1. Background
Cell death has essential roles in normal
physiological processes, as well as in disease pathology. Various
physiological phenomena, including embryonic development and the
immune system's selection of B and T cells, necessitate cell death
for their normal functioning. Normally, the clearance of dead cells
operates seamlessly, but this system may become overwhelmed when a
substantial number of cells die suddenly and accumulate, as
observed during infections, chronic inflammation and tissue damage
(1). Over recent decades,
researchers worldwide have identified numerous forms of cell death,
categorizing them into accidental cell death (ACD) and regulated
cell death (RCD) based on their underlying mechanisms. ACD, caused
by severe chemical, physical or mechanical stress, unfolds
uncontrollably, whereas RCD can be modulated through
pharmacological or genetic interventions (2). RCD, whether occurring under
physiological or pathological conditions, has a crucial role in
maintaining organismal homeostasis (3). Various forms of RCD occur
spontaneously in the absence of external perturbations as a
fundamental aspect of developmental processes or tissue turnover
and are often referred to as programmed cell death (4). Recent investigations have
characterized RCD into various modalities, encompassing programmed
necrosis, intrinsic apoptosis, extrinsic apoptosis, ferroptosis,
autophagy-dependent cell death, cellular pyroptosis, parthanatos,
entotic cell death, netotic cell death, lysosome-dependent cell
death, immunogenic cell death and necrosis driven by mitochondrial
permeability transition (3). Among
these, ferroptosis is an iron-dependent mode of cell death that was
discovered in 2012 and is characterized by the activation of
reactive oxygen species (ROS), iron aggregation, mitogen-activated
protein kinase pathway activation, reduction of cystine uptake and
glutathione (GSH) depletion (5).
Iron, beyond its role as a regulator of enzyme activity, oxidizes a
diverse array of substrates, inducing biological damage (6). Iron-mediated oxidative stress is a
central mechanism in ferroptosis that causes an overwhelming
accumulation of lethal lipid ROS (7,8).
Ferroptosis is therefore an iron-dependent modality of regulated
cell death characterized by an overwhelming iron-dependent
accumulation of lethal lipid ROS (9). With the ongoing scrutiny of
ferroptosis, evidence is accumulating that underscores its
involvement in iron or ROS-related diseases, including cancer,
neurodegenerative disorders, infections and inflammatory conditions
(10,11).
Head and neck cancer (HNC) comprises a heterogeneous
array of tumors that originate from the mucosal linings of the
upper aerodigestive tract and constitutes a significant global
health burden. With >880,000 new cases and >450,000 deaths
annually, HNC ranks as the sixth most prevalent cancer worldwide.
Among HNC, head and neck squamous cell carcinoma (HNSCC) is the
predominant subtype, accounting for >90% of HNC cases.
Characterized by rapid progression, extensive infiltration and poor
prognosis, HNSCC poses considerable therapeutic challenges
(12). Currently, the treatment
strategies for HNC are mainly based on radiotherapy, chemotherapy
and surgery (13). Long-standing
evidence dating back to the late 1950s links tobacco use to HNSCC
(14). Chronic heavy alcohol
consumption is another independent risk factor for HNC,
particularly HNSCC, and frequent alcohol consumption also
potentiates the carcinogenic effect of tobacco (15). Furthermore, the escalating incidence
of oropharyngeal cancers, attributed to human papillomavirus
infection, adds complexity to HNSCC epidemiology (16). Despite advances in therapeutic
strategies, the 5-year survival rate for patients with HNSCC
remains dishearteningly low, hovering between 50 and 60%, with up
to 30% of patients experiencing cancer relapse and treatment
failure (17). Although the median
age at diagnosis is typically ~60 years, a concerning trend has
emerged with a rising incidence of HNSCC among adults younger than
45 years, primarily attributable to increased rates of
oropharyngeal cancers associated with oncogenic human
papillomavirus (18).
Of note, dual consequences of ferroptosis have
emerged in tumorigenesis and tumor therapy, contingent upon tumor
type and stage. Iron, a pivotal nutrient for cell proliferation and
a cofactor for metabolic enzymes, can foster tumor initiation and
growth. Furthermore, ferroptosis may trigger tumorigenesis by
increasing the inflammatory response at an early stage (19). Conversely, recent experimental
endeavors underscore the tumor-suppressive effects of ferroptosis
inducers across diverse cancer models, highlighting the potential
of ferroptosis as a promising target for anti-cancer strategies
(20).
In recent years, numerous studies have revealed
abnormalities in ferroptosis in various tissues of different cancer
types and have demonstrated that certain drugs or natural compounds
can induce ferroptosis in cancer (21). In addition, increased levels of
transferrin (Tf) receptor (TFR)C1, which is responsible for
cellular iron uptake, and decreased abundance of Tf, which is
responsible for iron efflux, can lead to high intracellular levels
of iron ions and the induction of ferroptosis in HNSCC cells.
Furthermore, the TFRC gene is located in a genomic region (3q29)
that is frequently amplified in HNSCC (22). HNC is a complex cancer, for which
new drugs, combination therapies and prognosis predictors need to
be explored in depth. Targeting ferroptosis has potential in the
anti-cancer field; therefore, exploring the role of ferroptosis in
HNC is likely to provide a new direction for the diagnosis and
treatment of HNC. However, despite the promising findings obtained
to date, the relationship between ferroptosis and HNC remains
relatively unexplored, with few studies addressing this nexus. The
present review aimed to consolidate the existing understanding of
ferroptosis, encompassing its physiological mechanisms and its
involvement in HNC pathogenesis, treatment and prognosis. This
review aims to provide a comprehensive resource to inform future
studies investigating the intricate interplay between ferroptosis
and HNC.
2. Normal mechanisms of ferroptosis
Ferroptosis, a finely orchestrated process, revolves
around three pivotal pathways: Iron homeostasis, GSH regulation
(including its homeostasis and redox regulation), and lipid
metabolism. GSH regulation and lipid metabolism both involve
glutamate and interact with each other, and both can ultimately
influence ROS accumulation to regulate ferroptosis. The iron
homeostasis pathway regulates ferroptosis by controlling iron ion
levels. However, certain pathways, such as the nuclear factor
erythroid 2-related factor 2 (Nrf2)-related pathway, are associated
with all three pathways.
