cancer treatment
(63). A study indicated that
AS-IV inhibited angiogenesis in mouse models with xenografted
tumors treated with AS-IV by reducing protein levels of
heparin-binding epidermal growth factor (EGF)-like growth factor,
thrombospondin 2, amphiregulin, leptin, cellular communication
network factor 3 (also known as insulin-like growth factor binding
protein 9), EGF, coagulation factor III and the pro and active
forms of MMP-9 in tumors, and increasing protein levels of serpin
family E member 1 (also known as PAI-1) and platelet factor 4
(37). Another study indicated
that AS-IV significantly reduced tumor weight and inhibited tumor
microvessel formation in a nude mouse model of human HCC. They
observed that the expression of angiogenesis-related factors such
as fibroblast growth factor 2, VEGF, hepatocyte growth factor,
transferrin and coagulation factor VII was decreased by AS-IV,
indicating that the antitumor effects of AS-IV are mediated via
decreased microvessel count and expression of angiogenic- and
thrombosis-related factors (19).
The anticancer effects of AS-IV in different cancer
cell lines in vitro and different cancer models in
vivo are summarized in Tables
I and II.
 | Table I.Antitumor effects of AS-IV and
related mechanisms based on in vitro studies. |
Table I.
Antitumor effects of AS-IV and
related mechanisms based on in vitro studies.
| Author, year | Cancer type | Cell line | Concentration and
duration | Anticancer
effects | Molecular
target/biomarker | (Refs.) |
|---|
| Zhang, 2019 | Cervical
cancer | SiHa | 0.78125-800 µg/ml
for 24 h | ↓Invasion,
metastasis; ↓migration | TGF-β1/PI3K and
TGF-β1/MAPK, p38 | (22) |
| Sun, 2019 | Colorectal
cancer | HT29 and SW480 | 0–40 µg/ml for 24
h | ↓Growth, cell cycle
arrest; ↑apoptosis | Bax/Bcl-2, Cyt C
and Om, PARP, cleaved caspase-3 and cleaved caspase-9 | (23) |
| Jia, 2019 | Lung cancer | HCC827, A549 and
NCI-H1299 | 0–24 ng/ml for 48
h | ↑Cell death;
↓migration | Bcl-2 and Bax,
Akt/GSK-3β/β-catenin | (28) |
| Wang, 2018 | Colorectal
cancer | SW620 and
HCT116 | 50 and 100 ng/ml
for 24,48 and 72 h | ↓Cell
proliferation, G0/G1 cell cycle arrest | Cyclin D1 and CDK4,
B7-H3 | (30) |
| Su, 2020 | Liver cancer | SK-Hep1 and
Hep3B | 0–400 µM for 48
h | ↓Proliferation; ↑G1
phase arrest; ↑apoptosis; ↓invasion | Caspase-8,9, XIAP,
MCL1, C-FLIP | (31) |
| Lai, 2020 | Lung cancer | A549, H1299 | 0–16 ng/ml for 48
h | ↑Endoplasmic
reticulum stress; ↑autophagy | GRP78 and
Beclin1 | (38) |
| Xia, 2020 | Cervical
cancer | HeLa and SiHa
cells | 25 µM for 12 h | ↓Invasion;
↑autophagy | DCP1A and TMSB4X,
MGST3, AKR1C2 and ERLIN1 | (41) |
| Zhu, 2018 | Gastric cancer | BGC-823 and
MKN-74 | 0–40 µg/ml for
24,48 h | ↓Viability, EMT,
invasion, migration | PI3K/Akt/NF-κB
pathway | (46) |
| Han, 2020 | Glioma | U251 | 20–80 µg/ml for 48
h | ↓EMT, migration,
invasion, proliferation | Wnt/β-catenin
pathway | (47) |
| Wang, 2021 | Ovarian cancer | SKOV3 | 0.5–100 µg/ml for
24 h | ↓Cell viability,
invasion, migration | HMGB1-TLR4
signaling | (48) |
| Wang, 2017 | Gastric cancer | BGC-823 | 10–40 µmol/l for 48
h | ↓Proliferation,
migration, invasion | SOX2 and NANOG | (49) |
| Xu, 2018 | Lung cancer | A549 and H1299 | 80 nM for 48 h | ↓Growth, invasion,
migration, angiogenesis; ↑M2-macrophages | AMPK | (54) |
| Liu, 2020 | Colorectal
cancer | CT26 | 10–100 nM for 48
h | ↓Proliferation;
↑apoptosis | Promoting M2
macrophage polarization to the M1 phenotype | (55) |
| Zhang, 2014 | Lung cancer | 3LL-luc-IDO | 160 µg/ml for 24,
48 and 72 h | ↑Immune response,
regulatory T cells; ↑CTLs | CD8+CD28+,
indoleamine 2,3-dioxygenase | (58) |
| Wang, 2017 | Hepatic cancer | Bel-7402/FU | 0–100 µM for 24
h | ↓Cell viability,
AP-1 DNA binding activity; reverses drug resistance | JNK/c-Jun/AP-1
signaling pathway | (60) |
| Xie, 2016 | Colorectal
cancer | HCT116, SW480 | 1–15 ng/ml | ↓Viability;
↑sensitivity to cisplatin | NOTCH3 | (61) |
| Qin, 2017 | Hepatocellular
carcinoma | MHCC97-H and
Huh7 | 0–100 µg/ml for 24,
48 and 72 h | ↓Migration,
invasion, EMT |
Akt/GSK-3β/β-catenin | (66) |
| Zhao, 2019 | Vulvar squamous
cell carcinoma | SW962 | 0–800 µg/ml for 24,
48 and 72 h | ↓Cell viability,
proliferation; cell-cycle arrest; ↑apoptosis | Bax, Bcl-xl, Bcl-2
and cleaved-caspase 3, Beclin-1, LC3-B and P62, TGF-β/Smad | (69) |
| He, 2021 | Prostate
cancer | LNCap and PC-3 | 0–20 µM for 72
h | ↑Carboplatin
sensitivity | AKT/NF-κB signaling
pathway | (71) |
| Li, 2017 | Glioma | U251 | 10–80 µg/ml for 24
h | ↓Proliferation,
migration, invasion | MAPK/ERK | (75) |
| Jiang, 2017 | Breast cancer | MDA-MB-231 | 0–40 µg/ml for 24
h | ↓Viability,
invasion | Vav3 mediated
Rac1/MAPK signaling | (76) |
| Cheng, 2014 | Lung cancer | A549 | 0–90 µM for 24
h | ↓Viability,
adhesion, migration, invasion | PKC-α-ERK1/2-NF-κB
pathway | (77) |
| Min, 2022 | Liver cancer | Huh-7 | 0–150 µM for 24 or
48 h | ↓Proliferation,
migration, invasion |
TLR4/NF-κB/STAT3 | (81) |
| Li, 2022 | Gastric cancer | HGC-27 and
MKN-45 | 0–40 µg/ml for 24
or 48 h | ↓Viability, cell
proliferation, metastasis |
circDLST/miR-489-3p/EIF4A1 | (83) |
| Cui, 2020 | Liver cancer | SMMC-7721 and
Huh7 | 0–200 µg/ml for 24
h | ↑Apoptosis |
miR-150-5p/β-catenin | (85) |
| Li, 2022 | Lung cancer | A549 | 0–100 ng/ml for 24
h | Increase
sensitivity of bevacizumab; ↓autophagy | Akt/mTOR | (36) |
 | Table II.Antitumor effects and related
mechanisms of AS-IV based on xenografted animal studies. |
Table II.
