Introduction
The genus Clostridium is comprised of
Gram-positive, anaerobic, spore-forming bacteria. It is one of the
largest groups of bacteria, with >180 species capable of causing
bacteremia and a variety of invasive infections in humans (1). The most common species of this genus
is Clostridium perfringens (42%), followed by C.
septicum (14%), C. ramosum (9%), C.
clostridioforme (6%) and C. difficile (5%). By contrast,
C. paraputrificum has been identified in only 1% of cases,
which may explain why its clinical significance has not been fully
elucidated, yet (2).
C. paraputrificum is a participant in the
generally harmless commensal intestinal flora. As with other such
species, bacteremia can be triggered and cause injury to the
intestinal mucosa, facilitated by underlying diabetes,
malignancies, HIV infection, alcohol consumption, gastrointestinal
disorders or neutropenia (3,4). The
most frequently reported conditions with C. paraputrificum
as an etiological agent are sepsis, liver abscess, septic
arthritis, osteomyelitis, aspiration pneumonia, acute necrotizing
enterocolitis and colonic necrosis (1,5,6).
Clostridia appear as gram-positive, typically large,
straight or curved rods, with slightly rounded ends, measuring ~2-6
µm in length and 0.5-1 µm in diameter. Spores are oval and
subterminal or terminal. Pleomorphism forms are also common: Most
species of Clostridium are motile with peritrichous flagella
(7). A particular strain, M-21, has
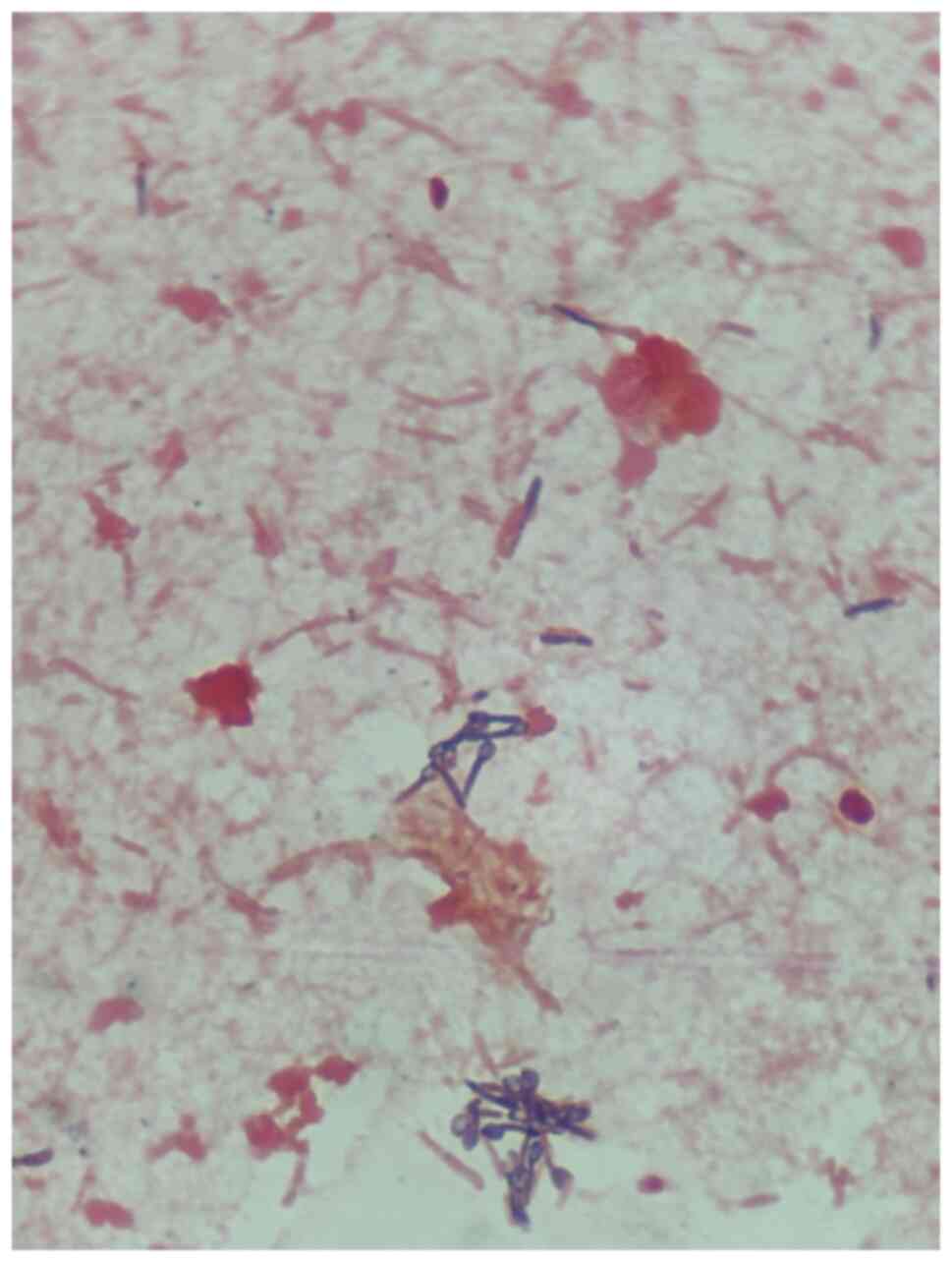
the capacity to produce gas by using chitin and glucose (8). In the present case, Gram-positive
anaerobic rods with spores were observed on the Gram-stained blood
cultures, and these were eventually identified as C.
paraputrificum.
The genome of C. paraputrificum consists of a
single circular chromosome. Certain strains may carry plasmids,
which are extrachromosomal DNA that can confer additional traits,
such as antibiotic resistance. 16S rRNA gene sequencing is used for
identifying C. paraputrificum (7).
The current study reported the case of a 70-year-old
patient with sepsis followed by septic shock caused by C.
paraputrificum, whose aggravating condition was likely
facilitated by a social background of poverty and alcohol
consumption rather than by pre-existing conditions previously