Iron homeostasis
Recent findings have illuminated the central role of
iron-dependent lipid peroxidation in ferroptosis, which hinges on
the peroxidation of phospholipids containing polyunsaturated fatty
acid (PUFA) moieties in conjunction with iron homeostasis. The
induction of ferroptosis entails specific pathways involving
redox-active iron and iron-dependent peroxidation enzymes (23). Iron metabolism is intricately
governed by hepatic mechanisms, which control systemic iron
homeostasis by producing and secreting factors pivotal for its
regulation. Key proteins, such as Tf, ferritins, hepcidin and
ferroportin (FPN), orchestrate this intricate regulation of iron
homeostasis to provide redox control and maintenance of the
systemic iron equilibrium (24).
The hepcidin-FPN axis is a linchpin in governing extracellular iron
homeostasis in both physiological and pathological states (25).
As an indispensable cofactor for various enzymes
participating in redox reactions, the dual existence of iron as
ferrous iron (Fe2+) and ferric iron (Fe3+)
underscores its significance. Fe3+ binds to Tf in the
serum and is then recognized by TFRC in the cell membrane. Ferritin
is a cytoplasmic iron storage protein composed of two subunits,
ferritin heavy chain 1 (FTH1) and ferritin light chain, which
regulate the storage of ferrous iron in cells. The process of
ferritinophagy is the degradation of FTH1 mediated by nuclear
receptor coactivator 4 (NCOA4) in autophagosomes, leading to the
release of iron bound by ferritin into free iron, which increases
the iron content and promotes ferroptosis. In addition, hepcidin, a
central regulatory molecule involved in systemic iron homeostasis,
is synthesized in hepatocytes and other cells and released into the
circulation. It blocks iron release from duodenal cells and
macrophages by binding to the iron export protein (FPN), a
transmembrane protein whose function is to transport iron from the
cell into the plasma. Under normal conditions, through the action
of FPN, ferrous iron is transported to the extracellular
environment and further oxidized to trivalent iron (26). In addition, Nrf2 is a regulatory
factor for cell redox balance and a protective anti-oxidant
response, as well as regulating glutamine metabolism related to
ferroptosis (27). Wei et al
(28) found that activation of the
Nrf2/heme oxygenase (HO)-1 pathway can increase Fe2+
levels in cancer cells and induce ferroptosis. The regulation of
iron homeostasis is therefore crucial in ferroptosis.
GSH regulation
In addition to processes related to iron
homeostasis, ferroptosis is influenced by GSH homeostasis and redox
regulation, a regulatory mechanism associated with important
cellular pathways. GSH is the essential reducing substrate for
glutathione peroxidase 4 (GPX4) and is crucial for inhibiting
ferroptosis. GSH is essential for life as evidenced by the
lethality caused by silencing γ-glutamate-cystine ligase, the
rate-limiting enzyme in GSH synthesis (29).
The impact of GSH levels on ferroptosis emerged from
the use of a ferroptosis inducer, erastin, which lowers the
intracellular level of GSH. In sensitive cells, this activates a
form of death morphologically identical to that induced by GPX4
knockout (9). The intracellular GSH
concentration is meticulously regulated through a multifaceted
homeostatic mechanism, wherein GSH steady-state levels are
modulated by the kinetics of specific enzyme reactions (30). The GSH concentration is controlled
by the rates of oxidation, conjugation, extrusion, uptake of
thiol-containing precursors and re-synthesis. The cystine-GSH-GPX4
axis is a pivotal system in combatting ferroptosis in mammals. In
the presence of catalytically active iron, the GSH/GPX4 axis
assumes a critical role in neutralizing the generation of specific
phospholipid hydroperoxides. The upstream component of this system
is the cystine-glutamate countertransporter (System Xc-), which
consists of the transporter protein solute carrier family 7 member
11 (SLC7A11), linked via a disulfide bond to the regulatory
subunit, SLC3A2. Operating as an amino acid transporter on the cell
membrane, System Xc-imports cystine while exporting glutamate,
thereby bolstering GSH synthesis (31). System Xc-transports intracellular
glutamate to the extracellular space and extracellular cystine into
the cell, which is then transformed into cystine for GSH synthesis.
GPX4 reduces the endogenous neutralization of lipid peroxide to
lipid alcohol, ultimately reducing ROS accumulation (21). Inhibition of system Xc-activates
ferroptosis in cell lines because of a lack of intracellular
cystine (32). Inhibition of
SLC7A11, a subunit of system Xc-, by drugs such as erastin leads to
GSH depletion and consequent inactivation of GPX4, which causes
lipid peroxidation-mediated ferroptosis. In addition, Wu et
al (33) found that Nrf2
enhances the resistance to ferroptosis in breast cancer cells by
upregulating SLC7A11 levels. Hence, the intricate interplay of GSH
homeostasis and redox regulation is a critical determinant in
ferroptosis.
Lipid metabolism
Ferroptosis is intricately intertwined with
glycolipid metabolism, encompassing glycolipid synthesis, storage,
degradation, peroxidation, transport and β-oxidation. A pivotal
step in ferroptosis promotion involves the peroxidation of PUFAs at
the bis-allylic position (34).
Under conditions of oxidative stress, increased synthesis of PUFA
promotes lipid peroxidation. PUFAs can be classified according to
the position of the first double bond on the methyl terminal end as
ω-6 [e.g., linoleic acid (LA; 18:2), γ-LA (GLA; 18:3), dihydro-GLA
(20:3), arachidonic acid (AA; 20:4) and adrenic acid (AdA; 22:4)]
or ω-3 [e.g., α-LA (18:3), eicosapentaenoic acid (20:5) and
docosahexaenoic acid (22:6)], and have amphiphilic properties to
maintain the fluidity of the cell membrane. Among them, AA and AdA
are the main substrates of lipid peroxidation in ferroptosis
(35).