Antitumor effects and related
mechanisms of AS-IV based on xenografted animal studies.
| Author, year | Cancer type | Xenografted animal
model | Formulation | Dose and
duration | Anticancer
effect | Molecular
target/biomarker | (Refs.) |
|---|
| Zhang, 2017 | Liver cancer | HepG2 cell-bearing
mice | AS-IV, curcumin,
cisplatin and AS-IV+curcumin | 20 mg/kg/day for 21
days | ↓Tumor growth | miR-122 and
miR-221 | (19) |
| Zhang, 2019 | Cervical
cancer | SiHa cell-bearing
mice | AS-IV, cisplatin
and cisplatin+AS-IV | 120 mg/kg/day for
21 days | ↓Tumor growth,
↓invasion | TGF-β1-mediated
PI3K and MAPK pathways | (22) |
| Sun, 2019 | Colorectal
cancer | HT29 cell-bearing
mice | AS-IV | 20 mg/kg for 30
days | ↓Tumor growth | PARP, p21 | (23) |
| Hu, 2017 | Osteosarcoma | 143B cell-bearing
mice | AS-IV, cisplatin
and AS-IV+cisplatin | 20 mg/kg/day for 28
days | ↓Tumor growth | Fas/FasL
signaling | (29) |
| Xia, 2020 | Cervical
cancer | SiHa cell-bearing
mice | AS-IV | 25 mg/kg/day for 35
days | ↓Invasion | DCP1A and
TMSB4X | (41) |
| Xu, 2018 | Lung cancer | LLC cell-bearing
mice | AS-IV | 40 mg/kg/day for 21
days | ↓Tumor growth,
↓invasion, ↓invasion, ↓migration, ↓angiogenesis | AMPK signaling
pathway | (54) |
| Liu, 2020 | Breast cancer | CT26 cell-bearing
mice | AS-IV, αPD1 and
AS-IV + αPD1 | 15 mg/kg/3 days for
9 days | ↓Tumor growth, ↑T
cell infiltration, ↑M2 macrophage polarization | TGF-β, IL-10,
VEGF-A, IFN-γ, IL-12 and TNF-α | (55) |
| Zhang, 2014 | Lung cancer | 3LL cell-bearing
mice | AS-IV, 1-MT,
paclitaxel | 40 mg/kg until
death | ↓Tumor growth | IDO | (58) |
| He, 2021 | Prostate
cancer | PC-3 cell-bearing
mice | AS-IV, carboplatin
and AS-IV+carboplatin | 40 mg/kg/day for 24
days | ↑Carboplatin
sensitivity | AKT/NF-κB signaling
pathway | (71) |
| Li, 2017 | Glioma | U251 cell-bearing
mice | AS-IV | 20 mg/kg/day for 7
days | ↓Tumor growth | MAPK/ERK signaling
pathway | (75) |
| Min, 2022 | Liver cancer | Huh-7 cell-bearing
mice | AS-IV | 20–100 mg/kg/3 days
for 40 days | ↓Tumor growth | TLR4/NF-κB/STAT3
signaling pathway | (81) |
| Li, 2022 | Gastric cancer | HGC-27 cell-bearing
mice | AS-IV,
circDLST | 40 mg/kg/day for 21
days | ↓Tumor growth |
circDLST/miR-489-3p/EIF4A1 | (83) |
| Cui, 2020 | Liver cancer |
SMMC-772cell-bearing mice | AS-IV,
miR-150-5p | Only treatment of
cells | ↓Tumor growth |
miR-150-5p/β-catenin | (85) |
Antitumor mechanisms and related signaling
pathways
The antitumor effects of AS-IV are involved in
various aspects of cancer pathogenesis, including cell
proliferation, invasion, migration, metastasis, immune responses
and apoptosis. Numerous studies have investigated the molecular
mechanisms, including the signaling pathways and target genes, by
which AS-IV inhibits tumor growth. The major signaling pathways
involved in the antitumor effects of AS-IV are discussed below.
AKT/GSK-3β/β-catenin signaling
pathway
Dysfunction of the AKT/GSK-3β/β-catenin (CTNNB1)
signaling pathway affects the proliferation, invasion and migration
of various cancer cells (64).
Activated AKT induces phosphorylation of GSK-3β, inhibiting
apoptosis and inducing the nuclear translocation of CTNNB1,
increasing the expression of EMT-associated transcription factors
such as Twist family BHLH transcription factor 1, zinc finger E-box
binding homeobox 1 and Snail family transcriptional repressor 1.
GSK-3β was found to promote tumor proliferation and growth via the
AKT pathway in NSCLCs (65).
A study indicated that AS-IV suppressed the
migration and invasion of HCC cells. In addition, the morphologies
of HCC cells were altered after treatment with AS-IV. Furthermore,
the levels of phosphorylated AKT (pAKT), GSK-3β and CTNNB1 were
decreased in AS-IV-treated HCC cells, indicating that AS-IV
suppressed the growth, invasion and migration of HCC cells by
targeting the AKT/GSK-3β/CTNNB1 pathway (66). When the A549 NSCLC cells were
treated with AS-IV at concentrations of 12 and 24 ng/ml for 48 h,
the phosphorylation of AKT, GSK-3β and CTNNB1 was significantly
inhibited, suggesting that AS-IV also suppressed lung cancer
progression by regulating the AKT/GSK-3β/CTNNB1 pathway (Fig. 3) (28).
