1. Introduction
Biofilm-associated infections in otorhinolaryngology
(ORL) pose a significant clinical challenge due to their inherent
resistance to antimicrobial therapy and immune defenses. These
infections contribute to chronic and recurrent conditions, such as
chronic rhinosinusitis, otitis media and tonsillitis, leading to
prolonged patient morbidity and a growing healthcare burden
(1-5).
Biofilms also frequently develop on tracheostomy and airway stents,
often forming within 1 week postoperatively, regardless of material
type. This can facilitate biofilm spread to the lower airways,
increasing the risk of pneumonia and sepsis (1-5).
Despite conventional treatment approaches, biofilm persistence in
ORL structures hinders disease resolution, necessitating
alternative therapeutic strategies (6-9).
Consequently, biofilm-associated infections pose significant
challenges, highlighting the urgent need for innovative
pharmacological strategies to disrupt biofilm formation.
The mechanisms underlying biofilm formation are
complex, involving changes in phenotypic traits, antibiotic
resistance and gene expression (1-3).
Pathogens, such as Pseudomonas aeruginosa (P.
aeruginosa) and Staphylococcus aureus (S.
aureus), are frequently implicated, exhibiting strong biofilm
formation in the sinuses and middle ears (1,4-6).
The intricate anatomy of ORL structures, including the narrow sinus
ostia, middle ear cavity and deep tonsillar crypts, fosters
bacterial adhesion and biofilm formation. These niches shield
bacteria from immune defenses and antibiotic penetration,
contributing to recurrent infections and treatment failures
(3,6).
Effectively managing biofilm-associated infections
requires innovative pharmacological approaches beyond conventional
antibiotics. Recent research highlights promising strategies, such
as quorum sensing inhibitors (QSIs), that disrupt bacterial
communication essential for biofilm formation (7-9).
Antibiofilm peptides, such as natural and synthetic cationic
peptides, effectively hinder biofilm growth and enhance
antimicrobial efficacy by disrupting bacterial membranes or
modulating gene expression (10,11).
Additionally, repurposing non-antibiotic drugs, such as statins and
non-steroidal anti-inflammatory drugs (NSAIDs), has emerged as a
potential for targeting biofilm pathogens while complementing
existing therapies (12-14).
Biofilm formation plays a crucial role in the
persistence of ORL infections, necessitating the development of
innovative therapeutic strategies. Advancing our understanding of
biofilm development mechanisms and exploring alternative approaches
(such as QSIs, antibiofilm peptides and drug repurposing) presents
promising opportunities to improve treatment outcomes. The present
review examines current drug mechanisms targeting biofilm formation
and highlights the need for further research into biofilms in ORL
infections.
2. Biofilm formation in ORL infections
Mechanisms of biofilm formation
ORL bacterial infections can arise from either
planktonic (free-floating) or biofilm-associated bacteria.
Planktonic bacteria are typically more susceptible to antibiotics
and immune defenses. Conversely, biofilm-associated bacteria form
structured communities encased in an extracellular polymeric
matrix, which offers increased resistance to antimicrobial agents
and immune system clearance (15,16).
This difference is crucial, as biofilm-associated infections are
often persistent and recurrent, playing a significant role in
chronic disease progression (15,16).
The mechanisms of biofilm formation in ORL involve
dynamic processes that manifest either as surface-attached
structures or free-floating aggregates, reflecting the broader
framework of biofilm formation. These processes are divided into
three key phases (attachment, growth and disaggregation) each
playing a critical role in biofilm persistence and enhancing
bacterial resistance to treatment (Fig.
1) (15,16).
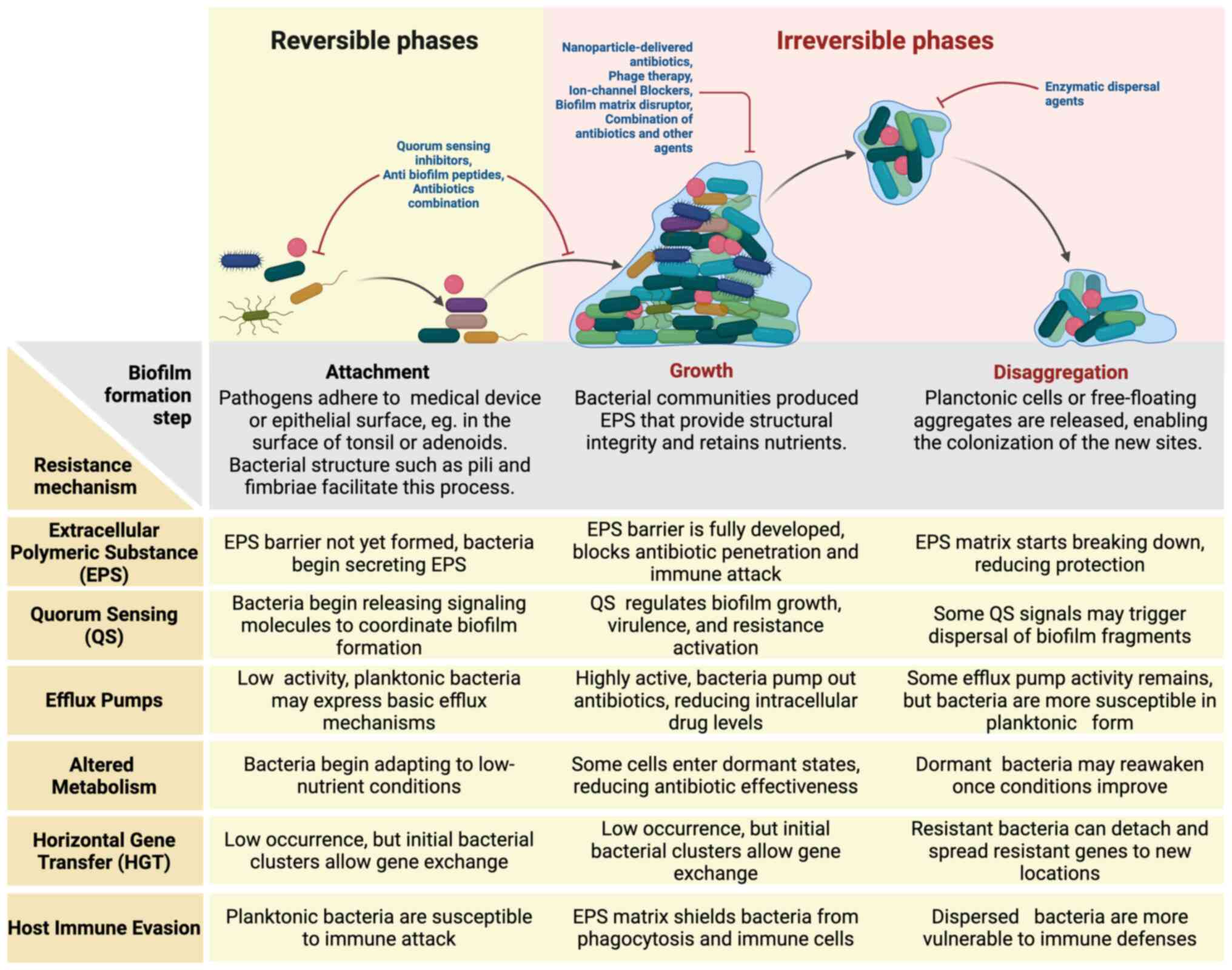 | Figure 1Biofilm formation, resistance
mechanisms and therapeutic strategies in ORL infections. There are
three key phases of biofilm development in otorhinolaryngologic
infections: Attachment, growth and disaggregation. During the
growth phase, bacteria produce extracellular polymeric substances,
enabling resistance through quorum sensing, efflux pumps, altered
metabolism, horizontal gene transfer and immune evasion. Various
antibiofilm strategies have been shown, targeting different stages,
including quorum sensing inhibitors, nanoparticle-based
antibiotics, phage therapy, and enzymatic dispersal agents. This
schematic highlights the persistence of biofilm infections and the
need for targeted therapies. |
The attachment phase represents the initial step in
biofilm formation, during which pathogens adhere to epithelial
surfaces or medical devices. Surface structures, such as pili and
fimbriae, play a key role in this process by enabling bacteria to
anchor firmly to surfaces (15,16).
In ORL, biofilms have been identified on the mucosal surfaces of
the tonsils and adenoids, as well as in the middle ear, where
pathogens, such as S. aureus and Haemophilus
influenzae can colonize (5,6,17,18).
The presence of biofilms in these regions is closely linked to
chronic conditions, such as chronic tonsillitis and otitis media,
highlighting the significance of the attachment phase in the
development and persistence of these infections (5,6,17,18).
In the growth phase, bacterial communities
proliferate and secrete extracellular polymeric substances (EPS),
which assemble into a protective matrix. This matrix not only
ensures structural stability but also retains nutrients and
establishes gradients of oxygen and metabolites, fostering diverse
microenvironments within the biofilm (19,20).
Quorum sensing (QS) regulates gene expression, enabling bacteria to
adjust their metabolism and enhance antibiotic resistance (19,21).
Biofilms in ORL infections mature over time, growing more resistant
and persistent. Consequently, they become less susceptible to
antimicrobial treatments and immune defenses (22).
Previous studies highlight that biofilm growth is
not limited to surface attachment. Free-floating aggregates formed
by bacterial EPS or host-derived polymers can develop within host
tissues and secretions. These aggregates exhibit features similar
to surface-attached biofilms, including antibiotic tolerance and
matrix production, expanding the conceptual understanding of
biofilm formation (15).
The final phase-disaggregation-involves the release
of planktonic cells or fragments from mature biofilms, facilitating
the colonization of new sites and contributing to infection
recurrence (23-25).