The involvement of AA/AdA derivatives in ferroptosis
requires the crucial roles of acyl-coenzyme A (CoA) synthetase
long-chain family member 4 (ACSL4) and lysophosphatidylcholine
acyltransferase 3 (LPCAT3). ACSL4 catalyzes the conversion of free
AA/AdA to CoA, facilitating their esterification into
phospholipids, whereas LPCAT3 catalyzes the biosynthesis of
AA/AdA-CoA and membrane phosphatidylethanolamine (PE), thereby
forming AA/AdA-PE (36).
Consequently, inhibition of ACSL4 or LPCAT3 attenuates ferroptosis
across various conditions. Of note, both ω-6 and ω-3 PUFAs
influence ferroptosis, with ω-6 PUFAs restoring ferroptosis
sensitivity in ACSL4-deficient cells (37), while dietary supplementation with a
mix of ω-6 and ω-3 PUFAs promotes ferroptosis-related inflammatory
bowel disease in mice (38). In
addition, Delesderrier et al (39) reported that ω-6 PUFAs have
relatively high susceptibility to lipid peroxidation and may
therefore be involved in ferroptosis. By contrast, ω-3 PUFAs
promote intracellular antioxidant synthesis and reduce the
formation of hydroperoxides, which induce ferroptosis.
In addition, the activity of the arachidonate
lipoxygenase (ALOX) family, consisting of six members (ALOXE3,
ALOX5, ALOX12, ALOX12B, ALOX15 and ALOX15B), plays a tissue- or
cell-dependent role in mediating the peroxidation of PUFAs to
produce lipid peroxides (AA/AdA-PE-OOHs), which leads to
ferroptosis. These lipid peroxides undergo secondary reactions,
yielding electrophilic oxidative truncations prone to interacting
with protein nucleophilic sites, thereby forming a network
regulating ferroptosis sensitivity (36).
Lipid storage and degradation are also intimately
linked to ferroptosis. Selective lipid droplet degradation via
lipid autophagy, mediated by RAB7A, member RAS oncogene family,
augments free fatty acid production, thereby promoting lipid
peroxidation and subsequent ferroptosis (40). Tumor protein D52-dependent lipid
storage inhibits RSL3 (a selective ferroptosis inducer)-induced
ferroptosis in hepatocellular carcinoma (HCC) cells (40). Periplasmicin 2, a member of the
lipodropin family, also inhibits ferroptosis in gastric cancer
cells (41). Acetyl-CoA carboxylase
α, a central enzyme involved in fatty acid β-oxidation and fatty
acid biosynthesis, plays an environmentally-dependent role in
promoting ferroptosis, suggesting that β-oxidation of lipids is
also associated with ferroptosis (36). Magtanong et al (42) identified monounsaturated fatty acids
(MUFAs) as suppressors of ferroptosis that act downstream of or in
parallel with GPX4 and that also block the accumulation of plasma
membrane lipid ROS. At the same time, exogenous MUFAs reduce PUFA
incorporation into phospholipids, ultimately leading to a
ferroptosis-resistant cellular state.
Besides, the redox regulation of ferroptosis
includes not only the GSH/GPX4 axis, but also ferroptosis
suppressor protein 1 (FSP1)-ubiquinol (CoQH2), cyclohydrolase-1
(GCH1)-tetrahydrobiopterin (BH4). The activity of FSP1 is not
affected by intracellular GSH levels, GPX4 activity or p53 protein
status. Instead, its activity is regulated by extra-mitochondrial
ubiquinone (also known as coenzyme Q10 or CoQ10), a molecule that
protects cells from iron-mediated oxidative damage. CoQ10 is
effective in preventing lipid peroxidation in its reduced state,
CoQH2, and FSP1 promotes the regeneration of CoQ10 by a mechanism
that is dependent on NADPH (43).
In addition, guanosine-5'-triphosphate (GTP) is involved in a key,
GPX4-independent regulatory system for iron oxidation via the
GCH1-BH4 pathway. BH4 is biosynthesized by GTP via three enzymatic
steps catalyzed by GCH1, 6-pyrrolyltetrahydropterin synthetase and
heptapterin reductase, respectively. GCH1 plays a rate-limiting
role and its expression level has a major impact on the ability of
cells to resist iron toxicity. If GCH1 expression is genetically or
pharmacologically inhibited, it leads to BH4 deficiency, which in
turn causes intracellular peroxide accumulation and iron
deposition. Conversely, overexpression of GCH1 increases BH4
biosynthesis, thereby decreasing ROS production (43). From these findings, lipid metabolism
emerges as a pivotal player in the intricate orchestration of
ferroptosis.
3. Ferroptosis and HNC
Ferroptosis in the prognosis of
HNC
Examination of the GEPIA database revealed the
survival rate of patients with tongue squamous cell carcinoma
(TSCC) with high levels of brain abundant membrane attached signal
protein 1 (BASP1) to be low, and that of patients with TSCC with
low BASP1 levels to be high. Subsequent statistical analysis of
paraffin-embedded TSCC tissues revealed that the level of BASP1 was
positively associated with the TSCC clinical stage and T-grade,
indicating its potential use as a prognostic indicator (44). BASP1 is an inhibitor of ferroptosis
in HNSCC cells that influences the immuno-oncological
microenvironment and is a potential predictive biomarker for
anti-tumor immunotherapy. Combining induced ferroptosis with BASP1
inhibition is a promising therapeutic strategy for overcoming
immunotherapy resistance (45). To
date, these biomarkers regarding the relationship between HNC and
ferroptosis have not been validated and used in a clinical setting,
and their sensitivity and specificity in the prognosis of HNC
cannot be determined. In addition, a 16-DNA methylation signature
associated with ferroptosis has been developed as a biomarker to
assess the prognosis of patients with HNSCC, including those with
OSCC (46). Furthermore, Wu et
al (47) have described a novel
long non-coding RNA signature related to ferroptosis that is
independent of expression levels and that offers potential for
predicting patient prognosis and clinical applications in
HNSCC.
Using a nasopharyngeal carcinoma (NPC)-affiliated
HNSCC database, the role of ferroptosis-related genes in HNSCC and
NPC was investigated and it was found that ferroptosis-related
genes may affect the tumor immune microenvironment in NPC.