The TGF-β1-mediated PI3K/AKT/mTOR
pathway and PI3K/MAPK pathway
The PI3K/AKT pathway is a common pathway regulating
the proliferation, apoptosis, metastasis and EMT of cancer cells,
which typically express high levels of PI3K and AKT and have high
levels of pAKT. Therefore, inactivation or inhibition of the
PI3K/AKT pathway may decrease cancer cell proliferation and
invasion. Indeed, inhibition of the PI3K/AKT pathway increased
apoptosis and inhibited metastasis of cancer cells (67). Several studies indicated that
Astragalus radix and AS-IV components exert antitumor
effects by suppressing the PI3K/AKT pathway (68). Another study investigated the
mechanism of action of AS-IV in the BGC-823 and MKN-74 cell lines,
examining the protein levels of pAKT, AKT, P65 and phosphorylated
P65 (pP65) by western blot after treatment with AS-IV with or
without 10 ng/ml of TGF-β1. They found that the pAKT/AKT and
pP65/P65 ratios were increased by TGF-β1 in BGC-823 and MKN-74
cells. However, this upregulation was attenuated by AS-IV,
indicating that AS-IV suppresses TGF-β1-induced activation of the
PI3K/Akt/NF-κB signaling pathway, inhibiting GC cell proliferation
and growth (46). In addition,
AS-IV was also found to reduce the proliferation of SW962 cells of
vulvar squamous cell carcinoma (VSCC) cells and induced cell-cycle
arrest in G0/G1 phase by upregulating P53 and P21 expression, and
downregulating cyclin D1 expression. Since TGF-β1 stimulated cell
proliferation, AS-IV treatment decreased the expression of TGF-βRII
and Smad4 in the SW962 cell line of VSCC. Thus, AS-IV may inhibit
cell proliferation through the TGF-β/Smad signaling pathway in VSCC
(69).
Another study investigated the effects of AS-IV on
tumor growth in vivo using xenograft tumor models created by
injecting the breast cancer cell line MCF7 subcutaneously into nude
mice. After treating mice with Sanhuang decoction, whose main
component is AS-IV, for four weeks, it was found to significantly
inhibit grafted tumor growth. Furthermore, the expression of PI3K,
AKT and mTOR was significantly decreased, indicating that Sanhuang
decoction/AS-IV inhibited cancer growth via the PI3K/AKT/mTOR
pathway (70). In addition, AS-IV
was reported to increase chemosensitivity to carboplatin in the
treatment of prostate cancer in cultured cells and tumor xenograft
through interfering with AKT/NF-κB signaling to suppress EMT, which
was induced by carboplatin (71).
The TGF-β1-mediated PI3K/MAPK signaling pathway is also involved in
the invasion and migration of cancer cells (72). One study assessed the effect of
AS-IV on the PI3K and MAPK pathways in cultured SiHa cervical
cancer cells, finding that AS-IV suppressed the phosphorylation of
P38, MAPK, PI3K, AKT and mTOR. Therefore, AS-IV may inhibit the
invasion of cancer cells, likely via the MAPK and PI3K pathways
(Fig. 3) (22).
The MAPK/ERK/NF-κB signaling
pathway
The MAPK/rat sarcoma virus (RAS)/ERK signaling
pathway regulates cell proliferation, apoptosis and invasion by
phosphorylating related targets in tumorigenesis. The RAS/MAPK
kinase/ERK pathway is the most important signaling cascade of all
MAPK pathways, regulating tumor cell survival and development
(73,74). This pathway is interrupted in
various cancers. Glioma is a common primary brain tumor
aggressively invading the central nervous system. After glioma U251
cells were treated with AS-IV, their proliferation and invasion
were decreased in a dose-dependent manner. In addition, a
wound-healing assay indicated that U251 cell migration was
significantly delayed by AS-IV (75). When they explored the underlying
molecular mechanisms, they indicated that AS-IV significantly
decreased the phosphorylation of MAPK and ERK, leading to the
inactivation of downstream targets of the MAPK/ERK cascade, such as
the MYC proto-oncogene BHLH transcription factor, which was
inhibited by AS-IV, indicating that AS-IV inactivated the MAPK/ERK
signaling pathway to prevent glioma cell invasion and migration
(Fig. 3) (75).
The Rac family small GTPase 1
(RAC1)/MAPK/ERK signaling pathway
AS-IV was found to inhibit the proliferation and
invasion of MDA-MB-231 cells and suppress breast tumor growth and
metastasis to the lungs. Its antitumor activity reflected the
inactivation of ERK1/2 and JNK, downregulation of MMP-2 and MMP-9,
decreased levels of activated RAC1 and inhibition of RAC1/MAPK
signaling, indicating the potential of AS-IV to treat metastatic
breast cancer (76).
The protein kinase C
(PKC)-α/ERK1-2/NF-κB signaling pathway
The migration and invasion ability of A549 lung
cancer cells was suppressed by AS-IV under in vitro culture
conditions. In addition, MMP-2, MMP-9 and integrin β1 expression
was found to be significantly decreased, but CDG1 expression was
significantly increased by AS-IV treatment. Further analysis
indicated that AS-IV significantly decreased TGF-β1, TNF-α and IL-6
expression. Of note, the PKC pathway inhibitor AEB071, ERK
inhibitor U0126 or NF-κB inhibitor PDTC were able to attenuate
AS-IV suppression of cell invasion and migration, indicating that
AS-IV inhibits migration and invasion of human lung cancer A549
cells by regulating the PKC-α/ERK1-2/NF-κB pathway (Fig. 3) (77).
Immune regulatory signaling pathways
in cancer
Antitumor immunity is typically mediated by
macrophages engulfing cancer cells or by CTLs or NK cells killing
the cancer cells. Tumor growth and progression are typically
suppressed by M1 macrophages, while M2-polarized macrophages have
been reported to promote tumor invasion, migration and metastasis
and can activate tumor-promoting genes in cancer cells (78). B7-H3 expression is upregulated in
various cancers, inhibiting CTLs and inducing immune escape of
cancers (79). After treatment
with AS-IV, B7-H3 expression was decreased, suppressing the
proliferation of colorectal cell lines SW620 and HCT116. Further
analysis indicated that AS-IV was able to suppress CD276 (also
known as B7-H3) expression by inducing the expression of miR-29c.
