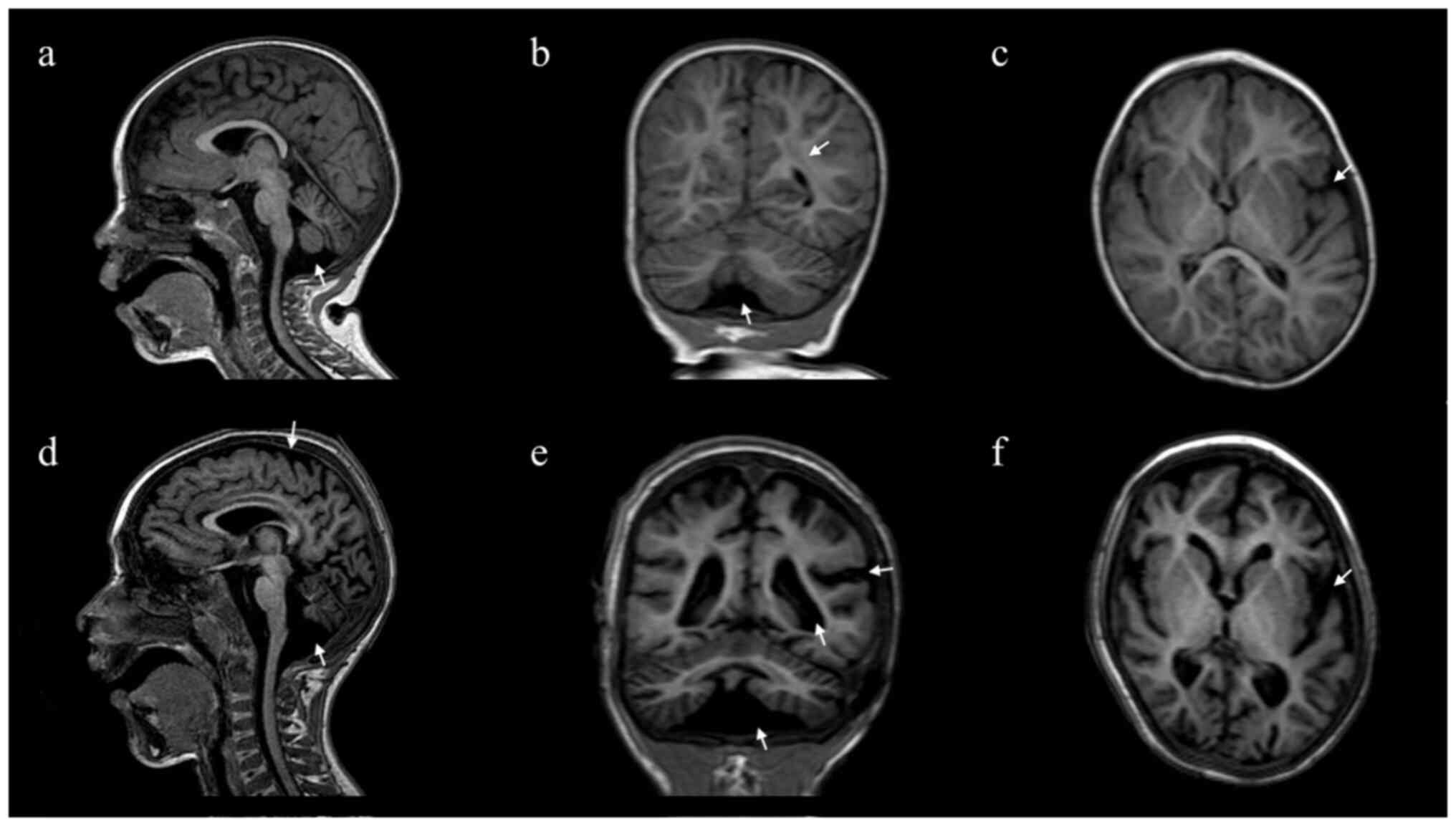
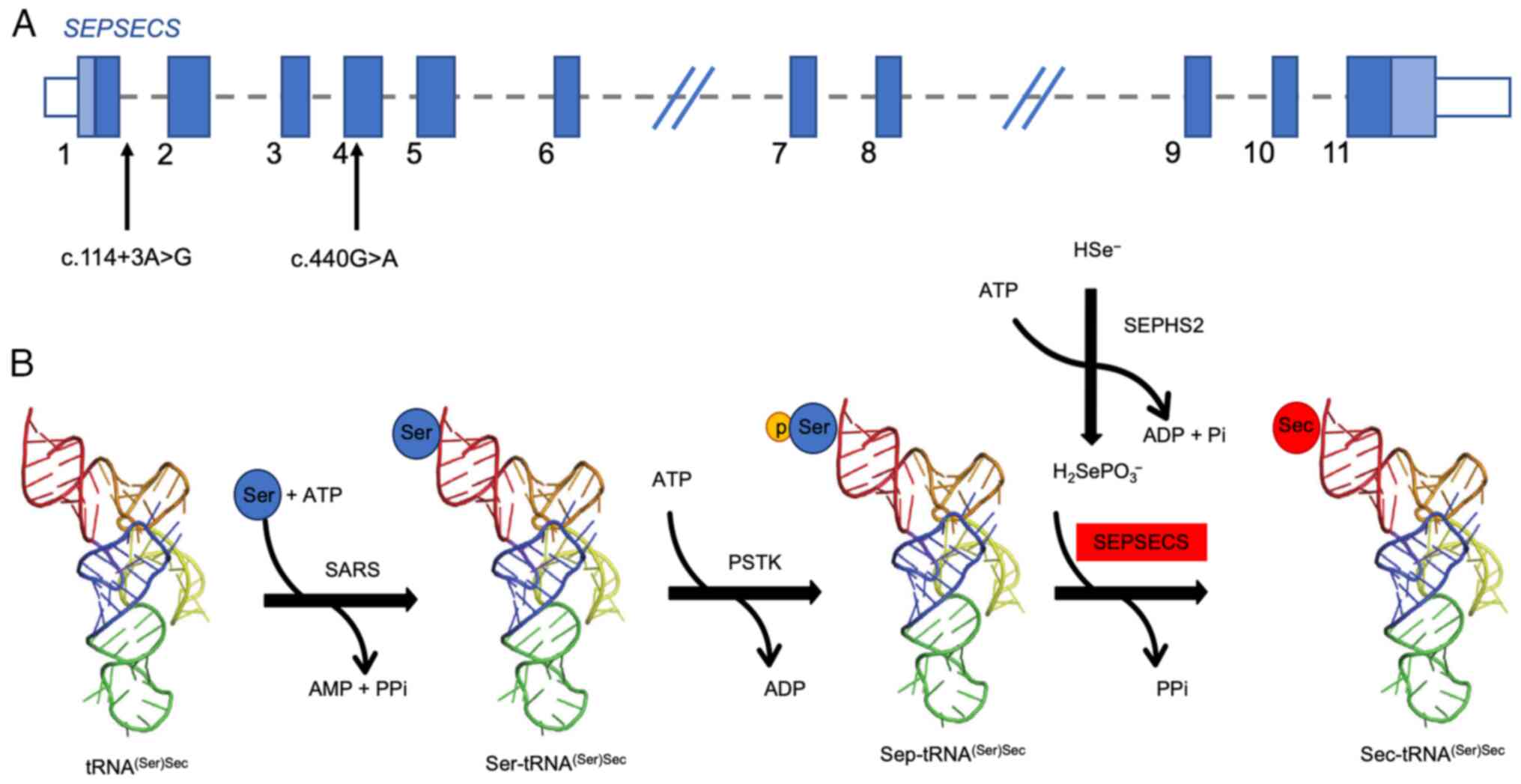
|
1
|
Ghasemi MR, Tehrani Fateh S, Moeinafshar
A, Sadeghi H, Karimzadeh P, Mirfakhraie R, Rezaei M, Hashemi-Gorji
F, Rezvani Kashani M, Fazeli Bavandpour F, et al: Broadening the
phenotype and genotype spectrum of novel mutations in
pontocerebellar hypoplasia with a comprehensive molecular
literature review. BMC Med Genomics. 17(51)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wirth EK, Bharathi BS, Hatfield D, Conrad
M, Brielmeier M and Schweizer U: Cerebellar hypoplasia in mice
lacking selenoprotein biosynthesis in neurons. Biol Trace Elem Res.