Screening identified the ferroptosis-related gene autophagy protein
5 (ATG5), as a significant independent prognostic marker. High
expression of ATG5 in patients with HNSCC is associated with worse
overall survival and poorer response and survival rates following
immune checkpoint inhibition therapy. Consequently, ATG5 is a key
immune infiltration-associated ferroptosis-associated factor that
has potential to be a prognostic biomarker and therapeutic target
in NPC and HNSCC (48).
Overall, studying the correlation between the
expression levels of ferroptosis-related genes and prognosis,
alongside the construction of precise prognostic models, holds
promise for providing diversified treatment options for patients
with HNC.
Ferroptosis in targeted therapy for
HNC
The main treatments currently available for HNC
include radiotherapy (RT), chemotherapy and surgery. In RT, ROS are
produced when water molecules absorb ionizing radiation. These ROS
further interact with PUFA, triggering a process of lipid
peroxidation and peroxidation of membrane phospholipids, which may
ultimately lead to ferroptosis (34). It may thus be expected that RT can
inhibit tumor progression by inducing iron-associated death.
Furthermore, RT resistance may decrease the effectiveness of RT, so
it is necessary to increase the radiosensitivity. Feng et al
(49) suggested that ferroptosis
inducers enhance the radiosensitivity of cells by inducing
ferroptosis, blocking the activation of the ferroptosis defense
system, increasing the total intracellular iron content, promoting
the production of ROS, decreasing the concentration of glutathione
and increasing the radiation resistance to lipid peroxidation in
cancer cells. Ma et al (50)
showed that the use of iron-oxide nanocarriers could enhance the
anti-tumor effect of cisplatin while reducing the side effects
produced by ROS. Cisplatin can produce hydrogen peroxide
(H2O2) in the cytoplasm through a series of
reactions in the tumor microenvironment (TME). Subsequently,
H2O2 can be converted to toxic hydroxyl
radicals via the Fenton reaction catalyzed by iron ions, which
triggers apoptosis and ferroptosis in tumor cells. It may be
assumed that ferroptosis, as a newly discovered form of cell death,
can be combined with other cancer therapeutic modalities to inhibit
tumor progression, which will need to be explored by future
experiments in HNC. However, the majority of HNC-based studies
investigating the role of ferroptosis in HNC treatment involve
targeted therapy.
In HNSCC, upregulated Nrf2 can directly bind to the
promoter region of SLC7A11, thereby inducing System Xc-. This
facilitates the translocation of extracellular cystine into the
cell, subsequently elevating GSH levels and upregulating GPX4
expression. Elevated GPX4 then inhibits ROS and lipid peroxidation
levels, ultimately diminishing the sensitivity of HNSCC cells to
ferroptosis. This leads to increased resistance to RT (51). Modulation of the
Nrf2/SLC7A11/ferroptosis pathway is therefore a potential
therapeutic avenue to enhance HNSCC sensitivity to RT.
Analysis of The Cancer Genome Atlas database
revealed elevated CDH4 expression in HNSCC and oral squamous cell
carcinoma (OSCC) tissues compared with normal tissues. In the CDH4
overexpression group, GSH was elevated, oxidized glutathione (GSSG)
was decreased and the GSH/GSSG ratio was elevated, which is
consistent with the ferroptosis mechanism. These findings confirmed
that CDH4 can decrease the sensitivity of cells to iron death and
suggested that the effect of CDH4 on cell proliferation may be due
to its inhibition of ferroptosis. CDH4 enhances the
epithelial-mesenchymal transition (EMT) pathway, thereby
diminishing OSCC cell sensitivity to ferroptosis and promoting
tumor proliferation, invasion and metastasis (52). Conversely, ferroptosis significantly
affects the overall survival of patients with HNSCC, primarily by
regulating extracellular matrix structure, humoral immune response
and vascular smooth muscle contraction, potentially via modulation
of cancer stem cell proliferation.
Several other genes, including ACSL1, SLC39A14, TFRC
and homo sapiens prion protein, exhibit close associations
with ferroptosis, HNSCC development and long-term patient
prognosis, underscoring their potential as therapeutic targets
(53). Notably, GPX4 is a negative
regulator of ferroptosis and its inhibitors induce ferroptosis in
tumor cells. Compounds that directly inactivate GPX4 and promote
ferroptosis are considered class II ferroptosis agonists. For
instance, (1S,3R)-RSL3 is a ferroptosis inducer with selective
cytotoxic effects on tumor cells with RAS mutations, which occur in
several cancers, including colorectal cancer, embryonal carcinoma,
alveolar rhabdomyosarcoma and melanoma (54,55).
RSL3 and trigonelline promote ferroptosis in HNC cells by
inhibiting Nrf2 gene expression, which increases resistance to
ferroptosis (56). In addition,
artesunate and dihydroartemisinin (DHA), semi-synthetic derivatives
of the traditional Chinese medicine component artemisinin, can also
induce ferroptosis in HNSCC cells. Artesunate can overcome
ferroptosis resistance in HNSCC by inhibiting the Nrf2-ARE pathway
(57). By contrast, DHA has
anti-tumor activity against HNSCC cells by inhibiting the cell
cycle, inducing ferroptosis and apoptosis, and inhibiting
angiogenesis (58). Furthermore,
consistent with lipid-ROS accumulation and increased iron ions in
ferroptosis, photodynamic therapy for oral tongue squamous cell
carcinoma (OTSCC) with a supramolecular nanodrug consisting of the
ferroptosis inducer erastin and photosensitizer chlorine 6
demonstrated enhanced anti-cancer effects by alleviating hypoxia
and promoting ROS production. In addition, the ROS and
O2 concentration were increased in the tumor cells and
SLC7A11 expression, which is upregulated in OTSCC, was inhibited.
These findings indicate that the ferroptosis-promoting photodynamic
therapy approach significantly enhances anti-cancer effects by
alleviating hypoxia and promoting ROS production (59). Fig.
1 summarizes some of the factors that target HNC to promote
ferroptosis.