These anticancer effects of AS-IV may be mediated by CTLs via the
B7-H3/NF-κB/CCND1 signaling pathway (Fig. 3) (30).
AS-IV has also been shown to induce immunity against
cancer as an adjunct (80).
Treatment of the cancer cell lines A549 and H1299 with M2-CM
promoted their migration ability, but M2-CM combined with AS-IV
significantly reduced their migration in wound-healing assays,
indicating that AS-IV inhibited cancer cell migration. After AS-IV
was administered to a lung tumor mouse model, the number of M2
macrophages decreased in the tumor tissue. Further analysis
indicated that AS-IV induced the transformation of M2 macrophages
into M1 macrophages mainly by inhibiting IL-13 and IL-4 and
suppressing AMPKα activation in M2 macrophages, since silencing
AMPKα partially attenuated the inhibitory effect of AS-IV on cancer
cell proliferation and invasion (Fig.
3) (54).
Other novel anticancer mechanisms and
related pathways
Suppressing M2 macrophage polarization
via the TLR4/NF-κB/signal transducer and activator of transcription
3 (STAT3) signaling pathway
Recently, a study reported that AS-IV suppressed HCC
cell proliferation and invasion by inducing macrophage
polarization. After macrophages were treated with AS-IV, CD206
levels in M2 macrophages were quantified by flow cytometry and the
levels of pSTAT3, pNF-κB and TLR4 in macrophages were determined.
AS-IV was found to decrease the M2 macrophage numbers and pSTAT3,
pNF-κB and TLR4 levels, suppressing HCC cell proliferation and
invasion by regulating the TLR4/NF-κB/STAT3 signaling pathway. In
addition, small interfering RNA targeting TLR4 inhibited Huh-7 cell
proliferation, indicating that AS-IV inhibited HCC tumor growth by
suppressing M2 macrophage polarization via the TLR4/NF-κB/STAT3
signaling pathway (Fig. 3)
(81).
Regulation of the pSMAD3C/3L and
NFE2-like BZIP transcription factor 2 (NFE2L2)/heme oxygenase 1
(HO-1) pathways
Recently, a study indicated that AS-IV decreased the
growth and multiplicity of primary liver cancer in mouse models.
After 20 weeks of AS-IV treatment, it was observed that AS-IV acted
on the TGF-β1/SMAD and NFE2L2 (also known as NRF2)/HO-1 pathways,
increasing the levels of pSMAD3C and pNRF2, HO-1 and NAD(P)H
quinone dehydrogenase 1 (NQO1) and decreasing the levels of
pSMAD2C, pSMAD2L and pSMAD3L, PAI-1 and smooth muscle α-actin in
mice with liver cancer. In vitro analysis also confirmed
that AS-IV regulated the levels of pSMAD3C, pSMAD3L, HO-1 and NQO1
via the pSMAD3C/3L and NRF2/HO-1 pathways in HSC-T6 and HepG2
cells, which was activated by TGF-β1. These findings suggest that
AS-IV promotes a switch in SMAD3 signaling from pSMAD3L-mediated
oncogenesis to pSMAD3C-mediated tumor suppression, increasing
levels of pSMAD3C, inhibiting pSMAD3L and pSMAD2C, and promoting
NRF2 phosphorylation via TGF-β1 signal mediation-specific pSMAD3
(Fig. 4) (82).
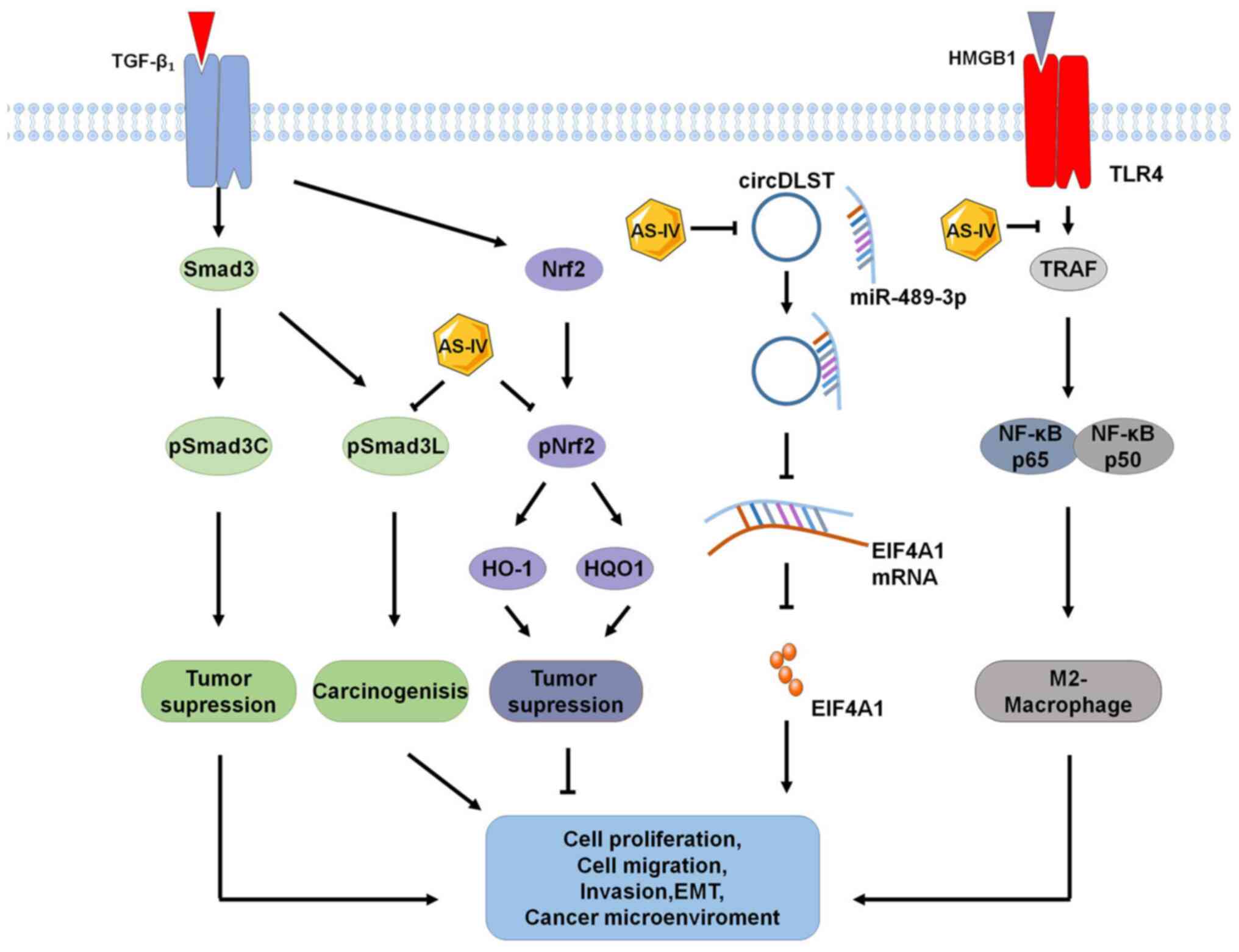 | Figure 4.Novel signaling pathways for the
antitumor effects of AS-IV. Recently identified signaling pathways
for the anticancer effects of AS-IV include pSMAD3C/3L, NRF2/HO-1,
circDLST/miR-489-3p/EIF4A1 and HMGB1/TLR4, which inhibits
M2-macrophage polarization. AS-IV, astragaloside-IV; TLR4,
Toll-like receptor 4; HO-1, heme oxygenase 1; HQO-1, heme quinone
oxidoreductase-1; EIF4A1, eukaryotic translation initiation factor
4A1; TRAF, tumor necrosis factor receptor-associated factor;
pSMAD3C, phosphor-SMAD family member 3C; pSMAD3L, phosphor-SMAD
family member 3L; HMGB1, high mobility group box 1; circDLST,
circRNA dihydrolipoamide S-succinyltransferase. |
Targeting the
circDLST/miR-489-3p/eukaryotic translation initiation factor 4A1
(EIF4A1) pathway
Circular RNAs have been reported to have important
roles in regulating malignant cancer progression. In most cancer
cells, circRNA dihydrolipoamide S-succinyltransferase (circDLST)
and EIF4A1 are highly expressed, while miR-489-3p expression is
low. In the cultured GC cell lines HGC-27 and MKN-45 treated with
AS-IV, it suppressed cell proliferation and metastasis by
downregulating circDLST, increasing miR-489-3p expression and
inhibiting EIF4A1 expression, preventing cancer progression.
Conversely, inhibition of miR-489-3p decreased the antitumor