This dispersal process is often triggered by environmental changes,
such as nutrient depletion or antibiotic exposure (23-25).
Recent findings challenge the traditional model by showing that
disaggregation can occur in both surface-attached and free-floating
biofilms, further complicating treatment strategies (15). Understanding the formation of
biofilms is essential, as their structural complexity directly
contributes to antibiotic resistance and complicates treatment.
This highlights the need for strategies specifically designed to
address the unique challenges posed by biofilms, as discussed in
the following section.
In summary, biofilm formation involves dynamic and
adaptive processes encompassing surface-associated and
free-floating mechanisms. This revised framework highlights the
importance of developing therapies that target biofilms in all
forms. Understanding these processes is crucial for developing
effective strategies to combat chronic ORL infections.
Pathogens involved in ORL
biofilms
Numerous bacterial and fungal pathogens are
frequently linked to biofilm formation in ORL infections. Key
bacterial species, such as P. aeruginosa, Streptococcus
pneumoniae (S. pneumoniae) and S. aureus, are
commonly implicated in chronic conditions such as rhinosinusitis,
otitis media and tonsillitis (Table
I) (4,5,18,22,26-29).
These pathogens adhere to epithelial surfaces and form biofilms,
enabling them to evade host immune defenses and antimicrobial
treatments. Haemophilus influenzae is a significant pathogen
in recurrent otitis media, with its biofilm-forming ability
associated with persistent middle ear infections (18). In fungal biofilms, Candida
spp. and Aspergillus spp. are often implicated in chronic
fungal sinusitis, particularly in immunocompromised patients or
those with underlying conditions, such as allergic fungal sinusitis
(30-33).
 | Table ICommon pathogens involved in
biofilm-associated ENT infections. |
Table I
Common pathogens involved in
biofilm-associated ENT infections.
| Pathogen | Infection type | Antibiotic
resistance features | (Refs.) |
|---|
| Pseudomonas
aeruginosa | Chronic
rhinosinusitis, otitis media | Multidrug
resistance, quorum sensing, efflux pumps | (4,5,17,18,26,52) |
| Staphylococcus
aureus | Tonsillitis, otitis
media, sinus infections |
Methicillin-resistance, EPS production,
efflux pumps | (5,17,18,35,38,39) |
| Haemophilus
influenzae | Otitis media, sinus
infections | β-lactam
resistance, biofilm-forming ability | (5,17,18) |
| Streptococcus
pneumoniae | Otitis media, sinus
infections | Penicillin
resistance, efflux pumps, biofilm formation | (4,5,18) |
| Moraxella
catarrhalis | Otitis media, sinus
infections | β-lactam
resistance, biofilm development | (5,18) |
| Klebsiella
pneumoniae | Chronic
rhinosinusitis, sinus infections | Carbapenem
resistance, extended-spectrum beta-lactamase production | (4,5,38,39) |
| Escherichia
coli | Sinus infections,
tonsillitis | β-lactam
resistance, efflux pumps, quorum sensing | (3,17,38,39) |
| Candida
spp. | Chronic fungal
sinusitis | Azole resistance,
biofilm growth on mucosal surfaces | (30-33) |
| Aspergillus
spp. | Fungal sinusitis,
chronic invasive infections | Azole resistance,
biofilm-like growth in immunocompromised hosts | (30-33) |
| Proteus
mirabilis | Otitis media, sinus
infections | Biofilm production,
multidrug resistance | (5,18) |
| Acinetobacter
baumannii | Chronic
rhinosinusitis, sinus infections | Carbapenem
resistance, efflux pumps, biofilm-related resistance | (38,39) |
| Enterococcus
faecalis | Chronic ear
infections, sinus infections | Vancomycin
resistance, iron acquisition systems facilitating biofilm
growth | (38) |
Specific environmental and host-related factors
significantly influence the prevalence of biofilm-forming
pathogens. Recurrent infections, prior antibiotic use and medical
procedures such as tympanostomy tube placement create ideal
conditions for biofilm formation. Additionally, biofilms are more
prevalent in patients with chronic inflammation or structural
abnormalities, as these conditions impair normal mucosal defenses
(34-36).
This persistence highlights the need for targeted therapies that
simultaneously disrupt biofilm structure and inhibit microbial
activity to achieve effective treatment outcomes.
3. Challenges in treating biofilm-associated
infections
Challenges related to biofilm
development and treatment agents
A significant challenge in treating
biofilm-associated infections is the increased antibiotic
resistance observed within biofilms. Biofilm-associated infections
hinder effective treatment by restricting antibiotic penetration,
fostering antibiotic resistance, and altering bacterial metabolic
activity, which reduces susceptibility to therapeutic agents.
Biofilm matrices act as physical barriers, shielding embedded
bacteria from antibiotics, while the metabolic dormancy of biofilm
cells further diminishes their responsiveness to treatment.
Bacteria within biofilms often exhibit significantly
higher resistance levels than their planktonic counterparts,
primarily due to the protective biofilm matrix and the presence of
resistance genes, such as those encoding multidrug efflux pumps
(37-39).
The EPS matrix acts as a formidable barrier to drug penetration.
The dense structure of the biofilm, composed of polysaccharides,
proteins and extracellular DNA (eDNA), hinders the diffusion of
antimicrobial agents and reduces their efficacy (40-42).
Consequently, even when antibiotics are administered, they often
fail to achieve the concentrations required to effectively
eliminate bacteria within the biofilm (43,44).
This resistance complicates treatment strategies, as conventional
antibiotics often cannot penetrate the biofilm adequately, enabling
bacterial persistence and leading to chronic infections.
Additionally, biofilms exhibit phenotypic variations
that give rise to subpopulations capable of withstanding
antimicrobial treatments, making elimination more difficult. Within
biofilms, cells often exist in diverse metabolic states, with
numerous entering a dormant or slow-growing phase. This
heterogeneity significantly reduces the effectiveness of
antibiotics, which typically target actively dividing cells
(15,42). To address these challenges,
innovative therapeutic strategies (such as combining physical
disruption, enzymatic degradation, and targeted drug delivery
systems) are essential for improving treatment outcomes.
Current pharmacological strategies focus on three
key objectives-preventing biofilm formation, disrupting established
biofilms, and enhancing the susceptibility of biofilm-embedded
pathogens to antimicrobial agents. Innovative approaches, such as
antibiotic-based strategies, QSIs, antibiofilm peptides, enzymatic
dispersal agents and repurposed drugs, offer promising avenues for
addressing the challenges posed by biofilm-associated
infections.
Challenges related to anatomical and
clinical aspects
The persistence of biofilm-associated infections in
ORL is closely linked to the distinct anatomical features of the
ear, nose and throat, which create protective niches ideal for
microbial colonization. For instance, the sinonasal cavities,
characterized by narrow ostia and complex mucosal folds, create an
environment conducive to biofilm formation and immune evasion
(43,44). In chronic rhinosinusitis, biofilms
drive persistent inflammation and antibiotic resistance, leading to
recurrent infections even after prolonged medical treatment
(43,44). Similarly, in chronic tonsillitis,
the tonsillar crypts act as reservoirs for bacterial biofilms,
harboring deep-seated microbial communities that shield pathogens
from host defenses and antimicrobial agents (45).
In the middle ear, biofilms are a key factor in
recurrent otitis media, particularly in children. Their shorter and
more horizontal Eustachian tubes make it easier for pathogen entry
and persistence (46-48).
Biofilm-forming bacteria, such as Haemophilus influenzae and
S. pneumoniae, often lead to chronic infections that are
challenging to eliminate, necessitating tympanostomy tube placement
(46-48).
The resistance of these biofilms to antibiotic penetration results
in frequent recurrences and extended disease duration, posing a
significant challenge in clinical management (46-48).
The complex ear, nose and throat anatomy,
characterized by narrow and intricate spaces, poses significant
challenges for interventional procedures. These confined regions
impede the effective delivery of enzymatic dispersal agents,
rendering antibiotic-only approaches frequently inadequate for
managing such infections (46-48).
These limitations necessitate a multifaceted treatment approach.
Surgical interventions, including precise mechanical debridement,
are often utilized to physically remove biofilm-affected
tissues.
Given the significant challenges posed by
biofilm-associated infections (such as antibiotic resistance, poor
drug penetration and metabolic adaptations) conventional treatments
often prove inadequate. Therefore, novel therapeutic strategies
aimed at inhibiting biofilm formation, disrupting established
biofilms, and eliminating biofilm-associated bacteria have been
explored. The following sections outline promising pharmacological
approaches, including antibiotic-based strategies, QSIs, enzymatic
dispersal agents, antibiofilm peptides and drug repurposing. These
strategies are designed to enhance treatment efficacy and improve
patient outcomes.
4. Antibiotic-based strategies
Conventional antibiotics
Conventional antibiotics have long been the
foundation for treating bacterial infections, including those
associated with biofilms. However, their efficacy is often limited
in biofilm environments. Biofilm-associated bacteria exhibit
significantly higher resistance to antibiotics than planktonic
bacteria due to factors such as hindered penetration of antibiotics
through the biofilm matrix and altered metabolic activity of
bacteria embedded within the biofilm structure (37-39).
Vancomycin, a standard treatment for methicillin-resistant S.
aureus, is less effective against biofilm-associated
infections, highlighting the need for alternative strategies to
enhance its efficacy (49-51).
The protective EPS matrix and the presence of persister cells
within biofilms hinder antibiotic penetration and reduce metabolic
activity, further diminishing antibiotic effectiveness. Moreover,
prolonged or inappropriate antibiotic use increases the risk of
resistance development, leading to persistent and recurrent
infections. This resistance poses significant challenges in
clinical management.