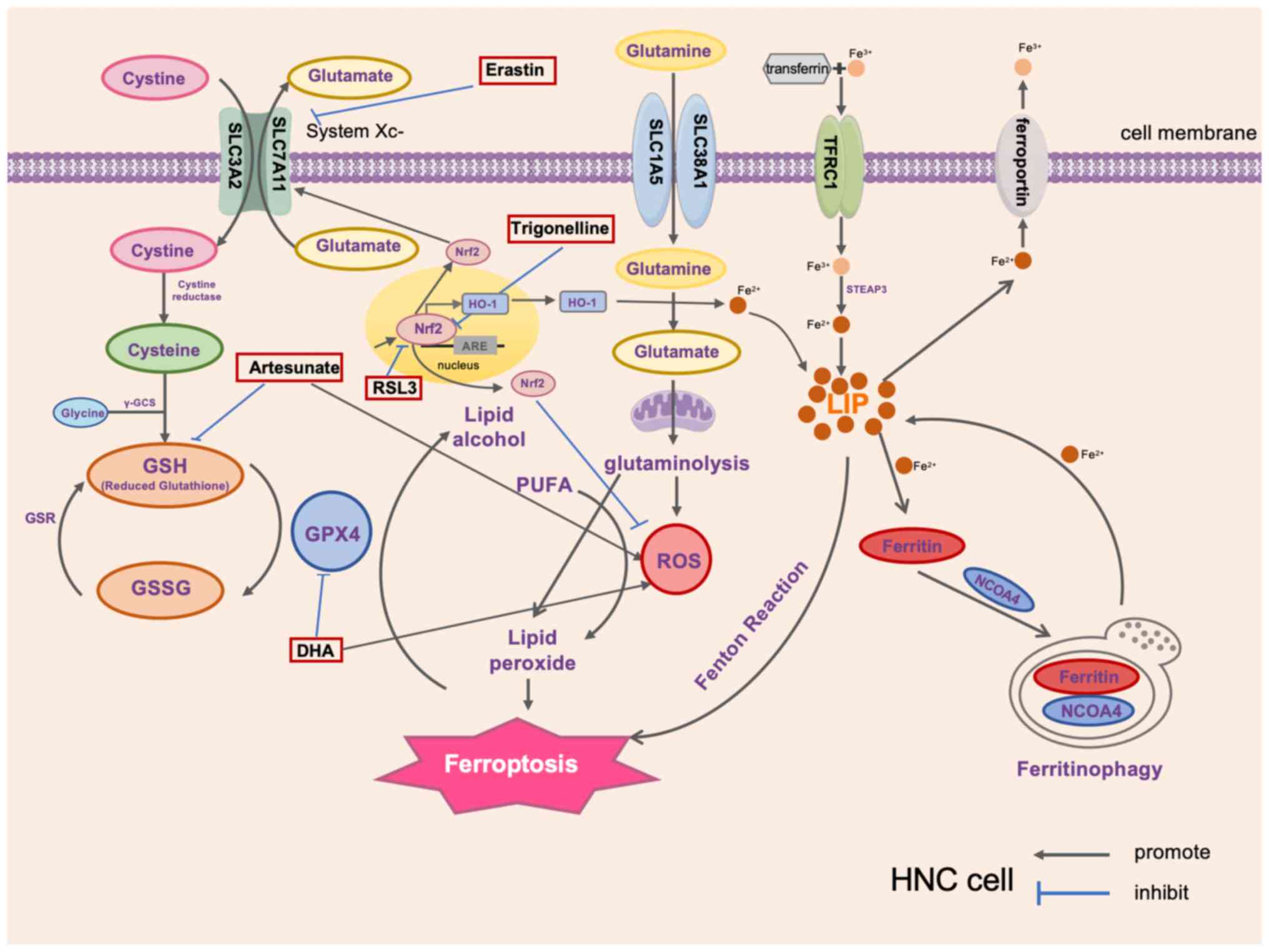 | Figure 1Critical ferroptosis factors in HNC
and therapeutic drugs that target ferroptosis. HNC, head and neck
cancer; SLC3A2, recombinant solute carrier family 3 member 2;
γ-GCS, γ-glutamylcysteine synthetase; GSR, GSH reductase; GSH,
glutathione; GSSG, oxidized GSH; GPX4, GSH peroxidase 4; Nrf2,
nuclear factor erythroid 2-related factor 2; ARE, antioxidant
response element; HO-1, heme oxygenase-1; PUFA, polyunsaturated
fatty acid; ROS, reactive oxygen species; TFRC1, transferrin
receptor 1; STEAP3, six-transmembrane epithelial antigen of
prostate; LIP, labile iron pool; NCOA4, nuclear receptor
coactivator 4; DHA, dihydroartemisinin; RSL3, a selective
ferroptosis inducer. |
Drugs that target ferroptosis can be combined with
radiotherapy, chemotherapy and immunotherapy for the treatment of
cancers (34,49,50).
It has been reported that mesenchymal cancer cells are
metastasis-prone cells that have been found to be highly sensitive
to ferroptosis (43). Therefore, it
may be speculated that inducing ferroptosis can inhibit the
metastatic spread of HNC. Raudenská et al (22) suggested that the mesenchymal
subtypes subgroup of HNSCC characterized by elevated expression of
EMT-related genes may be the most sensitive to ferroptosis. It may
be hypothesized that patients with HNC with elevated expression of
EMT-related genes may benefit more from ferroptosis-targeting
therapies. Future in-depth studies in this area will help develop
new drugs for HNC. In theory, targeting ferroptosis in HNC may be
combined with other treatments to improve HNC outcomes. It may be
speculated that criteria such as iron levels, ferroptosis-related
gene expression and mutations may be available to determine the
clinical applicability of ferroptosis-targeted therapy for cancer.
To date, the ferroptosis inducers sulfasalazine, altretamine,
sorafenib and statins have been approved by the Food and Drug
Administration as anticancer drugs for the treatment of cancers
(60). However, the potential
adverse effects of ferroptosis inducers during tumor therapy remain
elusive and future in-depth studies in this area are still needed.