activity of AS-IV. These findings indicated that AS-IV inhibits
cancer cell growth and progression via a novel
circDLST/miR-489-3p/EIF4A1 mechanism with circDLST-mediated
downregulation of EIF4A1. AS-IV was also found to suppress the
growth of HCC in vitro and in vivo by upregulating
miR-150-5p and repressing β-catenin through a mechanism of
miR-150-5p/β-catenin (83–85).
Suppressing the HMGB1/TLR4 signaling
pathway to inhibit M2-macrophage polarization-induced cancer
progression
AS-IV treatment suppressed M2 macrophage marker
expression of CD206, C-C motif chemokine ligand 24 (CCL24),
peroxisome proliferator-activated receptor γ (PPRAγ), arginase 1
and IL-10 in the ovarian cancer line SKOV3, indicating that AS-IV
inhibits macrophage M2 polarization to improve the antitumor effect
in the TME. In addition, AS-IV was indicated to inhibit the
expression of HMGB1 and TLR4 and decrease the expression of M2
markers TGF-β, MMP-9 and IL-10 in co-cultures of
ovarian cancer cells with macrophages. Blocking HMGB1 signaling
suppressed M2 macrophage-induced ovarian cancer cell proliferation,
invasion and migration, suggesting HMGB1 reversed the inhibitory
effect of AS-IV on M2-macrophage polarization-induced malignant
ovarian cancer cell progression. These findings indicate that AS-IV
may protect against ovarian cancer cell progression by suppressing
HMGB1/TLR4 signaling (Fig. 4)
(48,86).
Conclusion and future perspectives
The antitumor effects of AS-IV have been
demonstrated in vitro in cultured cancer cells and in
vivo in animal cancer models at different stages of cancer
progression. In each of these processes, AS-IV appears to increase
tumor cell apoptosis and autophagy, inhibit tumor cell
proliferation, invasion, migration and EMT, increase CTL immune
activities and promote the transformation of M2 macrophages into M1
macrophages. The antitumor effects of AS-IV are mainly via
upregulation or downregulation of different signaling pathways or
various recently identified novel mechanisms. In addition, AS-IV
increases the sensitivity of the cancer cells to cisplatin and
other drugs, increasing chemotherapy and immunotherapy efficacy.
These findings highlight the antitumor role of AS-IV and the
potential of AS-IV as an antitumor drug or adjuvant for use in
cancer therapy. However, current studies have their limitations and
future priorities should be on developing and utilizing AS-IV as a
new clinical drug. First, while several signaling pathways were
found to be associated with the action of AS-IV, the direct targets
and specific molecular networks of AS-IV for antitumor effects
require to be further elucidated. In addition, the dose of AS-IV
used in numerous studies varies greatly. Therefore, the safe and
effective dose of AS-IV requires to be accurately established.
Future studies should determine the effective doses of AS-IV to
produce antitumor effects. Furthermore, to date, only a small
number of studies have investigated the toxicity and the potential
adverse effects of AS-IV in vivo and in vitro.
Therefore, the toxicity and side effects of AS-IV should be studied
to enable safer clinical applications.
Finally, while A. membranaceus Bunge or TCM
decoctions containing A. membranaceus Bunge have been used
in the clinic to treat cancer patients, no single AS-IV compound
has been tested in the treatment of the patients. The main reason
why a single compound AS-IV has not yet been investigated in
clinical trials may be that the safe and effective dose, toxicity
and potential adverse effects of AS-IV have not been determined
accurately. This issue can probably be resolved by a biotech
company to carefully design and perform in vitro and in
vivo antitumor experiments of AS-IV based on the Food and Drug
Administration guidelines for the registration of a new drug.
Subsequently, clinical trials are required to further confirm the
safety and efficacy of AS-IV in cancer patients.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science
Foundation of China (grant nos. 81571241 and 82004402), Shandong
Provincial Natural Science Foundation (grant no. ZR2019ZD39) and
the Quancheng Talent Scholar Fund of Jinan City in Shandong of
China (5150 Talent Plan, 2018 to FH).