158:203–210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bellinger FP, Raman AV, Reeves MA and
Berry MJ: Regulation and function of selenoproteins in human
disease. Biochem J. 422:11–22. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ben-Zeev B, Hoffman C, Lev D, Watemberg N,
Malinger G, Brand N and Lerman-Sagie T: Progressive
cerebellocerebral atrophy: A new syndrome with microcephaly, mental
retardation, and spastic quadriplegia. J Med Genet.
40(e96)2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Agamy O, Ben Zeev B, Lev D, Marcus B, Fine
D, Su D, Narkis G, Ofir R, Hoffmann C, Leshinsky-Silver E, et al:
Mutations disrupting selenocysteine formation cause progressive
cerebello-cerebral atrophy. Am J Hum Genet. 87:538–544.
2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Makrythanasis P, Nelis M, Santoni FA,
Guipponi M, Vannier A, Béna F, Gimelli S, Stathaki E, Temtamy S,
Mégarbané A, et al: Diagnostic exome sequencing to elucidate the
genetic basis of likely recessive disorders in consanguineous
families. Hum Mutat. 35:1203–1210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alazami AM, Patel N, Shamseldin HE, Anazi
S, Al-Dosari MS, Alzahrani F, Hijazi H, Alshammari M, Aldahmesh MA,
Salih MA, et al: Accelerating novel candidate gene discovery in
neurogenetic disorders via whole-exome sequencing of prescreened
multiplex consanguineous families. Cell Rep. 10:148–161.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Anttonen AK, Hilander T, Linnankivi T,
Isohanni P, French RL, Liu Y, Simonović M, Söll D, Somer M,
Muth-Pawlak D, et al: Selenoprotein biosynthesis defect causes
progressive encephalopathy with elevated lactate. Neurology.
85:306–315. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu X, Petrovski S, Xie P, Ruzzo EK, Lu
YF, McSweeney KM, Ben-Zeev B, Nissenkorn A, Anikster Y, Oz-Levi D,
et al: Whole-exome sequencing in undiagnosed genetic diseases:
Interpreting 119 trios. Genet Med. 17:774–781. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iwama K, Sasaki M, Hirabayashi S, Ohba C,
Iwabuchi E, Miyatake S, Nakashima M, Miyake N, Ito S, Saitsu H and
Matsumoto N: Milder progressive cerebellar atrophy caused by
biallelic SEPSECS mutations. J Hum Genet. 61:527–531.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pavlidou E, Salpietro V, Phadke R,
Hargreaves IP, Batten L, McElreavy K, Pitt M, Mankad K, Wilson C,
Cutrupi MC, et al: Pontocerebellar hypoplasia type 2D and optic
nerve atrophy further expand the spectrum associated with
selenoprotein biosynthesis deficiency. Eur J Paediatr Neurol.
20:483–438. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Olson HE, Kelly M, LaCoursiere CM, Pinsky
R, Tambunan D, Shain C, Ramgopal S, Takeoka M, Libenson MH, Julich
K, et al: Genetics and genotype-phenotype correlations in early
onset epileptic encephalopathy with burst suppression. Ann Neurol.
81:419–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
van Dijk T, Vermeij JD, van Koningsbruggen
S, Lakeman P, Baas F and Poll-The BT: A SEPSECS mutation in a
23-year-old woman with microcephaly and progressive cerebellar
ataxia. J Inherit Metab Dis. 41:897–898. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hengel H, Buchert R, Sturm M, Haack TB,
Schelling Y, Mahajnah M, Sharkia R, Azem A, Balousha G, Ghanem Z,
et al: First-line exome sequencing in Palestinian and Israeli Arabs
with neurological disorders is efficient and facilitates disease
gene discovery. Eur J Hum Genet. 28:1034–1043. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arrudi-Moreno M, Fernández-Gómez A and
Peña-Segura JL: A new mutation in the SEPSECS gene related to
pontocerebellar hypoplasia type 2D. Med Clin (Barc). 156:94–95.