In conclusion, further investigation into the expression of
ferroptosis-related genes in HNC tumor cells holds promise for
developing targeted therapeutic approaches aimed at inducing
ferroptosis, thereby offering a novel direction for HNC
treatment.
4. Conclusions and perspective
Ferroptosis, as a newly recognized form of cell
death, has become an important research field in tumor development
and treatment. In HNC, because cell death can act as a second
messenger to guide the immune system and the tissue
microenvironment to ensure tissue repair and homeostasis, the
various cell death types can have multiple effects on treatment
response (22). In HNC, however,
the interplay between the various cell death pathways has not been
identified. Both basic and clinical research has explored the role
of ferroptosis in cancers, to advance cancer prevention, diagnosis,
prognosis and treatment. Despite this growing interest, there
remains a notable scarcity of studies focusing on ferroptosis in
HNC. Current research predominantly centers on leveraging
ferroptosis to target and sensitize HNC cells to chemotherapy and
to unravel the underlying mechanisms.
It is evident that certain cells undergo adaptations
to evade ferroptosis and understanding these adaptations is of
paramount importance in identifying biomarkers sensitive to
ferroptosis. The identification of such biomarkers promises to
significantly augment our understanding of ferroptosis promotion in
tumors, including HNC. However, there are potential mechanisms by
which HNC cells may develop resistance to ferroptosis-inducing
therapies. To the best of our knowledge, studies applying
ferroptosis-inducing therapies to HNC are currently lacking,
raising doubts about the resistance pathway.
It has been shown that CD8+ T cells could
promote ferroptosis in mouse melanoma tumor cells during cancer
immunotherapy (61). Ferroptosis
can reduce the number of immune cells to suppress the immune
function of immune cells (62).
Combined use of cinnamaldehyde dimer, which causes depletion of
intracellular GSH in breast cancer cells, and sorafenib resulted in
a significant enhancement of ferroptosis in ‘cold’ tumors and
triggered a strong immune response in vivo (63). These studies have shown that
ferroptosis can both promote and impair immune function. Fan et
al (64) showed that inducing
cancer cells to undergo ferroptosis may promote the expression of
their immunogenicity and in turn the anticancer activity of immune
cells. In addition, in small cell lung cancer, the release of
interferon γ (IFNγ) from the CD8+ T cell populations
reduces SLC3A2 and SLC7A11 expression, thereby promoting lipid
peroxidation as well as ferroptosis of cancer cells (65). In HCC, inhibition of apolipoprotein
C-1, a key protein in lipid metabolism, can also promote M1
polarization via the ferroptosis pathway and reshape the tumor
immune microenvironment and improve anti-programmed cell death 1
immunotherapy for HCC (66).
However, the existing studies did not point out the relationship
between ferroptosis and immunotherapy for HNC and the role
regarding the association between HNC cells and surrounding stromal
and immune cells affecting ferroptosis. Ferroptosis may play a role
in immunotherapy for other cancers, and in the future,
ferroptosis-related immunogenicity could broaden the scope of
immunotherapy and provide personalized therapeutic options for
patients with HNC. Certain non-coding RNAs may also be involved in
ferroptosis: Huang et al (67) reported that long non-coding RNAs can
affect ferroptosis by modulating GPX4 activity, Fe2+
levels, cysteine metabolism and ROS levels. The impact of hypoxia
is a common feature of solid tumors and the hypoxic TME is of a
certain relevance to ferroptosis. The related pathways between
ferroptosis under hypoxia mainly include the Nrf2/HO-1 signalling
pathway and the p62/kelch-like ech-associated protein-1/Nrf2
signalling pathway. Meanwhile, certain factors also participate in
the occurrence of ferroptosis under hypoxia, such as
hypoxia-inducible factor-1, NCOA4 and divalent metal transporter
1(68). However, how non-coding
RNAs regulate ferroptosis and how the hypoxic TME influences
ferroptosis in HNC remains elusive.
As a more superficial tumor, HNC can be studied by
targeting ferroptosis and by combining ferroptosis with light
stimulation to further explore the clinical application of
photodynamic therapy. The study of ferroptosis and its regulation
with respect to cancer therapy has great potential to deliver
therapeutic advances; however, few studies (including basic and
clinical trials) related to ferroptosis in HNC have been reported
recently. In-depth studies from multiple perspectives, such as
epigenetics, gene mutation, TME and tumor immunity, are warranted
to understand the regulatory mechanism of ferroptosis in HNC cells.
Findings from such studies will provide new ideas and strategies
for the treatment of HNC.
Acknowledgements
The authors would like to acknowledge Dr Shuangping
Liu at the Chronic Disease Research Center (Medical College, Dalian
University, Dalian, Liaoning, China) for her assistance with the
editing and revising of the manuscript.
Funding
Funding: The present study was funded by the Bengbu Medical
University Science and Technology Programme (2022byzd164) and the
Scientific Research Fund Project of Anhui Medical University
(2023xkj195).
Availability of data and materials
Not applicable.
Authors' contributions
XW designed and organized this manuscript. KL and TS
wrote the basic sections of the manuscript. SX, WW and YF revised
the manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the nomenclature committee on cell death 2018. Cell Death Differ.
25:486–541. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Conradt B: Genetic control of programmed
cell death during animal development. Annu Rev Genet. 43:493–523.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang X, Huang Z, Xie Z, Chen Y, Zheng Z,
Wei X, Huang B, Shan Z, Liu J, Fan S, et al: Homocysteine induces
oxidative stress and ferroptosis of nucleus pulposus via enhancing
methylation of GPX4. Free Radic Biol Med. 160:552–565.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mahoney-Sánchez L, Bouchaoui H, Ayton S,
Devos D, Duce JA and Devedjian JC: Ferroptosis and its potential
role in the physiopathology of Parkinson's disease. Prog Neurobiol.
196(101890)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Johnson DE, Burtness B, Leemans CR, Lui
VWY, Bauman JE and Grandis JR: Head and neck squamous cell
carcinoma. Nat Rev Dis Primers. 6(92)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hammond EC and Horn D: Smoking and death
rates: Report on forty-four months of follow-up of 187,783 men. 2.