Availability of data and materials
Not applicable.
Authors' contributions
FH conceived and designed this review. LZ, ML, ZC,
JW, GZ and FH were major contributors in writing the manuscript and
performed the literature search and selection. KC, JZ, LD, YL and
CJ wrote sections of the manuscript and prepared the figures. All
authors have read and approved the final manuscript. All authors
are responsible for all aspects of the work and approved the
submission in its current form. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ghaznavi H, Shirvaliloo M, Zarebkohan A,
Shams Z, Radnia F, Bahmanpour Z, Sargazi S, Saravani R, Shirvalilou
S, Shahraki O, et al: An updated review on implications of
autophagy and apoptosis in tumorigenesis: Possible alterations in
autophagy through engineered nanomaterials and their importance in
cancer therapy. Mol Pharmacol. 100:119–143. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown JM and Attardi LD: The role of
apoptosis in cancer development and treatment response. Nat Rev
Cancer. 5:231–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang C, Hu Y, Xiao W and Tian Z: Chimeric
antigen receptor- and natural killer cell receptor-engineered
innate killer cells in cancer immunotherapy. Cell Mol Immunol.
18:2083–2100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling CQ, Yue XQ and Ling C: Three
advantages of using traditional Chinese medicine to prevent and
treat tumor. J Integr Med. 12:331–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Lou Y, Wang J, Yu C and Shen W:
Research status and molecular mechanism of the traditional Chinese
medicine and antitumor therapy combined strategy based on tumor
microenvironment. Front Immunol. 11:6097052021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang K, Chen Q, Shao Y, Yin S, Liu C, Liu
Y, Wang R, Wang T, Qiu Y and Yu H: Anticancer activities of TCM and
their active components against tumor metastasis. Biomed
Pharmacother. 133:1110442021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen F, Zhong Z, Tan HY, Guo W, Zhang C,
Tan CW, Li S, Wang N and Feng Y: Uncovering the anticancer
mechanisms of Chinese herbal medicine formulas: Therapeutic
alternatives for liver cancer. Front Pharmacol. 11:2932020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen T, Yang P and Jia Y: Molecular
mechanisms of astragaloside-IV in cancer therapy (review). Int J
Mol Med. 47:132021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14:482019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Zhang Q, Chen Y, Liang CL, Liu H,
Qiu F and Dai Z: Antitumor effects of immunity-enhancing
traditional Chinese medicine. Biomed Pharmacother. 121:1095702020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Royal Botanic Gardens, Kew. https://mpns.science.kew.org/mpns-portal/?_ga=1.111763972.1427522246.1459077346
|
|
14
|
Chang X, Chen X, Guo Y, Gong P, Pei S,
Wang D, Wang P, Wang M and Chen F: Advances in chemical
composition, extraction techniques, analytical methods, and
biological activity of astragali radix. Molecules. 27:10582022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Z, Lou Y, Kong M, Luo Q, Liu Z and Wu
J: A systematic review of phytochemistry, pharmacology and
pharmacokinetics on astragali radix: Implications for astragali
radix as a personalized medicine. Int J Mol Sci. 20:14632019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang CH, Yang X, Wei JR, Chen NM, Xu JP,
Bi YQ, Yang M, Gong X, Li ZY, Ren K, et al: Ethnopharmacology,
phytochemistry, pharmacology, toxicology and clinical applications
of radix astragali. Chin J Integr Med. 27:229–240. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Qu L, Dong Y, Han L, Liu E, Fang S,
Zhang Y and Wang T: A review of recent research progress on the
astragalus genus. Molecules. 19:18850–18880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong S, Ou S, Liu Y, Xie M, Mei T, Zhang
Y, Zhang J, Wang Q and Yang B: Surface-enhanced raman spectroscopy
analysis of astragalus saponins and identification of metabolites
after oral administration in rats by ultrahigh-performance liquid
chromatography/quadrupole time-of-flight mass spectrometry
analysis. Front Pharmacol. 13:8284492022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Tang D, Zang W, Yin G, Dai J, Sun
YU, Yang Z, Hoffman RM and Guo X: Synergistic inhibitory effect of
traditional Chinese medicine astragaloside IV and curcumin on tumor
growth and angiogenesis in an orthotopic nude-mouse model of human
hepatocellular carcinoma. Anticancer Res. 37:465–473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Wu C, Gao L, Du G and Qin X:
Astragaloside IV derived from Astragalus membranaceus: A
research review on the pharmacological effects. Adv Pharmacol.
87:89–112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong AGW, Duan R, Wang HY, Kong XP, Dong
TTX, Tsim KWK and Chan K: Evaluation of the pharmaceutical
properties and value of astragali radix. Medicines (Basel).
5:462018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Zhou J, Qin X, Huang H and Nie C:
Astragaloside IV inhibits the invasion and metastasis of SiHa
cervical cancer cells via the TGF-β1-mediated PI3K and MAPK
pathways. Oncol Rep. 41:2975–2986. 2019.PubMed/NCBI
|
|
23
|
Sun P, Liu Y, Wang Q and Zhang B:
Astragaloside IV inhibits human colorectal cancer cell growth.
Front Biosci (Landmark Ed). 24:597–606. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim C and Kim B: Anti-cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10:10212018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia L, Lv D, Zhang S, Wang Z and Zhou B:
Astragaloside IV inhibits the progression of non-small cell lung
cancer through the Akt/GSK-3β/β-catenin pathway. Oncol Res.
27:503–508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu T, Fei Z and Wei N: Chemosensitive
effects of astragaloside IV in osteosarcoma cells via induction of
apoptosis and regulation of caspase-dependent Fas/FasL signaling.
Pharmacol Rep. 69:1159–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang S, Mou J, Cui L, Wang X and Zhang Z:
Astragaloside IV inhibits cell proliferation of colorectal cancer
cell lines through down-regulation of B7-H3. Biomed Pharmacother.