Combination therapies of antibiotics
with antibiofilm properties
Combination therapy has become a promising approach
for treating biofilm-associated infections. This approach involves
using two or more antimicrobial agents that target different
aspects of bacterial physiology or biofilm structure. For example,
the combination of colistin and tobramycin has shown greater
efficacy in eliminating biofilm-forming P. aeruginosa than
treatments with single agents, as it targets the metabolic
heterogeneity of bacteria within biofilms (52).
Synergistic effects occur when two antibiotics with
distinct mechanisms of action are combined. For example, combining
aminoglycosides, which disrupt protein synthesis, with
beta-lactams, which inhibit cell wall synthesis, enhances
antibacterial activity (53,54).
Synergy is further amplified when one antibiotic exhibits strong
biofilm penetration. Notably, rifampin, known for its ability to
penetrate biofilm and target dormant bacterial populations, has
been effectively combined with vancomycin or daptomycin to treat
biofilm-associated infections caused by Staphylococcus
species (55-57).
These combinations improve biofilm penetration, target both
metabolically active and dormant bacterial cells, and enhance
treatment efficacy while reducing the risk of resistance
development.
Combining antibiotics with biofilm-targeting agents,
such as chelating agents, has emerged as a promising therapeutic
strategy. For example, combining edetic acid (PubChem CID: 6049)
with broad-spectrum antibiotics, such as minocycline has shown the
ability to disrupt biofilm integrity and enhance antibiotic
penetration, thereby improving treatment outcomes (58). Furthermore, topical antibiotics and
silver-containing wound dressings have shown efficacy in reducing
biofilm formation and promoting wound healing in infected tissues,
although supporting evidence remains limited (59,60).
Recent advancements have yielded antibiotics with
targeted antibiofilm properties, designed to either disrupt the
biofilm matrix or inhibit mechanisms critical to biofilm formation.
For example, macrolides, such as azithromycin, exert antibiofilm
effects by interfering with QS, a key bacterial communication
system that regulates biofilm formation and maintenance. By
inhibiting QS, macrolides reduce biofilm integrity and increase
bacterial susceptibility to other antibiotics, particularly in
infections caused by P. aeruginosa and E. coli
(61,62). Tigecycline, a glycyl-cycline
antibiotic, is effective against multidrug-resistant pathogens and
exhibits strong biofilm penetration, targeting both Gram-positive
and Gram-negative bacteria (63).
It has proven particularly effective against MDR Acinetobacter
baumannii and Enterobacteriaceae species (63). Additionally, combining
ceftolozane/tazobactam inhibits biofilm formation and eradicates
established biofilms formed by P. aeruginosa under both
aerobic and anaerobic conditions (64).
Effectively managing biofilm-associated infections
in ORL requires a comprehensive understanding of the limitations of
conventional antibiotic therapies, alongside the adoption of
combination strategies and innovative agents with antibiofilm
properties. By combining these approaches, healthcare providers can
enhance treatment efficacy and improve patient outcomes.
5. Biofilm formation disruptor
QSIs
Bacteria communicate using chemical signaling
molecules called autoinducers, whose concentration rises with
increasing cell density. This process, known as QS, involves
synthesizing, releasing, detecting, and responding to autoinducers.
QS serves as a bacterial communication system that regulates gene
expression based on population density, playing a crucial role in
biofilm formation and virulence (65). In Gram-negative bacteria, such as
P. aeruginosa, QS systems comprise genes encoding
transcriptional regulators (R genes) and autoinducer synthesis
enzymes (I genes). The I genes, such as lasI and
rhlI, facilitate the production of specific autoinducers,
including 3-oxo-C12-HSL and N-butyryl-l-homoserine lactone (C4-HSL)
(65). These autoinducers play a
pivotal role in regulating the expression of virulence factors and
promoting biofilm formation.
In ORL infections, targeting QS presents a promising
strategy for combating biofilm-associated infections, particularly
in chronic conditions such as rhinosinusitis and otitis media. QSIs
disrupt the signaling pathways bacteria use to communicate and
coordinate their behaviors, which are essential for biofilm
formation. By disrupting these pathways, QSIs can suppress the
expression of virulence factors and inhibit biofilm maturation,
rendering bacteria more vulnerable to antibiotics and the host
immune system (8,66). For instance, QSIs can block the
production of key signaling molecules, such as N-acyl homoserine
lactones (AHLs), indole, autoinducer-2 (AI-2), autoinducing
peptide, and diffusible signal factors, which are crucial for
regulating biofilm development in pathogens such as P.
aeruginosa (8,66). By inhibiting these communication
systems, QSIs reduce bacterial virulence and destabilize biofilms,
ultimately enhancing treatment outcomes for chronic infections.
Several compounds have shown efficacy as QSIs.
Natural substances, such as furanone, baicalin and iberin, have
been shown to disrupt QS in various bacterial species, including
P. aeruginosa, by interfering with the synthesis of AHLs
(67-69).
The application of QSIs holds particular promise in treating
chronic rhinosinusitis and otitis media. Chronic rhinosinusitis,
for instance, is often linked to biofilm-forming bacteria,
contributing to persistent inflammation and recurrent infections
(70,71). By targeting QS mechanisms, QSIs can
disrupt biofilm structures, thereby enhancing the efficacy of
conventional antibiotic therapies (9).
Similarly, in otitis media, biofilm formation by
pathogens such as S. pneumoniae and Haemophilus
influenzae often complicate treatment (72). For instance, the LuxS/AI-2 QS system
in S. pneumoniae plays a critical role in biofilm formation
and pathogenicity in middle ear infections (72). QSIs have been shown to reduce
bacterial loads and improve patient outcomes (73), making them a promising therapeutic
strategy for biofilm-associated infections in ORL. By targeting and
disrupting bacterial communication systems, QSIs can enhance the
efficacy of conventional antibiotics and provide a precise approach
to treating chronic infections.
QSIs can potentially prevent biofilm formation, but
their clinical application is hindered by challenges such as
bacterial adaptation and off-target effects. Although certain QSIs,
such as azithromycin, exhibit biofilm-disrupting capabilities,
their efficacy in treating chronic ORL infections requires further
study. Similarly, enzymatic dispersal agents, including DNase I and
Dispersin B, have shown promise in degrading biofilms in
vitro. However, issues related to their stability and methods
for localized delivery pose significant barriers to their broader
adoption in clinical settings.
Biofilm dispersal agents and enzymatic
disruption of EPS
Biofilm dispersal agents and enzymatic approaches
focus on disrupting the EPS that forms the structural backbone of
biofilms. Enzymatic methods offer a direct approach to EPS
disruption. DNase I degrades eDNA, a key structural component that
stabilizes biofilms, thereby compromising their integrity (74,75).
eDNA is essential for maintaining biofilm stability and integrity,
and its degradation by DNase I disrupts biofilm structure,
promoting dispersal and enhancing bacterial susceptibility to
antimicrobials (74,75). Furthermore, treating DNase I can
prevent biofilm reformation by degrading eDNA, which inhibits
bacterial adhesion and aggregation (74,75).
Dispersin B is an enzyme that targets and hydrolyzes
β-1,6-linked N-acetylglucosamine, a key polysaccharide in biofilm
matrices, effectively dispersing biofilms formed by various
bacteria, including S. aureus and P. aeruginosa. When
combined with antibiotics, Dispersin B exhibits a synergistic
effect, significantly reducing bacterial viability compared with
treatment with antibiotics alone (76). Similarly, alginate lyase degrades
alginate, a major component of P. aeruginosa biofilms. This
enzymatic degradation disrupts biofilm structure and improves
antibiotic penetration, particularly in cystic fibrosis-related
infections (77,78).
Nitric oxide (NO) disrupts biofilms by acting as a
signaling molecule that triggers dispersal in P. aeruginosa.
Research indicates that low sublethal concentrations of NO donors,
such as sodium nitroprusside, effectively induce biofilm dispersal
while enhancing bacteria susceptibility to antimicrobial agents
(79). This process is associated
with changes in bacterial metabolism and oxidative stress
responses, destabilizing biofilm structures and facilitating
sessile cell transition to a planktonic state. Consequently,
treatment efficacy against biofilm-associated infections is
significantly enhanced (79).
Combining biofilm-disruption strategies (such as
enzymatic agents and chemical dispersal compounds) with
conventional antimicrobial therapies can significantly improve
treatment outcomes in chronic infections. However, further research
is needed to optimize delivery systems and ensure their safety and
efficacy across various clinical settings. Additionally, future
research should investigate whether combining biofilm dispersal
agents with conventional saline irrigation in biofilm-associated
sinonasal cavity infections can enhance mucociliary clearance,
reduce inflammation, and ultimately improve patients' quality of
life.
Antibiofilm peptides and bacterial
membrane disruptors
Antibiofilm peptides function by compromising the
structural integrity of biofilms and the membranes of the bacteria
they contain. These peptides can penetrate the biofilm matrix
(composed of polysaccharides, proteins and eDNA) leading to biofilm
destabilization and dispersal (80). Furthermore, certain peptides
directly interact with bacterial membranes, inducing membrane
disruption and subsequent cell lysis (80,81).
This dual mechanism, targeting both the biofilm matrix and
bacterial membranes, enhances the effectiveness of antibiofilm
peptides in combating persistent infections.
Numerous antibiofilm peptides have exhibited
significant potential for clinical applications. Among these,
LL-37, a human cathelicidin peptide, stands out for its
broad-spectrum antimicrobial and antibiofilm activities, disrupting
bacterial membranes and modulating immune defenses. Notably, LL-37
has shown promise in disrupting biofilms formed by S. aureus
and P. aeruginosa, enhancing the effectiveness of
conventional antibiotics (82). In
a PREVIOUS study, LL-37 effectively reduced S. aureus
biofilm colony counts by over 4 logs at concentrations of 10 µM or
higher within 24 h, outperforming conventional antibiotics such as
gentamicin and vancomycin (82).