Death rates by cause. J Am Med Assoc. 166:1294–1308.
1958.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hashibe M, Brennan P, Benhamou S,
Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova
E, Fernandez L, et al: Alcohol drinking in never users of tobacco,
cigarette smoking in never drinkers, and the risk of head and neck
cancer: Pooled analysis in the international head and neck cancer
epidemiology consortium. J Natl Cancer Inst. 99:777–789.
2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mehanna H, Beech T, Nicholson T, El-Hariry
I, McConkey C, Paleri V and Roberts S: Prevalence of human
papillomavirus in oropharyngeal and nonoropharyngeal head and neck
cancer-systematic review and meta-analysis of trends by time and
region. Head Neck. 35:747–755. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Canning M, Guo G, Yu M, Myint C, Groves
MW, Byrd JK and Cui Y: Heterogeneity of the head and neck squamous
cell carcinoma immune landscape and its impact on immunotherapy.
Front Cell Dev Biol. 7(52)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sturgis EM and Cinciripini PM: Trends in
head and neck cancer incidence in relation to smoking prevalence:
An emerging epidemic of human papillomavirus-associated cancers?
Cancer. 110:1429–1435. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dai E, Han L, Liu J, Xie Y, Kroemer G,
Klionsky DJ, Zeh HJ, Kang R, Wang J and Tang D: Autophagy-dependent
ferroptosis drives tumor-associated macrophage polarization via
release and uptake of oncogenic KRAS protein. Autophagy.
16:2069–2083. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hassannia B, Vandenabeele P and Vanden
Berghe T: Targeting ferroptosis to iron out cancer. Cancer Cell.
35:830–849. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C
and Li B: Ferroptosis, a new form of cell death: Opportunities and
challenges in cancer. J Hematol Oncol. 12(34)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Raudenská M, Balvan J and Masařík M: Cell
death in head and neck cancer pathogenesis and treatment. Cell
Death Dis. 12(192)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24(449)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gao G, Li J, Zhang Y and Chang YZ:
Cellular iron metabolism and regulation. In: Chang YZ (ed). Brain
Iron Metabolism and CNS Diseases. Advances in Experimental Medicine
and Biology. Vol 1173. Springer Singapore, Singapore, pp21-32,
2019.
|
|
25
|
Rochette L, Gudjoncik A, Guenancia C,
Zeller M, Cottin Y and Vergely C: The iron-regulatory hormone
hepcidin: A possible therapeutic target? Pharmacol Ther. 146:35–52.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fujimaki M, Furuya N, Saiki S, Amo T,
Imamichi Y and Hattori N: Iron supply via NCOA4-mediated ferritin
degradation maintains mitochondrial functions. Mol Cell Biol.
39:e00010–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Do MT, Kim HG, Choi JH and Jeong HG:
Metformin induces microRNA-34a to downregulate the
Sirt1/Pgc-1α/Nrf2 pathway, leading to increased susceptibility of
wild-type p53 cancer cells to oxidative stress and therapeutic
agents. Free Radic Biol Med. 74:21–34. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wei R, Zhao Y, Wang J, Yang X, Li S, Wang
Y, Yang X, Fei J, Hao X, Zhao Y, et al: Tagitinin C induces
ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal
cancer cells. Int J Biol Sci. 17:2703–2717. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dalton TP, Chen Y, Schneider SN, Nebert DW
and Shertzer HG: Genetically altered mice to evaluate glutathione
homeostasis in health and disease. Free Radic Biol Med.
37:1511–1526. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Oestreicher J and Morgan B: Glutathione:
Subcellular distribution and membrane transport 1.
Biochem Cell Biol. 97:270–289. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rochette L and Vergely C: Coronary artery
disease: Can aminothiols be distinguished from reactive oxygen
species? Nat Rev Cardiol. 13:128–130. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Belalcázar AD, Ball JG, Frost LM,
Valentovic MA and Wilkinson J IV: Transsulfuration is a significant
source of sulfur for glutathione production in human mammary
epithelial cells. ISRN Biochem. 2013(637897)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu X, Liu C, Li Z, Gai C, Ding D, Chen W,
Hao F and Li W: Regulation of GSK3β/Nrf2 signaling pathway
modulated erastin-induced ferroptosis in breast cancer. Mol Cell
Biochem. 473:217–228. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kagan VE, Mao G, Qu F, Angeli JP, Doll S,
Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al: Oxidized
arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem
Biol. 13:81–90. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mayr L, Grabherr F, Schwärzler J,
Reitmeier I, Sommer F, Gehmacher T, Niederreiter L, He GW, Ruder B,
Kunz KTR, et al: Dietary lipids fuel GPX4-restricted enteritis
resembling Crohn's disease. Nat Commun. 11(1775)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Delesderrier E, Monteiro JDC, Freitas S,
Pinheiro IC, Batista MS and Citelli M: Can iron and polyunsaturated
fatty acid supplementation induce ferroptosis? Cell Physiol
Biochem. 57:24–41. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bai Y, Meng L, Han L, Jia Y, Zhao Y, Gao
H, Kang R, Wang X, Tang D and Dai E: Lipid storage and lipophagy
regulates ferroptosis. Biochem Biophys Res Commun. 508:997–1003.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun X, Yang S, Feng X, Zheng Y, Zhou J,
Wang H, Zhang Y, Sun H and He C: The modification of ferroptosis
and abnormal lipometabolism through overexpression and knockdown of
potential prognostic biomarker perilipin2 in gastric carcinoma.
Gastric Cancer. 23:241–259. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Magtanong L, Ko PJ, To M, Cao JY, Forcina
GC, Tarangelo A, Ward CC, Cho K, Patti GJ, Nomura DK, et al:
Exogenous monounsaturated fatty acids promote a
ferroptosis-resistant cell state. Cell Chem Biol. 26:420–432.e9.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu Y, Lu S, Wu LL, Yang L, Yang L and
Wang J: The diversified role of mitochondria in ferroptosis in
cancer. Cell Death Dis. 14(519)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li Y, Wu T, Jiao Z and Yang A: BASP1 is
up-regulated in tongue squamous cell carcinoma and associated with
a poor prognosis. Asian J Surg. 45:1101–1106. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pan X, Xu X, Wang L, Zhang S, Chen Y, Yang
R, Chen X, Cheng B, Xia J and Ren X: BASP1 is a prognostic
biomarker associated with immunotherapeutic response in head and
neck squamous cell carcinoma. Front Oncol.