associated with this rare bacterial infection.
Materials and methods
Identification of bacteria
To test for suspected bacterial infections, blood
culture vials were incubated in a BD BACTEC FX Blood Culture system
(BD Biosciences) for seven days according to hospital and
laboratory protocols. After 48 h of incubation, one of the two
blood cultures showed the growth of bacteria. The identification of
the bacteria was achieved with matrix-assisted laser desorption
ionisation (MALDI)-time-of-flight technology (MALDI Biotyper;
Bruker Daltonics), which is used to sequence proteins, map
biomolecules in tissues, identify microorganisms and to analyze
several thousand biochemical assays in a day (9). Gram staining was also performed, as
well as various cultures from urine and bed sores secretions to
screen for multi-drug resistant bacteria. The test was performed
according to standard procedures.
Toxins A and B for Clostridioides difficile
were investigated by means of immunochromatographic assay using a
rapid test kit supplied by DDDiagnostic. The stool sample was
processed to obtain a suspension (a small amount from the stool
sample was mixed with the buffer solution). This was then exposed
to specific antibodies for Clostridioides difficile's A and
B toxins for 10-20 min, after which the results were displayed on
the test kit. A control line appeared, confirming that the test was
performed correctly, and the presence of toxins would have been
indicated by additional lines.
Antibiogram methodology
For the antibiogram, the method of microdilutions in
broth (Micronaut system) on Bruker plates was used. The specificity
of this method was 99% according to the laboratory staff and other
published research (10). Genetic
assays could not be performed, as our laboratory is not equipped
with the necessary kits.
Case report
A 70-year-old male patient was first brought to the
emergency room of ‘Dr. N. Oblu’ Emergency Neurology Hospital (Iași,
Romania) in February 2024. The patient's symptoms had started ~3
days prior and on admission, the patient's general condition was
deteriorating, with apparent confusion and inability to maintain
orthostatism. The patient was an impoverished rural resident with a
history of cardiovascular disease (heart failure) and chronic
alcohol consumption.