Bacteriocins, ribosomally synthesized peptides
produced by bacteria, exhibit potent antibacterial effects against
closely related species. For example, bacteriocins derived from
Lactobacillus species have been shown to effectively inhibit
biofilm formation by pathogenic bacteria in various settings
(83,84). Specifically, Lactobacillus
acidophilus VB1 exhibits antibacterial and antibiofilm
activities against pathogens linked to chronic otitis media
(85). This strain exerts this
effect through co-aggregation with pathogens and the production of
metabolites, such as cell-free supernatants and biosurfactants,
which disrupt biofilm formation and synergistically enhance the
efficacy of antibiotics such as ciprofloxacin (85).
Synthetic antimicrobial peptides (AMPs) also show
significant potential for eliminating biofilms by disrupting
bacterial membranes or inhibiting biofilm-related gene expression.
For instance, peptide 1018 has proven effective in eliminating
mature biofilms formed by both Gram-negative and Gram-positive
bacteria at concentrations below the minimum inhibitory
concentration. At 0.8 µg/ml, peptide 1018 induced biofilm
dispersal, while higher concentrations (10 µg/ml) achieved
near-complete elimination of biofilm-associated cells (80). Moreover, synthetic peptides can be
engineered to enhance their stability and broaden their efficacy
against diverse biofilm-forming pathogens. A notable example is the
novel antibiofilm peptide, BiF, which was developed through
hybridization with a lipid-binding motif. This dual-function
antimicrobial-antibiofilm peptide hybrid has shown effectiveness
against biofilm-forming Staphylococcus epidermidis (86).
Antibiofilm peptides hold significant potential in
preventing and managing recurrent otitis media, a prevalent ear
infection often associated with biofilm formation in the middle
ear. Recurrent otitis media is often linked to biofilm formation on
the tympanic membrane and within the middle ear, resulting in
persistent infections that are challenging to treat using
conventional antibiotics (5,6,17,18).
Further investigation is warranted to determine whether the
targeted delivery of LL-37 and other AMPs, combined with standard
treatment protocols, could significantly reduce the recurrence of
otitis media and sinus cavity infections.
Ion channel blockers
Ion channel blockers offer a novel therapeutic
strategy for treating biofilm-associated infections, particularly
chronic ORL infections. These agents target bacterial ion transport
systems, which are essential for maintaining biofilm integrity. By
disrupting these systems, ion channel blockers can destabilize
biofilms, thereby enhancing the effectiveness of conventional
treatments.
Bacterial biofilms utilize ion gradients to regulate
various cellular processes, including nutrient uptake, waste
removal and membrane potential maintenance, while also facilitating
antibiotic resistance mechanisms. For instance, NorA, an efflux
pump belonging to the major facilitator superfamily of
transporters, expels toxic compounds, including antimicrobial
agents, from bacterial cells (87-89).
Overexpression of NorA in S. aureus enhances its resistance
to antimicrobial treatments and facilitates biofilm formation
(87-89).
Similarly, FeoB in Enterococcus faecalis mediates iron
uptake, enabling enhanced biofilm growth under iron-rich conditions
(90,91). Disrupting these ion transport
systems can compromise biofilm formation and stability, making
bacteria more susceptible to antimicrobial agents.
Gallium compounds have emerged as promising ion
channel blockers due to their ability to mimic iron and disrupt
iron-dependent bacterial processes. Iron serves as a crucial
cofactor for bacterial enzymes involved in DNA synthesis,
respiration and virulence factor production. The structural
similarity of gallium to ferric iron (Fe³+) allows it to
compete for uptake through bacterial iron transport systems, such
as the FeoB transporter and siderophore-mediated pathways (92,93).
Unlike iron, gallium cannot be reduced from Fe³+ to
Fe²+ under physiological conditions, which inhibit key
iron-dependent metabolic enzymes, including ribonucleotide
reductase and superoxide dismutase (93). This disruption impairs bacterial DNA
synthesis, oxidative stress defense and overall biofilm stability.
Consequently, gallium not only prevents biofilm formation but also
enhances bacterial susceptibility to antibiotics. Studies have
shown that gallium compounds, such as gallium nitrate, effectively
inhibit P. aeruginosa biofilms and exhibit synergistic
effects when combined with conventional antimicrobial agents
(92,93).
Calcium channel blockers and proton pump inhibitors
(PPIs) destabilize biofilms by disrupting calcium- and
cation-dependent signaling pathways. Agents such as the calcium
channel blockers nimodipine and verapamil, alongside PPI
dexlansoprazole, block calcium channels associated with the
cyclic-di-GMP signaling cascade (94,95).
This disruption reduces EPS production, weakens biofilm structure,
and promotes the dispersal of biofilms formed by S. aureus,
P. aeruginosa and Candida albicans (94,95).
Ion channel blockers represent a promising strategy
for managing biofilm-associated infections in ORL. Future research
should explore their applicability, safety and efficacy when
combined with conventional antibiotic therapy for chronic
rhinosinusitis and otitis media.
6. Drug repurposing strategies
Drug repurposing, or drug repositioning, involves
exploring existing medications for new therapeutic applications
beyond their original use. This strategy is particularly beneficial
for biofilm-associated infections, as it enables the use of
non-antibiotic drugs with antibiofilm properties. Leveraging the
established safety profiles of these drugs, as well as their
mechanisms of action, clinicians may improve treatment outcomes for
conditions such as recurrent tonsillitis and otitis media.
Drug repurposing for biofilm management leverages
the ability of certain non-antibiotic drugs to disrupt biofilm
formation and stability. These drugs can exert anti-inflammatory
effects, modulate immune defenses, or interfere with bacterial
signaling pathways, ultimately impairing biofilm formation and
persistence.
Several drug classes have been identified as
promising candidates for repurposing in biofilm-associated
infections. Statins, such as atorvastatin and simvastatin, have
been shown to inhibit biofilm formation in various bacterial
species, including S. aureus and P. aeruginosa.
Atorvastatin suppresses the toll-like receptor 4 (TLR4)/MyD88/NF-κB
signaling pathway, promoting anti-inflammatory responses (96,97).
Simvastatin shows bacteriostatic and bactericidal activity against
methicillin-sensitive and methicillin-resistant S. aureus
and reduces biofilm formation (98).
Similarly, NSAIDs not only reduce inflammation but
may also disrupt biofilm integrity by modifying the bacterial
microenvironment. Combining ibuprofen with fluoroquinolones
enhances the inhibition of biofilm-associated regulators, including
Alg44 and AlgT/U, as well as the MexB and OprM efflux pump
(12,99). Another study showed that diclofenac
significantly reduced biofilm formation, with inhibition rates
ranging from 22.67-70%, compared with controls (100).
Drug repurposing offers a promising approach for
managing chronic ORL infections, such as recurrent tonsillitis and
otitis media, where biofilm formation drives antibiotic resistance
and treatment failure. Combining repurposed drugs with conventional
antibiotics can enhance biofilm disruption, reduce bacterial load,
and minimize recurrence. However, current evidence on NSAID and
statins primarily stems from in vitro studies, highlighting
the need for further in vivo and clinical
investigations.
7. Emerging and experimental therapies
Advancements in biofilm-associated infection
management have led to the development of innovative and
experimental therapeutic strategies. Notably, nanotechnology-based
therapies and phage therapy offer distinct mechanisms to disrupt
biofilm formation and enhance treatment efficacy.
Nanotechnology
Nanotechnology-based therapies leverage
nanoparticles to enhance drug delivery and enhance the penetration
of antibiotics and antibiofilm agents. These nanoparticles can be
engineered to improve the solubility, stability and bioavailability
of therapeutic agents. By facilitating the targeted delivery of
antibiotics to infection sites, nanoparticles help overcome
biofilm-related barriers to treatment (101-103).
Studies have shown that silver nanoparticles exhibit significant
antimicrobial and antibiofilm properties, effectively disrupting
biofilm formation by pathogens such as P. aeruginosa and
S. aureus (101,102,104,105). Magnetite nanoparticles combined
with antibiotics, such as streptomycin and neomycin, and
encapsulated within biopolymeric spheres exhibit promising
antimicrobial properties and enhanced biocompatibility. These
characteristics make them highly suitable for targeted delivery
systems in ENT infection (106).
Furthermore, polymeric nanoparticles can optimize the controlled
release of antibiofilm agents, promoting sustained therapeutic
effects while minimizing the need for frequent administration
(107).
Nanotechnology-based drug delivery systems offer a
promising approach to addressing the limitations of conventional
antibiotics in ORL biofilm infections. Nanoparticles, such as
silver nanoparticles and liposomal antibiotic carriers, have been
shown to penetrate biofilms and enhance antimicrobial efficacy. For
instance, in chronic rhinosinusitis, nanoparticle-based drug
formulations have shown improved retention in sinonasal mucosa,
enhancing local drug concentrations and reducing systemic toxicity
(106,108). However, several challenges, such
as rapid clearance by mucociliary mechanisms, potential cytotoxic
effects on the respiratory epithelium, and the lack of large-scale
clinical trials, limit their immediate clinical application.
Additionally, the regulatory approval process for phage therapy
remains complex due to strain-specific variations and the need for
personalized phage cocktails. Although nanoparticle-based
formulations have shown efficacy in preclinical models of chronic
rhinosinusitis and otitis media, their clinical validation remains
limited (106,108). Future clinical trials evaluating
the safety and efficacy of nanocarriers in targeting
biofilm-associated ORL infections are essential to advance their
translational potential.