2021.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
16
|
Nicita F, Travaglini L, Bombelli F, Tosi
M, Pro S, Bertini E and D'Amico A: Novel SEPSECS pathogenic
variants featuring unusual phenotype of complex movement disorder
with thin corpus callosum: A case report. Neurol Genet.
8(e661)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Martínez-Martín Á, García-García J,
Díaz-Maroto Cicuéndez I, Quintanilla-Mata ML and Segura T: Bringing
light into the darkness: Autosomal recessive cerebellar ataxia due
to a recessive mutation in the SEPSECS gene. Neurologia (Engl Ed).
37:709–710. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramadesikan S, Hickey S, De Los Reyes E,
Patel AD, Franklin SJ, Brennan P, Crist E, Lee K, White P, McBride
KL, et al: Biallelic SEPSECS variants in two siblings with
pontocerebellar hypoplasia type 2D underscore the relevance of
splice-disrupting synonymous variants in disease. Cold Spring Harb
Mol Case Stud. 8(a006165)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rong T, Yao R, Deng Y, Lin Q, Wang G, Wang
J, Jiang F and Jiang Y: Case Report: A relatively mild phenotype
produced by novel mutations in the SEPSECS Gene. Front Pediatr.
9(805575)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schlüter A, Rodríguez-Palmero A, Verdura
E, Vélez-Santamaría V, Ruiz M, Fourcade S, Planas-Serra L, Martínez
JJ, Guilera C, Girós M, et al: Diagnosis of Genetic White Matter
Disorders by Singleton Whole-Exome and Genome Sequencing Using
Interactome-Driven Prioritization. Neurology. 98:e912–e923.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao R, Zhang L and Lu H: Analysis of the
Clinical Features and Imaging Findings of Pontocerebellar
Hypoplasia Type 2D Caused by Mutations in SEPSECS Gene. Cerebellum.
22:938–946. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Barone R, Alaimo S, Messina M, Pulvirenti
A and Bastin J: MIMIC-Autism Group. Ferro A, Frye RE and Rizzo R: A
Subset of Patients With Autism Spectrum Disorders Show a
Distinctive Metabolic Profile by Dried Blood Spot Analyses. Front
Psychiatry. 9(636)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Barone R, Carchon H, Jansen E, Pavone L,
Fiumara A, Bosshard NU, Gitzelmann R and Jaeken J: Lysosomal enzyme
activities in serum and leukocytes from patients with
carbohydrate-deficient glycoprotein syndrome type IA
(phosphomannomutase deficiency). J Inherit Metab Dis. 21:167–172.
1998.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Richards S, Aziz N, Bale S, Bick D, Das S,
Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al: ACMG
Laboratory Quality Assurance Committee. Standards and guidelines
for the interpretation of sequence variants: A joint consensus
recommendation of the American College of Medical Genetics and
Genomics and the Association for Molecular Pathology. Genet Med.
17:405–424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mailloux RJ, Jin X and Willmore WG: Redox
regulation of mitochondrial function with emphasis on cysteine
oxidation reactions. Redox Biol. 2:123–139. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Puppala AK, French RL, Matthies D, Baxa U,
Subramaniam S and Simonović M: Structural basis for early-onset
neurological disorders caused by mutations in human selenocysteine
synthase. Sci Rep. 6(32563)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Muraresku CC, McCormick EM and Falk MJ:
Mitochondrial Disease: Advances in clinical diagnosis, management,
therapeutic development, and preventative strategies. Curr Genet
Med Rep. 6:62–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ferreira GC and McKenna MC: L-Carnitine
and Acetyl-L-carnitine Roles and Neuroprotection in Developing
Brain. Neurochem Res. 42:1661–1675. 2017.PubMed/NCBI View Article : Google Scholar
|