On admission, the patient was presenting with signs
suggestive of an ischemic stroke, yet the brain CT scan showed no
evidence of stroke or recent hemorrhage (data not shown). Instead,
the chest CT revealed areas of alveolar condensation affecting the
medium lobe of the right lung almost entirely (aspect suggestive of
lobar pneumonia), as well as in posterior, basal, bilateral areas,
including left pleurisy of ~10 mm and pulmonary scleroemphysema
(data not shown). These results, together with the neurological
examination, excluded a neurological emergency, and other possible
causes were also excluded based on further specialized
investigations (cardiology, gastroenterology, pneumology,
nephrology). It was not possible to include CT images, as CT
scanning is a service outsourced to another hospital, and the
results were received as already interpreted.
Paraclinical tests indicated leukocytosis
[leukocytes, 33,020/mm3 (normal range,
6,000-13,500/mm3)] with neutrophilia [neutrophils, 92.6%
(normal range, 30-75%)], C-reactive protein, 317 mg/l (normal
range, <0.5 mg/l), coagulation disorders and hepatocytolysis
[alanine aminotransferase, 351 U/l (normal range, 5-40 U/l); and
aspartate aminotransferase, 143 U/l (normal range, 5-37 U/l)], as
well as evidence of nitrogen retention expressed as high serum urea
[158 mg/dl (normal range, 15-50 mg/dl)] and elevated creatinine [4
mg/dl (normal range, 0.6-1.1 mg/dl)] (Table I). On clinical examination, the
patient presented muscle hypertonia and a stiff neck. The suspicion
of meningitis was thus raised and the patient was subsequently
transferred to the ‘Sf. Parascheva’ Clinical Hospital of Infectious
Diseases (Iași, Romania).
 | Table IEvolution of biological
parameters. |
Table I
Evolution of biological
parameters.
| Item (normal
ranges) | 1st day | 2nd day | 3rd day | 4th day | 5th day |
|---|
| Blood count | | | | | |
|
WBC,
mm3 (4,000-10,000) | 18,140 | - | - | - | 11,530 |
|
NEUT, %
(45-80) | 94.2 | - | - | - | 85.7 |
|
LYMPH, %
(20-45) | 2.4 | - | - | - | 4.4 |
|
HB, g/dl
(13.2-17.3) | 12.7 | - | - | - | 10.7 |
|
HCT, %
(39-51) | 37.5 | - | - | - | 321 |
|
PLT,
mm3 (150,000-380,000) | 340,000 | - | - | - | 197,000 |
| Inflammatory
markers | | | | | |
|
CRP, mg/l
(0-5) | 375.68 | 195.62 | - | - | - |
|
Procalcitonin,
ng/ml (<0.5) | >10 | - | - | - | - |
|
Fibrinogen,
g/l (2-4) | 4.66 | - | - | - | - |
| Biochemistry | | | | | |
|
Urea, mg/dl
(15-50) | 170 | 235 | 277 | 293 | 227 |
|
Creatinine,
mg/dl (0.6-1.1) | 3.95 | 5.18 | 5.99 | 7.25 | 4.84 |
|
Total
protein, g/l (67-87) | 56.64 | - | - | - | 50.98 |
| Liver markers | | | | | |
|
ALT, U/l
(5-40) | 150 | - | - | 111 | 65 |
|
AST, U/l
(5-37) | 348 | - | - | 152 | 111 |
|
Total
bilirubin, mg/dl (0.2-1) | 0.65 | - | - | - | 0.40 |
On arrival, the patient was afebrile,
hemodynamically stable, with slightly elevated blood pressure
[123/86 mmHg (normal range, <120/80 mmHg)] and heart rate [100
bpm (normal range, 60-90 bpm)], and with low oxygen saturation [90%
(normal range, 95-100%)], and this was addressed with oxygen
therapy (4 l/min). The cardiopulmonary auscultation revealed
crackling rales on the right hemithorax and diminished vesicular
murmur on the left. The patient's abdomen was depressed, painless
on deep palpation and diuresis was present. In addition, grade I
calcaneal and grade I-II sacral eschars were noted, while mucous
membranes were normally colored. The patient was conscious, able to
open his eyes and perform eye tracking, and able to use his limbs
spontaneously. However, he became uncooperative and his condition
appeared to be deteriorating ~6 h after admission.