102:1037–1044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su CM, Wang HC, Hsu FT, Lu CH, Lai CK,
Chung JG and Kuo YC: Astragaloside IV induces apoptosis,
G1-phase arrest and inhibits anti-apoptotic signaling in
hepatocellular carcinoma. In vivo. 34:631–638. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim MO, Lee HS, Chin YW, Moon DO and Ahn
JS: Gartanin induces autophagy through JNK activation which
extenuates caspase-dependent apoptosis. Oncol Rep. 34:139–146.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amaravadi RK, Kimmelman AC and Debnath J:
Targeting autophagy in cancer: Recent advances and future
directions. Cancer Discov. 9:1167–1181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Russell RC, Yuan HX and Guan KL: Autophagy
regulation by nutrient signaling. Cell Res. 24:42–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu
HL, Yang C and Liu HF: p62 links the autophagy pathway and the
ubiqutin-proteasome system upon ubiquitinated protein degradation.
Cell Mol Biol Lett. 21:292016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Li G, Chen M and Cai R:
Astragaloside IV enhances the sensibility of lung adenocarcinoma
cells to bevacizumab by inhibiting autophagy. Drug Dev Res.
83:461–469. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li QW, Zhang GL, Hao CX, Ma YF, Sun X,
Zhang Y, Cao KX, Li BX, Yang GW and Wang XM: SANT, a novel Chinese
herbal monomer combination, decreasing tumor growth and
angiogenesis via modulating autophagy in heparanase overexpressed
triple-negative breast cancer. J Ethnopharmacol. 266:1134302021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai ST, Wang Y and Peng F: Astragaloside
IV sensitizes non-small cell lung cancer cells to cisplatin by
suppressing endoplasmic reticulum stress and autophagy. J Thorac
Dis. 12:3715–3724. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang B, Yang N, Chen Y, Zhu M, Lian Y,
Xiong Z, Wang B, Feng L and Jia X: An integrated strategy for
effective-component discovery of astragali radix in the treatment
of lung cancer. Front Pharmacol. 11:5809782021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qu X, Gao H, Zhai J, Sun J, Tao L, Zhang
Y, Song Y and Hu T: Astragaloside IV enhances cisplatin
chemosensitivity in hepatocellular carcinoma by suppressing MRP2.
Eur J Pharm Sci. 148:1053252020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia C, He Z and Cai Y: Quantitative
proteomics analysis of differentially expressed proteins induced by
astragaloside IV in cervical cancer cell invasion. Cell Mol Biol
Lett. 25:252020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang X: EMT: New signals from the
invasive front. Oral Oncol. 47:686–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ribatti D: Epithelial-mesenchymal
transition in morphogenesis, cancer progression and angiogenesis.
Exp Cell Res. 353:1–5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kozak J, Forma A, Czeczelewski M, Kozyra
P, Sitarz E, Radzikowska-Büchner E, Sitarz M and Baj J: Inhibition
or reversal of the epithelial-mesenchymal transition in gastric
cancer: Pharmacological approaches. Int J Mol Sci. 22:2772020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu J and Wen K: Astragaloside IV inhibits
TGF-β1-induced epithelial-mesenchymal transition through inhibition
of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother
Res. 32:1289–1296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han J, Shen X, Zhang Y, Wang S and Zhou L:
Astragaloside IV suppresses transforming growth factor-β1-induced
epithelial-mesenchymal transition through inhibition of
Wnt/β-catenin pathway in glioma U251 cells. Biosci Biotechnol
Biochem. 84:1345–1352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang X, Gao S, Song L, Liu M, Sun Z and
Liu J: Astragaloside IV antagonizes M2 phenotype macrophage
polarization-evoked ovarian cancer cell malignant progression by
suppressing the HMGB1-TLR4 axis. Mol Immunol. 130:113–121. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang ZF, Ma DG, Zhu Z, Mu YP, Yang YY,
Feng L, Yang H, Liang JQ, Liu YY, Liu L and Lu HW: Astragaloside IV
inhibits pathological functions of gastric cancer-associated
fibroblasts. World J Gastroenterol. 23:8512–8525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C,
Lin F, Liao H, You Z and Liu L: Prognostic impact of
tumor-associated macrophage infiltration in non-small cell lung
cancer: A systemic review and meta-analysis. Oncotarget.
7:34217–34228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cuccarese MF, Dubach JM, Pfirschke C,
Engblom C, Garris C, Miller MA, Pittet MJ and Weissleder R:
Heterogeneity of macrophage infiltration and therapeutic response
in lung carcinoma revealed by 3D organ imaging. Nat Commun.
8:142932017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sawa-Wejksza K, Dudek A, Lemieszek M,
Kaławaj K and Kandefer-Szerszeń M: Colon cancer-derived conditioned
medium induces differentiation of THP-1 monocytes into a mixed
population of M1/M2 cells. Tumour Biol. 40:10104283187978802018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li N, Qin J, Lan L, Zhang H, Liu F, Wu Z,
Ni H and Wang Y: PTEN inhibits macrophage polarization from M1 to
M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer
Biol Ther. 16:297–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL,
Gong WY, Dong JC and Liu BJ: Astragaloside IV inhibits lung cancer
progression and metastasis by modulating macrophage polarization
through AMPK signaling. J Exp Clin Cancer Res. 37:2072018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu F, Ran F, He H and Chen L:
Astragaloside IV exerts anti-tumor effect on murine colorectal
cancer by re-educating tumor-associated macrophage. Arch Immunol
Ther Exp (Warsz). 68:332020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen J, Ye X, Pitmon E, Lu M, Wan J,
Jellison ER, Adler AJ, Vella AT and Wang K: IL-17 inhibits
CXCL9/10-mediated recruitment of CD8+ cytotoxic T cells
and regulatory T cells to colorectal tumors. J Immunother Cancer.
7:3242019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Huang LF, Yao YM, Li JF, Zhang SW, Li WX,
Dong N, Yu Y and Sheng ZY: The effect of astragaloside IV on immune
function of regulatory T cell mediated by high mobility group box 1
protein in vitro. Fitoterapia. 83:1514–1522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang A, Zheng Y, Que Z, Zhang L, Lin S,
Le V, Liu J and Tian J: Astragaloside IV inhibits progression of
lung cancer by mediating immune function of Tregs and CTLs by
interfering with IDO. J Cancer Res Clin Oncol. 140:1883–1890. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu W, Chen H and Wang D: Protective role
of astragaloside IV in gastric cancer through regulation of
microRNA-195-5p-mediated PD-L1. Immunopharmacol Immunotoxicol.