Phage therapy
Phage therapy is an innovative approach that employs
bacteriophages (viruses that specifically infect and eliminate
bacteria) to target biofilm-specific pathogens. This method is
particularly promising in addressing antibiotic resistance, as
bacteriophages can effectively disrupt biofilms while eliminating
resistant bacterial strains, all without harming beneficial
microbiota (109-111).
Studies have shown the efficacy of phage therapy in treating
chronic infections caused by biofilm-forming bacteria, such as
P. aeruginosa, S. aureus and Klebsiella sp.,
in chronic wound infection (109-111).
The high specificity of bacteriophages enables targeted treatment,
minimizing collateral damage to surrounding tissues and reducing
the adverse effects often associated with conventional antibiotics
(112). By enhancing the efficacy
of existing treatments and offering alternatives in the face of
increasing antibiotic resistance, phage therapy has significant
potential for improving patient outcomes in chronic ORL
conditions.
It has been previously revealed that phages can
effectively disrupt biofilms in patients with chronic otitis media
and sinus infections by degrading EPS and lysing bacterial cells
(113). However, the clinical
application of phage therapy in ORL faces significant challenges,
including concerns about phage stability in mucosal environments,
potential immune responses and regulatory constraints (114). Although compassionate-use cases
have yielded promising outcomes, large-scale clinical trials are
needed to develop standardized phage formulations, dosing protocols
and comprehensive safety profiles (114). Addressing challenges, such as
mucosal penetration, host immune interactions and large-scale
production, is crucial to integrating these innovative therapies
into mainstream ORL clinical practice.
8. Conclusion and future directions
The present review highlights the pivotal role of
biofilm formation in the persistence and recurrence of ORL
infections, particularly its contribution to antimicrobial
resistance and treatment failure. Emerging pharmacological
strategies, such as QSIs, antibiofilm peptides, enzymatic dispersal
agents and repurposed drugs, offer promising approaches to disrupt
biofilm structural integrity and enhance treatment outcomes
(Tables II and III).
 | Table IIAnti-biofilm therapies and their
mechanisms of action. |
Table II
Anti-biofilm therapies and their
mechanisms of action.
| Therapy type | Mechanism of
action | Example agents | (Refs.) |
|---|
| Quorum sensing
inhibitors | Disrupt bacterial
communication, preventing biofilm formation and virulence factor
expression. | Furanones,
Baicalin, Iberin, Azithromycin | (8,65-67) |
| Enzymatic dispersal
agents | Degrade
extracellular polymeric substances and eDNA, breaking biofilm
structure. | DNase I, Dispersin
B, Alginate lyase | (74-77) |
| Anti-biofilm
peptides | Penetrate biofilm,
disrupt bacterial membranes, and modulate gene expression to
inhibit biofilm formation. | LL-37,
Bacteriocins, Synthetic AMPs (e.g., peptide 1018) | (81,82,84) |
| Drug repurposing
strategies | Reuse existing
drugs with anti-inflammatory or biofilm-disrupting properties,
enhancing antibiotic effects. | Statins
(Atorvastatin, Simvastatin), NSAIDs (Ibuprofen) | (12,97,100) |
| Combination
antibiotic therapy | Target different
aspects of bacterial physiology to penetrate biofilm and eradicate
bacteria. | Colistin +
Tobramycin, Vancomycin + Rifampin | (52,54) |
| Nanotechnology
approaches | Deliver drugs using
nanoparticles to enhance penetration, stability, and antimicrobial
action. | Silver
nanoparticles, Magnetite-based carriers | (102,105-107) |
| Phage therapy | Use bacteriophages
to target and lyse bacteria specifically within lyse bacteria
specifically within biofilms. biofilms. | Bacteriophages for
P. aeruginosa, S. aureus | (111,112) |
| Ion channel
blockers | Disrupt ion
gradients and efflux pump activity, weakening biofilm
integrity. | Gallium compounds,
Verapamil, Proton pump inhibitors | (87-89,92,95) |
| Biofilm matrix
disruptors | Target EPS
components to weaken matrix stability, enabling antibiotic
penetration. | Chelating agents
(e.g., EDTA), Nitric oxide donors | (89) |
| Immunomodulatory
approaches | Enhance immune
responses to target biofilm-associated infections and modulate
inflammation. | Immunostimulatory
peptides, Toll-like receptor modulators | (10,13,97) |
 | Table IIISummary of effectiveness and
limitation of anti-biofilm therapies. |
Table III
Summary of effectiveness and
limitation of anti-biofilm therapies.
| Therapy type | Effectiveness | Limitations |
|---|
| Quorum sensing
Inhibitors | Effective in early
biofilm stages; reduces virulence | Limited clinical
trials; potential bacterial resistance |
| Enzymatic dispersal
agents | Directly breaks
down biofilms; enhances antibiotic effects | Specificity issues;
potential immune response interference |
| Anti-biofilm
peptides | Broad-spectrum
activity; effective in resistant strains | Expensive;
potential cytotoxicity |
| Drug repurposing
strategies | Cost-effective;
enhances conventional therapy | Limited data on
long-term effects |
| Combination
antibiotic therapy | Synergistic effect;
useful for resistant infections | Risk of toxicity
and antibiotic resistance |
| Nanotechnology
approaches | High penetration;
effective at lower doses | Requires advanced
formulation; regulatory concerns |
| Phage therapy | Specific targeting;
minimal side effects | Narrow spectrum;
requires strain-specific selection |
| Ion channel
blockers | Weakens biofilm
structure; enhances antibiotic action | Limited in
vivo evidence; possible side effects |
| Biofilm matrix
disruptors | Directly weakens
biofilm structure | Risk of toxicity;
variable effectiveness |
| Immunomodulatory
approaches | Potential for
long-term infection control | Complexity of
immune modulation; needs further research |
Despite these advancements, translating these
findings into clinical practice remains challenging, necessitating
further research to validate safety, refine delivery systems, and
assess long-term efficacy. QSIs, for instance, exhibit potential in
preventing biofilm formation, but their clinical application is
hindered by concerns over bacterial adaptation and off-target
effects. Although certain QSIs, such as azithromycin, exhibit
biofilm-disrupting capabilities, their efficacy in treating chronic
ORL infections is still being studied (115). Similarly, enzymatic dispersal
agents, such as DNase I and Dispersin B, have shown promise in
degrading biofilms in vitro, but their stability and methods
for localized delivery remain significant barriers to their broader
clinical application (116).
Antibiofilm peptides exhibit broad-spectrum
activity, but their clinical use is hindered by high production
costs and potential cytotoxicity. While some synthetic AMPs have
advanced to preclinical testing, their pharmacokinetics and
potential immunogenicity require further validation before clinical
adoption (117). Drug repurposing
strategies, such as statins and NSAIDs, have garnered interest due
to their dual anti-inflammatory and biofilm-disrupting effects
(12-14).
However, their effectiveness in treating biofilm-associated ORL
infections remains unclear, as most supporting evidence derives
from in vitro studies and animal models rather than
large-scale human trials.
While in vitro and preclinical studies have
shown promising biofilm-targeting strategies, their efficacy and
practicality in real-world clinical settings remain largely
unverified. Future research must focus on conducting well-designed
clinical trials to assess the effectiveness, safety and
pharmacokinetics of these therapies in patients with chronic
biofilm-associated infections. Additionally, it is essential to
explore patient-specific factors (such as comorbidities, immune
responses and treatment adherence) to ensure successful clinical
implementation. Integrating advanced technologies, such as
nanocarriers, phage therapy and immunomodulatory approaches into
clinically relevant frameworks, will enhance treatment precision
and help translate laboratory findings into practical,
patient-centered applications.
Acknowledgements
The authors would like to thank Ms Rahmati Putri
Yaniafari (Nanyang Technological University, Singapore) for her
assistance in accessing several sources used in the present
review.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are
included in the figures and/or tables of this article.
Authors' contributions
MAE, INK and NMS conceptualized the study, drafted
and revised the manuscript. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Drenkard E and Ausubel FM: Pseudomonas
biofilm formation and antibiotic resistance are linked to
phenotypic variation. Nature. 416:740–743. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Behzadi P, Gajdács M, Pallós P, Ónodi B,
Stájer A, Matusovits D, Kárpáti K, Burián K, Battah B, Ferrari M,
et al: Relationship between biofilm-formation, phenotypic virulence
factors and antibiotic resistance in environmental Pseudomonas
aeruginosa. Pathogens. 11(1015)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen C, Liao X, Jiang H, Zhu H, Yue L, Li
S, Fang B and Liu Y: Characteristics of Escherichia coli biofilm
production, genetic typing, drug resistance pattern and gene
expression under aminoglycoside pressures. Environ Toxicol
Pharmacol. 30:5–10. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sanderson AR, Leid JG and Hunsaker D:
Bacterial biofilms on the sinus mucosa of human subjects with
chronic rhinosinusitis. Laryngoscope. 116:1121–1126.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mohammed RQ and Abdullah PB: Infection
with acute otitis media caused by Pseudomonas aeruginosa
(MDR) and Staphylococcus aureus (MRSA). Biochem Cell Arch.
20:905–908. 2020.
|
|
6
|
Bendouah Z, Barbeau J, Hamad WA and
Desrosiers M: Biofilm formation by Staphylococcus aureus and
Pseudomonas aeruginosa is associated with an unfavorable
evolution after surgery for chronic sinusitis and nasal polyposis.