A lumbar puncture was performed and it excluded an
inflammatory reaction. Considering the polymorphic symptomatology
and the general condition of the patient, the initial therapeutic
intention was to address a wide spectrum of bacteria. Thus,
treatment was initiated with ciprofloxacin 200 mg and imipenem 500
mg (taking into account creatinine levels when deciding on these
dosages), analgesic and antipyretic medication, a gastric protector
and hydro-electrolytic rebalancing infusions. Furthermore, the
patient's cultures for multi-drug resistant bacteria were
negative.
The patient was monitored and his general condition
continued to aggravate over the next 24 h. The patient's oxygen
needs increased to 10 l/min and creatinine levels rose to 6 mg/dl,
a sign of nitrogen retention suggestive of deteriorating kidney
function. In addition, the clinical observation of a rigid (wooden)
abdomen was made. A contrast CT of the abdomen and pelvis was
performed at the ‘Sf. Spiridon’ Emergency Clinical County Hospital
(Iasi, Romania). A surgical consultation was also provided, yet
neither revealed any evidence that would justify the need for
emergency surgery (e.g., no perforated gastric ulcer or mesenteric
ischemia). Considering also the patient's accelerating intestinal
transit, he was tested for toxins A and B of Clostridioides
difficile, with negative results. The test was performed using
immunochromatographic assay.
Taking into account the clinical sign of ‘wooden
abdomen’, in the absence of changes detectable by CT imaging, the
suspicion of localized tetanus was raised. By this time, three days
after admission, excoriations with grating lesions, bed sores at
the lumbar-sacral level and ‘diaper’ dermatitis had been noted by
visual examination, and these were considered possible gateways for
such an infection.
At four days after admission, due to creatinine
increasing further to 6.9 mg/dl under the initially established
therapy, the patient was transferred to the Nephrology Department
of ‘Dr C. I. Parhon’ Clinical Hospital (Iasi, Romania) where a
dialysis session was initiated. Serological analysis for
Clostridium tetani was performed and the result was
negative. After localized tetanus was ruled out, the patient's
earlier blood samples (collected prior to the initiation of