13(1021262)2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xu Y, Hong M, Kong D, Deng J, Zhong Z and
Liang J: Ferroptosis-associated DNA methylation signature predicts
overall survival in patients with head and neck squamous cell
carcinoma. BMC Genomics. 23(63)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wu C, Liu F, Chen H, Liu Q, Song C, Cheng
K, Gao Z and Fan C: Identification of ferroptosis-related lncRNA
pairs for predicting the prognosis of head and neck squamous cell
carcinoma. J Oncol. 2022(7602482)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shi M, Du J, Shi J, Huang Y, Zhao Y and Ma
L: Ferroptosis-related gene ATG5 is a novel prognostic biomarker in
nasopharyngeal carcinoma and head and neck squamous cell carcinoma.
Front Bioeng Biotechnol. 10(1006535)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Feng Y, Li X, Yang B, Li M, Du Y, Wang J,
Liu S, Gong L, Li L and Gao L: The role of ferroptosis in
radiotherapy and combination therapy for head and neck squamous
cell carcinoma (review). Oncol Rep. 51(79)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ma P, Xiao H, Yu C, Liu J, Cheng Z, Song
H, Zhang X, Li C, Wang J, Gu Z and Lin J: Enhanced cisplatin
chemotherapy by iron oxide nanocarrier-mediated generation of
highly toxic reactive oxygen species. Nano Lett. 17:928–937.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Feng L, Zhao K, Sun L, Yin X, Zhang J, Liu
C and Li B: SLC7A11 regulated by NRF2 modulates esophageal squamous
cell carcinoma radiosensitivity by inhibiting ferroptosis. J Transl
Med. 19(367)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Xie J, Lan T, Zheng DL, Ding LC and Lu YG:
CDH4 inhibits ferroptosis in oral squamous cell carcinoma cells.
BMC Oral Health. 23(329)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Liu F, Tang L, Li Q, Chen L, Pan Y, Yin Z,
He J and Tian J: Single-cell transcriptomics uncover the key
ferroptosis regulators contribute to cancer progression in head and
neck squamous cell carcinoma. Front Mol Biosci.
9(962742)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Codenotti S, Poli M, Asperti M, Zizioli D,
Marampon F and Fanzani A: Cell growth potential drives ferroptosis
susceptibility in rhabdomyosarcoma and myoblast cell lines. J
Cancer Res Clin Oncol. 144:1717–1730. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sui X, Zhang R, Liu S, Duan T, Zhai L,
Zhang M, Han X, Xiang Y, Huang X, Lin H and Xie T: RSL3 drives
ferroptosis through GPX4 inactivation and ROS production in
colorectal cancer. Front Pharmacol. 9(1371)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Shin D, Kim EH, Lee J and Roh JL: Nrf2
inhibition reverses resistance to GPX4 inhibitor-induced
ferroptosis in head and neck cancer. Free Radic Biol Med.
129:454–462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li S, Liu Y, Li J, Zhao X and Yu D:
Mechanisms of ferroptosis and application to head and neck squamous
cell carcinoma treatments. DNA Cell Biol. 40:720–732.
2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lin R, Zhang Z, Chen L, Zhou Y, Zou P,
Feng C, Wang L and Liang G: Dihydroartemisinin (DHA) induces
ferroptosis and causes cell cycle arrest in head and neck carcinoma
cells. Cancer Lett. 381:165–175. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhu T, Shi L, Yu C, Dong Y, Qiu F, Shen L,
Qian Q, Zhou G and Zhu X: Ferroptosis promotes photodynamic
therapy: Supramolecular photosensitizer-inducer nanodrug for
enhanced cancer treatment. Theranostics. 9:3293–3307.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang H, Cheng Y, Mao C, Liu S, Xiao D,
Huang J and Tao Y: Emerging mechanisms and targeted therapy of
ferroptosis in cancer. Mol Ther. 29:2185–2208. 2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang W, Green M, Choi JE, Gijón M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al:
CD8+ T cells regulate tumour ferroptosis during cancer
immunotherapy. Nature. 569:270–274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen X, Kang R, Kroemer G and Tang D:
Ferroptosis in infection, inflammation, and immunity. J Exp Med.
218(e20210518)2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhou Z, Liang H, Yang R, Yang Y, Dong J,
Di Y and Sun M: Glutathione depletion-induced activation of
dimersomes for potentiating the ferroptosis and immunotherapy of
‘cold’ tumor. Angew Chem Int Ed Engl. 61(e202202843)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fan X, Fan YT, Zeng H, Dong XQ, Lu M and
Zhang ZY: Role of ferroptosis in esophageal cancer and
corresponding immunotherapy. World J Gastrointest Oncol.
15:1105–1118. 2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Niu X, Chen L, Li Y, Hu Z and He F:
Ferroptosis, necroptosis, and pyroptosis in the tumor
microenvironment: Perspectives for immunotherapy of SCLC. Semin
Cancer Biol. 86:273–285. 2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Huang Y, Wang S, Ke A and Guo K:
Ferroptosis and its interaction with tumor immune microenvironment
in liver cancer. Biochim Biophys Acta Rev Cancer.
1878(188848)2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Huang J, Wang J, He H, Huang Z, Wu S, Chen
C, Liu W, Xie L, Tao Y, Cong L and Jiang Y: Close interactions
between lncRNAs, lipid metabolism and ferroptosis in cancer. Int J
Biol Sci. 17:4493–4513. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Gao X, Hu W, Qian D, Bai X, He H, Li L and
Sun S: The mechanisms of ferroptosis under hypoxia. Cell Mol
Neurobiol. 43:3329–3341. 2023.PubMed/NCBI View Article : Google Scholar
|