Otolaryngol Head Neck Surg. 134:991–996. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carradori S, Di Giacomo N, Lobefalo M,
Luisi G, Campestre C and Sisto F: Biofilm and quorum sensing
inhibitors: The road so far. Expert Opin Ther Pat. 30:917–930.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Bian Z and Wang Y: Biofilm
formation and inhibition mediated by bacterial quorum sensing. Appl
Microbiol Biotechnol. 106:6365–6381. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou L, Zhang Y, Ge Y, Zhu X and Pan J:
Regulatory mechanisms and promising applications of quorum
sensing-inhibiting agents in control of bacterial biofilm
formation. Front Microbiol. 11(589640)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ridyard KE and Overhage J: The potential
of human peptide LL-37 as an antimicrobial and anti-biofilm agent.
Antibiotics (Basel). 10(650)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Martínez M, Polizzotto A, Flores N,
Semorile L and Maffía PC: Antibacterial, anti-biofilm and in vivo
activities of the antimicrobial peptides P5 and P6.2. Microb
Pathog. 139(103886)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paes Leme RC and da Silva RB:
Antimicrobial activity of non-steroidal anti-inflammatory drugs on
biofilm: Current evidence and potential for drug repurposing. Front
Microbiol. 12(707629)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schelz Z, Muddather HF and Zupkó I:
Repositioning of HMG-CoA reductase inhibitors as adjuvants in the
modulation of efflux pump-mediated bacterial and tumor resistance.
Antibiotics (Basel). 12(1468)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kumar A, Alam A, Grover S, Pandey S,
Tripathi D, Kumari M, Rani M, Singh A, Akhter Y, Ehtesham NZ and
Hasnain SE: Peptidyl-prolyl isomerase-B is involved in
Mycobacterium tuberculosis biofilm formation and a generic target
for drug repurposing-based intervention. NPJ Biofilms Microbiomes.
5(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sauer K, Stoodley P, Goeres DM,
Hall-Stoodley L, Burmølle M, Stewart PS and Bjarnsholt T: The
biofilm life cycle: Expanding the conceptual model of biofilm
formation. Nat Rev Microbiol. 20:608–620. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Irie Y, Borlee BR, O'Connor JR, Hill PJ,
Harwood CS, Wozniak DJ and Parsek MR: Self-produced
exopolysaccharide is a signal that stimulates biofilm formation in
Pseudomonas aeruginosa. Proc Natl Acad Sci USA.
109:20632–20636. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Parastan R, Kargar M, Solhjoo K and
Kafilzadeh F: Staphylococcus aureus biofilms: Structures,
antibiotic resistance, inhibition, and vaccines. Gene Rep.
20(100739)2020.
|
|
18
|
Galli J, Calò L, Ardito F, Imperiali M,
Bassotti E, Fadda G and Paludetti G: Biofilm formation by
Haemophilus influenzae isolated from adeno-tonsil tissue
samples, and its role in recurrent adenotonsillitis. Acta
Otorhinolaryngol Ital. 27:134–138. 2007.PubMed/NCBI
|
|
19
|
Davenport EK, Call DR and Beyenal H:
Differential protection from tobramycin by extracellular polymeric
substances from Acinetobacter baumannii and
Staphylococcus aureus biofilms. Antimicrob Agents Chemother.
58:4755–4761. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Serra DO, Klauck G and Hengge R: Vertical
stratification of matrix production is essential for physical
integrity and architecture of macrocolony biofilms of Escherichia
coli. Environ Microbiol. 17:5073–5088. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Parsek MR and Greenberg EP:
Acyl-homoserine lactone quorum sensing in gram-negative bacteria: A
signaling mechanism involved in associations with higher organisms.
Proc Natl Acad Sci USA. 97:8789–8793. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singh GB, Malhotra S, Yadav SC, Kaur R,
Kwatra D and Kumar S: The role of biofilms in chronic otitis
media-active squamosal disease: An evaluative study. Otol Neurotol.
42:e1279–e1285. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dar D, Dar N, Cai L and Newman DK: Spatial
transcriptomics of planktonic and sessile bacterial populations at
single-cell resolution. Science. 373(eabi4882)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cornforth DM, Dees JL, Ibberson CB, Huse
HK, Mathiesen IH, Kirketerp-Møller K, Wolcott RD, Rumbaugh KP,
Bjarnsholt T and Whiteley M: Pseudomonas aeruginosa
transcriptome during human infection. Proc Natl Acad Sci USA.
115:E5125–E5134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dal Co A, Van Vliet S and Ackermann M:
Emergent microscale gradients give rise to metabolic cross-feeding
and antibiotic tolerance in clonal bacterial populations. Philos
Trans R Soc Lond B Biol Sci. 374(20190080)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Boase S, Foreman A, Cleland E, Tan L,
Melton-Kreft R, Pant H, Hu FZ, Ehrlich GD and Wormald PJ: The
microbiome of chronic rhinosinusitis: Culture, molecular
diagnostics and biofilm detection. BMC Infect Dis.
13(210)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kostić M, Ivanov M, Babić SS, Tepavčević
Z, Radanović O, Soković M and Ćirić A: Analysis of tonsil tissues
from patients diagnosed with chronic tonsillitis-microbiological
profile, biofilm-forming capacity and histology. Antibiotics
(Basel). 11(1747)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee MR, Pawlowski KS, Luong A, Furze AD
and Roland PS: Biofilm presence in humans with chronic suppurative
otitis media. Otolaryngol Head Neck Surg. 141:567–571.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hoa M, Syamal M, Schaeffer MA, Sachdeva L,
Berk R and Coticchia J: Biofilms and chronic otitis media: An
initial exploration into the role of biofilms in the pathogenesis
of chronic otitis media. Am J Otolaryngol. 31:241–245.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Karthikeyan P and Nirmal Coumare V:
Incidence and presentation of fungal sinusitis in patient diagnosed
with chronic rhinosinusitis. Indian J Otolaryngol Head Neck Surg.
62:381–385. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bahethi R, Talmor G, Choudhry H, Lemdani
M, Singh P, Patel R and Hsueh W: Chronic invasive fungal
rhinosinusitis and granulomatous invasive fungal sinusitis: A
systematic review of symptomatology and outcomes. Am J Otolaryngol.
45(104064)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang SW, Luo CM and Cheng TC: Fungal
abscess of anterior nasal septum complicating maxillary sinus
fungal ball rhinosinusitis caused by Aspergillus flavus:
Case report and review of literature. J Fungi (Basel).
10(497)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Leszczyńska J, Stryjewska-Makuch G,
Lisowska G, Kolebacz B and Michalak-Kolarz M: Fungal sinusitis
among patients with chronic rhinosinusitis who underwent endoscopic
sinus surgery. Otolaryngol Pol. 72:35–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Marom T, Habashi N, Cohen R and Tamir SO:
Role of biofilms in post-tympanostomy tube otorrhea. Ear Nose
Throat J. 99 (1 Suppl):22S–29S. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Manasherob R, Mooney JA, Lowenberg DW,
Bollyky PL and Amanatullah DF: Tolerant small-colony variants form
prior to resistance within a Staphylococcus aureus biofilm
based on antibiotic selective pressure. Clin Orthop Relat Res.
479:1471–1481. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Habashi N, Marom T, Steinberg D, Zacks B
and Tamir SO: Biofilm distribution on tympanostomy tubes: An ex
vivo descriptive study. Int J Pediatr Otorhinolaryngol.
138(110350)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mah TF, Pitts B, Pellock B, Walker GC,
Stewart PS and O'Toole GA: A genetic basis for Pseudomonas
aeruginosa biofilm antibiotic resistance. Nature. 426:306–310.
2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kvist M, Hancock V and Klemm P:
Inactivation of efflux pumps abolishes bacterial biofilm formation.
Appl Environ Microbiol. 74:7376–7382. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tang M, Wei X, Wan X, Ding Z, Ding Y and
Liu J: The role and relationship with efflux pump of biofilm
formation in Klebsiella pneumoniae. Microb Pathog.
147(104244)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Powell LC, Abdulkarim M, Stokniene J, Yang
QE, Walsh TR, Hill KE, Gumbleton M and Thomas DW: Quantifying the
effects of antibiotic treatment on the extracellular polymer
network of antimicrobial resistant and sensitive biofilms using
multiple particle tracking. NPJ Biofilms Microbiomes.
7(13)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kosztołowicz T and Metzler R: Diffusion of
antibiotics through a biofilm in the presence of diffusion and
absorption barriers. Phys Rev E. 102(032408)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tuon FF, Dantas LR, Suss PH and Tasca
Ribeiro VST: Pathogenesis of the Pseudomonas aeruginosa
biofilm: A review. Pathogens. 11(300)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Denton O, Wan Y, Beattie L, Jack T,
McGoldrick P, McAllister H, Mullan C, Douglas CM and Shu W:
Understanding the role of biofilms in acute recurrent tonsillitis
through 3D bioprinting of a novel gelatin-PEGDA hydrogel.