antibiotic therapy) were further processed and Clostridium
paraputrificum was identified as gram-positive rods with spores
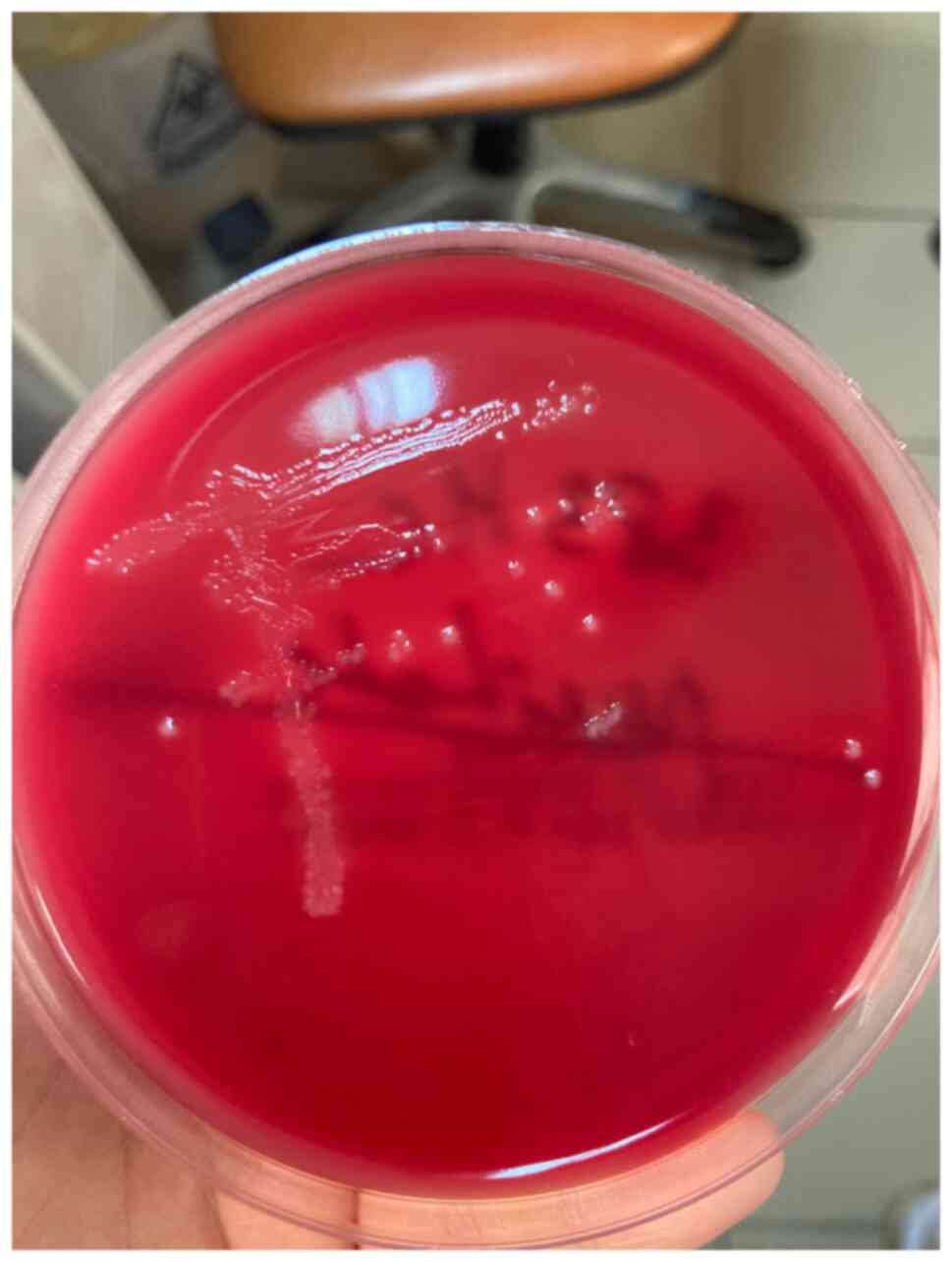
on Gram-stained cultures, as illustrated in Figs. 1 and 2.
After establishing the etiology from the blood
cultures, the resumption of diuresis and the decrease in creatinine
to 4.25 mg/dl, the patient was transferred to an intensive care
unit for patients with infectious diseases at the ‘Sf. Parascheva’
Clinical Hospital for Infectious Diseases (Iasi, Romania) five days
after admission. Antibiotic sensitivity tests for anaerobic
organisms showed that the C. paraputrificum strain was
sensitive to meropenem, metronidazole, penicillin G,
piperacillin/tazobactam, vancomycin, ampicillin,
amoxicillin/clavulanic acid and imipenem (Table II). The treatment was changed to
penicillin G and metronidazole.
 | Table IIAntimicrobial susceptibility test for
Clostridium paraputrificum. |
Table II
Antimicrobial susceptibility test for
Clostridium paraputrificum.
| Antibiotic | Minimum inhibitory
concentration, mg/l | Sensitivity |
|---|
| Clindamycin | 0.5 | Resistant |
| Meropenem
(carbapenem) | ≤0.5 | Sensitive |
| Metronidazole | 1 | Sensitive |
| Penicillin G | 0.5 | Sensitive |
|
Piperacillin/Tazobactam | ≤1/4 | Sensitive |
| Vancomycin | ≤2 | Sensitive |
| Ampicillin | 0.5 | Sensitive |
|
Amoxicillin/clavulanic acid | <0.5/0.25 | Sensitive |
| Imipenem
(carbapenem) | <0.5 | Sensitive |
At this point, ~6 h into the new antibiotic
treatment, the patient's general condition continued to deteriorate
both neurologically and hemodynamically (hypotension). Septic shock
was established, vasopressor support with noradrenaline was
initiated and it was deemed necessary to change the antibiotic
therapy to carbapenem (imipenem) and fluoroquinolone, in addition
to intravenous hydro-electrolytic rebalancing. After three days of
antibiotherapy with fluoroquinolone and carbapenem, the patient's
general condition improved and he was moved from intensive care to
a regular infectious diseases ward, with the indication for further
treatment and continuous monitoring. In total, the patient was
under this treatment for 10 days and standard therapy was also
provided according to the symptoms during this time. Eventually,
the patient had a favorable outcome.