43:443–451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang PP, Luan JJ, Xu WK, Wang L, Xu DJ,
Yang CY, Zhu YH and Wang YQ: Astragaloside IV downregulates the
expression of MDR1 in Bel-7402/FU human hepatic cancer cells by
inhibiting the JNK/c-Jun/AP-1 signaling pathway. Mol Med Rep.
16:2761–2766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xie T, Li Y, Li SL and Luo HF:
Astragaloside IV enhances cisplatin chemosensitivity in human
colorectal cancer via regulating NOTCH3. Oncol Res. 24:447–453.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dulloo I, Phang BH, Othman R, Tan SY,
Vijayaraghavan A, Goh LK, Martin-Lopez M, Marques MM, Li CW, Wang
de Y, et al: Hypoxia-inducible TAp73 supports tumorigenesis by
regulating the angiogenic transcriptome. Nat Cell Biol. 17:511–523.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu L, Zhou XM, Yang FF, Miao Y, Yin Y, Hu
XJ, Hou G, Wang QY and Kang J: TRIM22 confers poor prognosis and
promotes epithelial-mesenchymal transition through regulation of
AKT/GSK3β/β-catenin signaling in non-small cell lung cancer.
Oncotarget. 8:62069–62080. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH,
Wang YC, Ye BG, Cao MQ, Gao DM and Tang ZY: Astragaloside IV
inhibits metastasis in hepatoma cells through the suppression of
epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin
pathway. Oncol Rep. 37:1725–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y, Liu C, Xie Z and Lu H: Knockdown
of TRIM47 inhibits breast cancer tumorigenesis and progression
through the inactivation of PI3K/Akt pathway. Chem Biol Interact.
317:1089602020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li R, Song Y, Zhou L, Li W and Zhu X:
Downregulation of RAGE inhibits cell proliferation and induces
apoptosis via regulation of PI3K/AKT pathway in cervical squamous
cell carcinoma. Onco Targets Ther. 13:2385–2397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao Y, Wang L, Wang Y, Dong S, Yang S,
Guan Y and Wu X: Astragaloside IV inhibits cell proliferation in
vulvar squamous cell carcinoma through the TGF-β/Smad signaling
pathway. Dermatol Ther. 32:e128022019.PubMed/NCBI
|
|
70
|
Zhang XQ, Yao C, Bian WH, Chen X, Xue JX,
Zhu ZY, Ying Y, Xu YL and Wang C: Effects of astragaloside IV on
treatment of breast cancer cells execute possibly through
regulation of Nrf2 via PI3K/AKT/mTOR signaling pathway. Food Sci
Nutr. 7:3403–3413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
He Y, Zhang Q, Chen H, Guo Q, Zhang L,
Zhang Z and Li Y: Astragaloside IV enhanced carboplatin sensitivity
in prostate cancer by suppressing AKT/NF-κB signaling pathway.
Biochem Cell Biol. 99:214–222. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tanaka Y, Kobayashi H, Suzuki M, Kanayama
N and Terao T: Transforming growth factor-beta1-dependent urokinase
up-regulation and promotion of invasion are involved in
Src-MAPK-dependent signaling in human ovarian cancer cells. J Biol
Chem. 279:8567–8576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Anfuso CD, Motta C, Giurdanella G, Arena
V, Alberghina M and Lupo G: Endothelial PKCα-MAPK/ERK-phospholipase
A2 pathway activation as a response of glioma in a triple culture
model. A new role for pericytes? Biochimie. 99:77–87.
2014.PubMed/NCBI
|
|
74
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
75
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jiang K, Lu Q, Li Q, Ji Y, Chen W and Xue
X: Astragaloside IV inhibits breast cancer cell invasion by
suppressing Vav3 mediated Rac1/MAPK signaling. Int Immunopharmacol.
42:195–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Komohara Y, Fujiwara Y, Ohnishi K and
Takeya M: Tumor-associated macrophages: Potential therapeutic
targets for anti-cancer therapy. Adv Drug Deliv Rev. 99:180–185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Xie C, Liu D, Chen Q, Yang C, Wang B and
Wu H: Soluble B7-H3 promotes the invasion and metastasis of
pancreatic carcinoma cells through the TLR4/NF-κB pathway. Sci Rep.
6:275282016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hong F, Xiao W, Ragupathi G, Lau CB, Leung
PC, Yeung KS, George C, Cassileth B, Kennelly E and Livingston PO:
The known immunologically active components of astragalus account
for only a small proportion of the immunological adjuvant activity
when combined with conjugate vaccines. Planta Med. 77:817–824.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Min L, Wang H and Qi H: Astragaloside IV
inhibits the progression of liver cancer by modulating macrophage
polarization through the TLR4/NF-κB/STAT3 signaling pathway. Am J
Transl Res. 14:1551–1566. 2022.PubMed/NCBI
|
|
82
|
Zhang C, Li L, Hou S, Shi Z, Xu W, Wang Q,
He Y, Gong Y, Fang Z and Yang Y: Astragaloside IV inhibits
hepatocellular carcinoma by continually suppressing the development
of fibrosis and regulating pSmad3C/3L and Nrf2/HO-1 pathways. J
Ethnopharmacol. 279:1143502021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li F, Cao K, Wang M, Liu Y and Zhang Y:
Astragaloside IV exhibits anti-tumor function in gastric cancer via
targeting circRNA dihydrolipoamide S-succinyltransferase
(circDLST)/miR-489-3p/eukaryotic translation initiation factor
4A1(EIF4A1) pathway. Bioengineered. 13:10111–10122. 2022.PubMed/NCBI
|
|
84
|
Zhang J, Hou L, Liang R, Chen X, Zhang R,
Chen W and Zhu J: CircDLST promotes the tumorigenesis and
metastasis of gastric cancer by sponging miR-502-5p and activating
the NRAS/MEK1/ERK1/2 signaling. Mol Cancer. 18:802019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cui X, Jiang X, Wei C, Xing Y and Tong G:
Astragaloside IV suppresses development of hepatocellular carcinoma
by regulating miR-150-5p/β-catenin axis. Environ Toxicol Pharmacol.
78:1033972020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang L, Botchway BOA and Liu X: The
repression of the HMGB1-TLR4-NF-κB signaling pathway by safflower
yellow may improve spinal cord injury. Front Neurosci.
15:8038852021. View Article : Google Scholar : PubMed/NCBI
|