Bioengineering (Basel). 11(202)2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huang Y, Qin F, Li S, Yin J, Hu L, Zheng
S, He L, Xia H, Liu J and Hu W: The mechanisms of biofilm
antibiotic resistance in chronic rhinosinusitis: A review. Medicine
(Baltimore). 101(e32168)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Abu Bakar M, McKimm J, Haque SZ, Majumder
MAA and Haque M: Chronic tonsillitis and biofilms: A brief overview
of treatment modalities. J Inflam Res. 11:329–337. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schilder AGM, Chonmaitree T, Cripps AW,
Rosenfeld RM, Casselbrant ML, Haggard MP and Venekamp RP: Otitis
media. Nat Rev Dis Primers. 2(16063)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Duff AF, Jurcisek JA, Kurbatfinski N,
Chiang T, Goodman SD, Bakaletz LO and Bailey MT: Oral and middle
ear delivery of otitis media standard of care antibiotics, but not
biofilm-targeted antibodies, alter chinchilla nasopharyngeal and
fecal microbiomes. NPJ Biofilms Microbiomes. 10(10)2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Niedzielski A, Chmielik LP and Stankiewicz
T: The formation of biofilm and bacteriology in otitis media with
effusion in children: A prospective cross-sectional study. Int J
Environ Res Public Health. 18(3555)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Abdelhady W, Bayer AS, Seidl K, Moormeier
DE, Bayles KW, Cheung AL, Yeaman MR and Xiong YQ: Impact of
vancomycin on sarA-mediated biofilm formation: Role in persistent
endovascular infections due to methicillin-resistant
Staphylococcus aureus. J Infect Dis. 209:1231–1240.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rose WE and Poppens PT: Impact of biofilm
on the in vitro activity of vancomycin alone and in combination
with tigecycline and rifampicin against Staphylococcus
aureus. J Antimicrob Chemother. 63:485–488. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cho OH, Bae IG, Moon SM, Park SY, Kwak YG,
Kim BN, Yu SN, Jeon MH, Kim T, Choo EJ, et al: Therapeutic outcome
of spinal implant infections caused by Staphylococcus
aureus: A retrospective observational study. Medicine
(Baltimore). 97(e12629)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Herrmann G, Yang L, Wu H, Song Z, Wang H,
Høiby N, Ulrich M, Molin S, Riethmüller J and Döring G:
Colistin-tobramycin combinations are superior to monotherapy
concerning the killing of biofilm Pseudomonas aeruginosa. J
Infect Dis. 202:1585–1592. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
53
|
Giamarellou H, Zissis NP, Tagari G and
Bouzos J: In vitro synergistic activities of aminoglycosides and
new beta-lactams against multiresistant Pseudomonas
aeruginosa. Antimicrob Agents Chemother. 25:534–536.
1984.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Giamarellou H: Aminoglycosides plus
beta-lactams against gram-negative organisms. Evaluation of in
vitro synergy and chemical interactions. Am J Med. 80:126–137.
1986.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Olson ME, Slater SR, Rupp ME and Fey PD:
Rifampicin enhances activity of daptomycin and vancomycin against
both a polysaccharide intercellular adhesin (PIA)-dependent and
-independent Staphylococcus epidermidis biofilm. J
Antimicrob Chemother. 65:2164–2171. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zimmerli W and Sendi P: Role of rifampin
against staphylococcal biofilm infections in vitro, in animal
models, and in orthopedic-device-related infections. Antimicrob
Agents Chemother. 63:e01746–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Niska JA, Shahbazian JH, Ramos RI, Francis
KP, Bernthal NM and Miller LS: Vancomycin-rifampin combination
therapy has enhanced efficacy against an experimental
Staphylococcus aureus prosthetic joint infection. Antimicrob
Agents Chemother. 57:5080–5086. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ferreira Chacon JM, Hato de Almeida E, de
Lourdes Simões R, Lazzarin C, Ozório V, Alves BC, Mello de Andréa
ML, Santiago Biernat M and Biernat JC: Randomized study of
minocycline and edetic acid as a locking solution for central line
(port-a-cath) in children with cancer. Chemotherapy. 57:285–291.
2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Vermeulen H, van Hattem JM, Storm-Versloot
MN and Ubbink DT: Topical silver for treating infected wounds.
Cochrane Database Syst Rev: CD005486, 2007.
|
|
60
|
Jiang Y, Zhang Q, Wang H, Välimäki M, Zhou
Q, Dai W and Guo J: Effectiveness of silver and iodine dressings on
wound healing: A systematic review and meta-analysis. BMJ Open.
14(e077902)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Tateda K, Comte R, Pechere JC, Köhler T,
Yamaguchi K and Van Delden C: Azithromycin inhibits quorum sensing
in Pseudomonas aeruginosa. Antimicrob Agents Chemother.
45:1930–1933. 2001.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hoffmann N, Lee B, Hentzer M, Rasmussen
TB, Song Z, Johansen HK, Givskov M and Høiby N: Azithromycin blocks
quorum sensing and alginate polymer formation and increases the
sensitivity to serum and stationary-growth-phase killing of
Pseudomonas aeruginosa and attenuates chronic P.
aeruginosa lung infection in Cftr(-/-) mice. Antimicrob Agents
Chemother. 51:3677–3687. 2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Gupta S, Aruna C, Nagaraj S, Dias M and
Muralidharan S: In vitro activity of tigecycline against
multidrug-resistant gram-negative blood culture isolates from
critically ill patients. J Antimicrob Chemother. 67:1293–1295.
2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kostoulias X, Fu Y, Morris FC, Yu C, Qu Y,
Chang CC, Blakeway L, Landersdorfer CB, Abbott IJ, Wang L, et al:
Ceftolozane/tazobactam disrupts Pseudomonas aeruginosa
biofilms under static and dynamic conditions. J Antimicrob
Chemother. 80:372–380. 2025.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Miller MB and Bassler BL: Quorum sensing
in bacteria. Annu Rev Microbiol. 55:165–199. 2001.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Köhler T, Perron GG, Buckling A and van
Delden C: Quorum sensing inhibition selects for virulence and
cooperation in Pseudomonas aeruginosa. PLOS Pathog.
6(e1000883)2010.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Tsikopoulos A, Petinaki E, Festas C,
Tsikopoulos K, Meroni G, Drago L and Skoulakis C: In vitro
inhibition of biofilm formation on silicon rubber voice prosthesis:
Α systematic review and meta-analysis. ORL J Otorhinolaryngol Relat
Spec. 84:10–29. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jakobsen TH, Bragason SK, Phipps RK,
Christensen LD, van Gennip M, Alhede M, Skindersoe M, Larsen TO,
Høiby N, Bjarnsholt T and Givskov M: Food as a source for quorum
sensing inhibitors: Iberin from horseradish revealed as a quorum
sensing inhibitor of Pseudomonas aeruginosa. Appl Environ
Microbiol. 78:2410–2421. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Luo J, Dong B, Wang K, Cai S, Liu T, Cheng
X, Lei D and Chen Y, Li Y, Kong J and Chen Y: Baicalin inhibits
biofilm formation, attenuates the quorum sensing-controlled
virulence and enhances Pseudomonas aeruginosa clearance in a
mouse peritoneal implant infection model. PLoS One.
12(e0176883)2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Prince AA, Steiger JD, Khalid AN,
Dogrhamji L, Reger C, Eau Claire SE, Chiu AG, Kennedy DW, Palmer JN
and Cohen NA: Prevalence of biofilm-forming bacteria in chronic
rhinosinusitis. Am J Rhinol. 22:239–245. 2008.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Foreman A, Holtappels G, Psaltis AJ,
Jervis-Bardy J, Field J, Wormald PJ and Bachert C: Adaptive immune
responses in Staphylococcus aureus biofilm-associated
chronic rhinosinusitis. Allergy. 66:1449–1456. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yadav MK, Vidal JE, Go YY, Kim SH, Chae SW
and Song JJ: The LuxS/AI-2 quorum-sensing system of
Streptococcus pneumoniae is required to cause disease, and
to regulate virulence- and metabolism-related genes in a rat model
of middle ear infection. Front Cell Infect Microbiol.
8(138)2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Dawit G, Mequanent S and Makonnen E:
Efficacy and safety of azithromycin and amoxicillin/clavulanate for
otitis media in children: A systematic review and meta-analysis of
randomized controlled trials. Ann Clin Microbiol Antimicrob.
20(28)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Brown HL, Hanman K, Reuter M, Betts RP and
Van Vliet AHM: Campylobacter jejuni biofilms contain extracellular
DNA and are sensitive to DNase I treatment. Front Microbiol.
6(699)2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Tetz GV, Artemenko NK and Tetz VV: Effect
of DNase and antibiotics on biofilm characteristics. Antimicrob
Agents Chemother. 53:1204–1209. 2009.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gawande PV, Leung KP and Madhyastha S:
Antibiofilm and antimicrobial efficacy of
DispersinB®-KSL-W peptide-based wound gel against
chronic wound infection associated bacteria. Curr Microbiol.
68:635–641. 2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lamppa JW and Griswold KE: Alginate lyase
exhibits catalysis-independent biofilm dispersion and antibiotic
synergy. Antimicrob Agents Chemother. 57:137–145. 2013.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Daboor SM, Rohde JR and Cheng Z:
Disruption of the extracellular polymeric network of Pseudomonas
aeruginosa biofilms by alginate lyase enhances pathogen
eradication by antibiotics. J Cyst Fibros. 20:264–270.
2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Barraud N, Hassett DJ, Hwang SH, Rice SA,
Kjelleberg S and Webb JS: Involvement of nitric oxide in biofilm
dispersal of Pseudomonas aeruginosa. J Bacteriol.
188:7344–7353. 2006.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Reffuveille F, de la Fuente-Núñez C,
Mansour S and Hancock REW: A broad-spectrum antibiofilm peptide
enhances Antibiotic Action against bacterial biofilms. Antimicrob
Agents Chemother. 58:5363–5371. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Le CF, Fang CM and Sekaran SD:
Intracellular targeting mechanisms by antimicrobial peptides.
Antimicrob Agents Chemother. 61:e02340–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kang J, Dietz MJ and Li B: Antimicrobial
peptide LL-37 is bactericidal against Staphylococcus aureus
biofilms. PLoS One. 14(e0216676)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Jalilsood T, Baradaran A, Song AAL, Foo
HL, Mustafa S, Saad WZ, Yusoff K and Rahim RA: Inhibition of
pathogenic and spoilage bacteria by a novel biofilm-forming
Lactobacillus isolate: A potential host for the expression of
heterologous proteins. Microb Cell Fact. 14(96)2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Li J, Zhang Q, Zhao J, Zhang H and Chen W:
Lactobacillus-derived components for inhibiting biofilm formation
in the food industry. World J Microbiol Biotechnol.