Initially, despite the fact that the patient was
under appropriate antibiotics therapy started based on the
antibiogram results, his evolution was not favourable. For this
reason, it was assumed that the patient may be superinfected with a
multi-drug resistant nosocomial bacterium using his bed sores as a
gateway. After changing the antibiotherapy to carbapenemes and
floroquinolones, the patient's condition finally began to improve.
Of note, the patient's cultures for multi-drug resistant bacteria
were negative, but this does occur and such results do not
invalidate the existence of these bacteria. Clinicians are trained
to assess the patient's signs, symptoms and laboratory results
globally, which in this case proved life-saving. The patient was
clinically reassessed seven days after discharge, when the results
were satisfactory, and the liver and renal functions were back to
normal. This was the patient's final examination in our hospital,
after which further monitoring and care were provided by the
patient's general practitioner, as needed.
Discussion
The gastrointestinal tract hosts a multitude of
bacteria that form the intestinal microbiota. Clostridium
species dominate this commensal, normally innocuous microflora,
ensuring intestinal homeostasis, yet they also have the potential
to cause serious harm. Case reports discussing infection with C.
paraputrificum specifically are scarce in the literature. Only
14 such cases were described between 1961 and 2023, one implication
being that related mortality cannot be accurately established
(2,4).
C. paraputrificum has been identified as a
source of infection in patients with AIDS (1), colon necrosis (1), intestinal neoplasm (2,3),
appendicitis (4), liver abscess
(5), and septic arthritis (6). One peculiarity of the present case was
that the abdominopelvic CT with contrast excluded gastrointestinal
causes, unlike most other cases published so far (1-5).
Usually, risk factors for this particular infection are malignancy,
HIV infection, diabetes, alcohol consumption and gastrointestinal
pathology (1). Poor living
conditions were the more likely contributors to the patient's
bacterial infection in the present case, possibly enhanced by
alcohol consumption. Regarding the patient's history of heart
failure, it was not considered a risk factor for this particular
infection, although future case reports may provide evidence to
suggest otherwise.
With regard to treatment options, most studies
describe the resistance of C. paraputrificum to clindamycin
(1,2,4).
Instead, previous cases reported by others showed 99% of the
isolates were sensitive to metronidazole and 90% sensitive to
penicillin, which suggests that the initiation of empirical
treatment should include metronidazole to reduce the risk of
therapeutic failure.
In the case presented by Intra et al
(2), the patient had a substantial
gastrointestinal neoplasm (the pathology most related to C.
paraputrificum infection) and the isolated strain was resistant
to penicillin G. In a case reported by Mostel et al
(4), the patient was treated with
ampicillin-sulbactam and the evolution was good. Related to our
experience, this suggests that the sensitivity of C.
paraputrificum may differ among regions and may also depend on
the patient's comorbidities.
Another interesting aspect is that, usually, such
bacteria are not isolated in the hemoculture, and even when
clostridial infections are specifically considered,
Clostridioides difficile is the most common culprit and just
1% of cases are due to Clostridium paraputrificum (2). Establishing a definitive diagnosis
proved a formidable challenge for us, as no such case had been
previously encountered at our department. The patient's state
aggravated despite receiving antibiotic treatment that should have
been effective according to the antibiogram, so a nosocomial
infection caused by multidrug-resistant skin bacteria would have
appeared more likely, considering the patient's bedsores and the
hospitalization period.
It is noteworthy that the patient of the present
study was economically impoverished and living in precarious
conditions, while also being a long-term alcohol user. A social
background of poverty and hardship can have substantial
implications on health, including a compromised immune system
facilitating infection with bacteria that only rarely affect
patients with certain predisposing pathologies. Our sharing of this
case with the scientific community is, in a sense, an open
invitation to assess the role of socio-economic factors in rare
bacterial infections.