40(117)2024.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Algburi AR, Jassim SM, Popov IV, Weeks R
and Chikindas ML: Lactobacillus acidophilus VB1
co-aggregates and inhibits biofilm formation of chronic otitis
media-associated pathogens. Braz J Microbiol. 55:2581–2592.
2024.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Wongchai M, Wongkaewkhiaw S, Kanthawong S,
Roytrakul S and Aunpad R: Dual-function antimicrobial-antibiofilm
peptide hybrid to tackle biofilm-forming Staphylococcus
epidermidis. Ann Clin Microbiol Antimicrob.
23(44)2024.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Tintino SR, Souza VCAD, Silva JMAD,
Oliveira-Tintino CDDM, Pereira PS, Leal-Balbino TC, Pereira-Neves
A, Siqueira-Junior JP, da Costa JGM, Rodrigues FFG, et al: Effect
of vitamin K3 inhibiting the function of NorA efflux
pump and its gene expression on Staphylococcus aureus.
Membranes (Basel). 10(130)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Monteiro KLC, de Aquino TM and Mendonça
Junior FJB: An update on Staphylococcus aureus NorA efflux
pump inhibitors. Curr Top Med Chem. 20:2168–2185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Lu X, Wang G, Xie Y, Tang W, Liu B and
Zhang J: Efflux pump inhibitor combined with ofloxacin decreases
MRSA biofilm formation by regulating the gene expression of NorA
and quorum sensing. RSC Adv. 13:2707–2717. 2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Govindarajan DK, Meghanathan Y,
Sivaramakrishnan M, Kothandan R, Muthusamy A, Seviour TW and
Kandaswamy K: Enterococcus faecalis thrives in dual-species
biofilm models under iron-rich conditions. Arch Microbiol.
204(710)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Brunson DN, Colomer-Winter C, Lam LN and
Lemos JA: Identification of multiple iron uptake mechanisms in
Enterococcus faecalis and their relationship to virulence.
Infect Immun. 91(e0049622)2023.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Kaneko Y, Thoendel M, Olakanmi O, Britigan
BE and Singh PK: The transition metal gallium disrupts
Pseudomonas aeruginosa iron metabolism and has antimicrobial
and antibiofilm activity. J Clin Invest. 117:877–888.
2007.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Goss CH, Kaneko Y, Khuu L, Anderson GD,
Ravishankar S, Aitken ML, Lechtzin N, Zhou G, Czyz DM, McLean K, et
al: Gallium disrupts bacterial iron metabolism and has therapeutic
effects in mice and humans with lung infections. Sci Transl Med.
10(eaat7520)2018.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Nobile CJ, Ennis CL, Hartooni N, Johnson
AD and Lohse MB: A selective serotonin reuptake inhibitor, a proton
pump inhibitor, and two calcium channel blockers inhibit Candida
albicans biofilms. Microorganisms. 8(756)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Yu Q, Ding X, Xu N, Cheng X, Qian K, Zhang
B, Xing L and Li M: In vitro activity of verapamil alone and in
combination with fluconazole or tunicamycin against Candida
albicans biofilms. Int J Antimicrob Agents. 41:179–182.
2013.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Koushki K, Shahbaz SK, Mashayekhi K,
Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP
and Sahebkar A: Anti-inflammatory action of statins in
cardiovascular disease: The role of inflammasome and toll-like
receptor pathways. Clin Rev Allergy Immunol. 60:175–199.
2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kong F, Ye B, Lin L, Cai X, Huang W and
Huang Z: Atorvastatin suppresses NLRP3 inflammasome activation via
TLR4/MyD88/NF-κB signaling in PMA-stimulated THP-1 monocytes.
Biomed Pharmacother. 82:167–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Graziano TS, Cuzzullin MC, Franco GC,
Schwartz-Filho HO, de Andrade ED, Groppo FC and Cogo-Müller K:
Statins and antimicrobial effects: Simvastatin as a potential drug
against Staphylococcus aureus biofilm. PLoS One.
10(e0128098)2015.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Khodaparast S, Ghanbari F and Zamani H:
Evaluation of the effect of ibuprofen in combination with
ciprofloxacin on the virulence-associated traits, and efflux pump
genes of Pseudomonas aeruginosa. World J Microbiol
Biotechnol. 38(125)2022.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Abbas HA, Atallah H, El-Sayed MA and
El-Ganiny AM: Diclofenac mitigates virulence of multidrug-resistant
Staphylococcus aureus. Arch Microbiol. 202:2751–2760.
2020.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Severino P, Silveira EF, Loureiro K, Chaud
MV, Antonini D, Lancellotti M, Sarmento VH, da Silva CF, Santana
MHA and Souto EB: Antimicrobial activity of polymyxin-loaded solid
lipid nanoparticles (PLX-SLN): Characterization of physicochemical
properties and in vitro efficacy. Eur J Pharm Sci. 106:177–184.
2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wang XF, Zhang SL, Zhu LY, Xie SY, Dong Z,
Wang Y and Zhou WZ: Enhancement of antibacterial activity of
tilmicosin against Staphylococcus aureus by solid lipid
nanoparticles in vitro and in vivo. Vet J. 191:115–120.
2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Kotrange H, Najda A, Bains A, Gruszecki R,
Chawla P and Tosif MM: Metal and metal oxide nanoparticle as a
novel antibiotic carrier for the direct delivery of antibiotics.
Int J Mol Sci. 22(9596)2021.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Patro SK, Panda NK and Sharma M: Drug
repurposing for, ENT and head and neck, infectious and oncologic
diseases: Current practices and future possibilities. In: Sobti RC,
Lal SK and Goyal RK (eds). Drug Repurposing for Emerging Infectious
Diseases and Cancer. Singapore: Springer Nature, pp253-282,
2023.
|
|
105
|
Kora AJ and Arunachalam J: Assessment of
antibacterial activity of silver nanoparticles on Pseudomonas
aeruginosa and its mechanism of action. World J Microbiol
Biotechnol. 27:1209–1216. 2011.
|
|
106
|
Caciandone M, Niculescu AG, Grumezescu V,
Bîrcă AC, Ghica IC, Vasile BȘ, Oprea O, Nica IC, Stan MS, Holban
AM, et al: Magnetite nanoparticles functionalized with therapeutic
agents for enhanced ENT antimicrobial properties. Antibiotics
(Basel). 11(623)2022.PubMed/NCBI View Article : Google Scholar
|
|
107
|
García-Alvarez R, Izquierdo-Barba I and
Vallet-Regí M: 3D scaffold with effective multidrug sequential
release against bacteria biofilm. Acta Biomater. 49:113–126.
2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lee M, Park CG, Huh BK, Kim SN, Lee SH,
Khalmuratova R, Park JW, Shin HW and Choy YB: Sinonasal delivery of
resveratrol via mucoadhesive nanostructured microparticles in a
nasal polyp mouse model. Sci Rep. 7(40249)2017.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Cano EJ, Caflisch KM, Bollyky PL, Van
Belleghem JD, Patel R, Fackler J, Brownstein MJ, Horne B, Biswas B,
Henry M, et al: Phage therapy for limb-threatening prosthetic knee
Klebsiella pneumoniae infection: Case report and in vitro
characterization of anti-biofilm activity. Clin Infect Dis.
73:e144–e151. 2021.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Manoharadas S, Altaf M, Alrefaei AF,
Hussain SA, Devasia RM, Badjah Hadj AYM and Abuhasil MSA:
Microscopic analysis of the inhibition of staphylococcal biofilm
formation by Escherichia coli and the disruption of preformed
staphylococcal biofilm by bacteriophage. Microsc Res Tech.
84:1513–1521. 2021.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Morris J, Kelly N, Elliott L, Grant A,
Wilkinson M, Hazratwala K and McEwen P: Evaluation of bacteriophage
anti-biofilm activity for potential control of orthopedic
implant-related infections caused by Staphylococcus aureus.
Surg Infect (Larchmt). 20:16–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhao M, Li H, Gan D, Wang M, Deng H and
Yang QE: Antibacterial effect of phage cocktails and
phage-antibiotic synergy against pathogenic Klebsiella
pneumoniae. mSystems. 9(e0060724)2024.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Fong SA, Drilling A, Morales S, Cornet ME,
Woodworth BA, Fokkens WJ, Psaltis AJ, Vreugde S and Wormald PJ:
Activity of bacteriophages in removing biofilms of Pseudomonas
aeruginosa isolates from chronic rhinosinusitis patients. Front
Cell Infect Microbiol. 7(418)2017.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Gordon M and Ramirez P: Efficacy and
experience of bacteriophages in biofilm-related infections.
Antibiotics (Basel). 13(125)2024.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Lim DJ, Skinner D, Mclemore J, Rivers N,
Elder JB, Allen M, Koch C, West J, Zhang S, Thompson HM, et al:
In-vitro evaluation of a ciprofloxacin and azithromycin sinus stent
for Pseudomonas aeruginosa biofilms. Int Forum Allergy
Rhinol. 10:121–127. 2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Pakkulnan R, Thonglao N and Chareonsudjai
S: DNase I and chitosan enhance efficacy of ceftazidime to
eradicate Burkholderia pseudomallei biofilm cells. Sci Rep.
13(1059)2023.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Cresti L, Falciani C, Cappello G, Brunetti
J, Vailati S, Melloni E, Bracci L and Pini A: Safety evaluations of
a synthetic antimicrobial peptide administered intravenously in
rats and dogs. Sci Rep. 12(19294)2022.PubMed/NCBI View Article : Google Scholar
|