In conclusion, C. paraputrificum is an
uncommon species of Clostridium and it is rarely evidenced
in patients' cultured samples. The present study reported an
unusual case of C. paraputrificum infection in a patient who
developed septic shock while under therapy with penicillin G and
metronidazole. To the best of our knowledge, this is the first
reported case without any associated gastrointestinal pathology,
and where a social background of poverty may have been a risk
factor instead.
Prior reviews and studies recommend metronidazole as
empirical treatment (1-6),
yet the patient of the present study did not show any improvement
while on it, so metronidazole may not be effective in every case.
Switching to carbapenem produced good outcomes, including recovery
from shock and normalization of renal function, mental status and
mobility, suggesting that carbapenem could be considered as the
first line of treatment in similar cases. Further research is
needed to establish the impact of bacteremia with C.
paraputrificum, as well as the definition of the pathogenesis
and the investigation of risk factors.
Acknowledgements
Not applicable.
Funding
Funding: This research received no external funding.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Conceptualization: CM, CEF and BBM; methodology: AR
and CEF; software: AR; validation: CEF, BBM, ERIB and CM;
investigation: CEF, EM, BBM and LM; resources: LM; writing-original
draft preparation: CEF and BBM; writing-review and editing: CM, MO
and CEF; visualization: MO and CM; supervision: MO and CM. BBM and
CEF confirm the authenticity of all the raw data. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent to
participate in this study.
Patient consent for publication
The patient provided written informed consent for
the publication of this case report and all accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shinha T and Hadi C: Clostridium
paraputrificum Bacteremia Associated with Colonic Necrosis in a
Patient with AIDS. Case Rep Infect Dis. 2015(312919)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Intra J, Milano A, Sarto C and Brambilla
P: A rare case of Clostridium paraputrificum bacteremia in a
78-year-old Caucasian man diagnosed with an intestinal neoplasm.
Anaerobe. 66(102292)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rodríguez RT, Solís Marquínez MN, Álvarez
MDCC, Fernández Suárez J, Fernández Domínguez J, Rodríguez BI and
Rodríguez Álvarez FJ: Clostridium paraputrificum bacteremia in a
64-year-old woman with colon carcinoma. Anaerobe.
81(102716)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mostel Z, Hernandez A and Tatem L:
Clostridium paraputrificum bacteremia in a patient with presumptive
complicated appendicitis: A case report. IDCases.
27(e01361)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kwon YK, Cheema FA, Maneckshana BT, Rochon
C and Sheiner PA: Clostridium paraputrificum septicemia and liver
abscess. World J Hepatol. 10:388–395. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vijayvargiya P, Garrigos ZE, Rodino KG,
Razonable RR and Abu Saleh OM: Clostridium paraputrificum septic
arthritis and osteomyelitis of shoulder: A case report and review
of literature. Anaerobe. 62(102105)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Simůnek J, Kopecný J, Hodrová B and
Bartonová H: Identification and characterization of Clostridium
paraputrificum, a chitinolytic bacterium of human digestive tract.
Folia Microbiol (Praha). 47:559–564. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Evvyernie D, Yamazaki S, Morimoto K,
Karita S, Kimura T, Sakka K and Ohmiya K: Identification and
characterization of Clostridium paraputrificum M-21, a
chitinolytic, mesophilic and hydrogen-producing bacterium. J Biosci
Bioeng. 89:596–601. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schroeter J, Wilkemeyer I, Schiller RA and
Pruss A: Validation of the microbiological testing of tissue
preparations using the BACTEC™ blood culture system. Transfus Med
Hemother. 39:387–390. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vrioni G, Tsiamis C, Oikonomidis G,
Theodoridou K, Kapsimali V and Tsakris A: MALDI-TOF mass
spectrometry technology for detecting biomarkers of antimicrobial
resistance: Current achievements and future perspectives. Ann
Transl Med. 6(240)2018.PubMed/NCBI View Article : Google Scholar
|