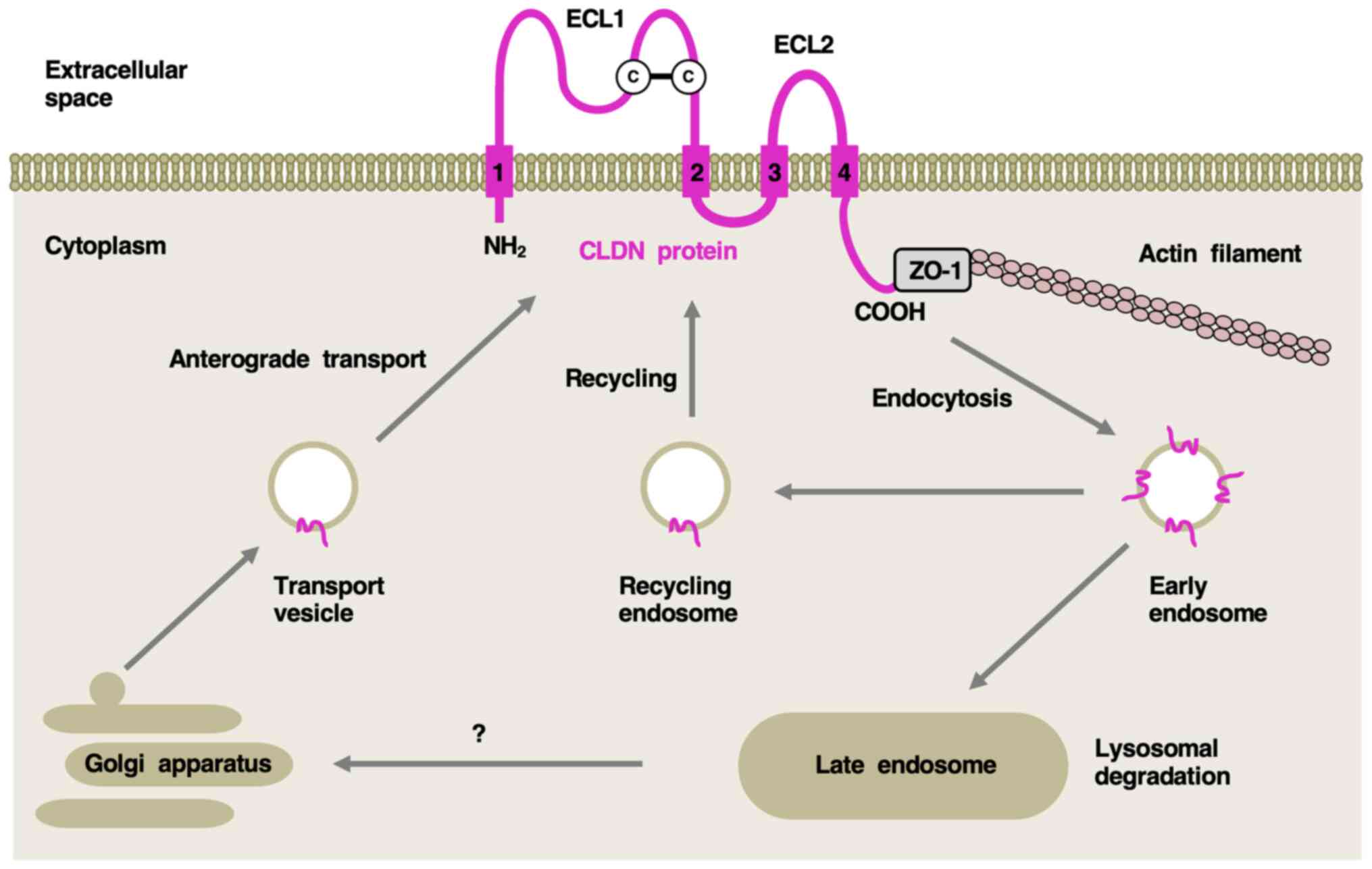
CLDN isoforms at intercellular tight junctions have
four transmembrane domains. The first extracellular folding loop
stabilizes the paracellular interface and the C-terminal
cytoplasmic region interacts with the zona occludens 1 scaffold
protein for the assembly of other tight junction proteins (7,8).
CLDN proteins that form homo- and heterotypical as well as
trans/cis complexes regulate paracellular barrier or permeability
functions at endothelial, epidermal, gastrointestinal, renal and
other interfaces to maintain organ and/or whole-body homeostasis
(9-15). Tight junction functions are
dynamically regulated by junctional CLDN isoforms that undergo
antegrade transport from the Golgi apparatus to the plasma
membrane, endocytosis to early endosomes, and sorting to recycling
endosomes for trafficking back to the cell surface or late
endosomes for lysosomal degradation (Fig. 1).
Because CLDN proteins at non-junctional basolateral
membranes in tumor cells are accessible targets for antibody-based
therapeutic modalities irrespective of their oncogenic or tumor
suppressive function and signaling, anti-CLDN monoclonal antibodies
(mAbs), antibody-drug conjugates (ADCs) and bispecific antibodies
(bsAbs; including bispecific T cell engagers), as well as
CLDN-directed chimeric antigen receptor (CAR) T cells, have been
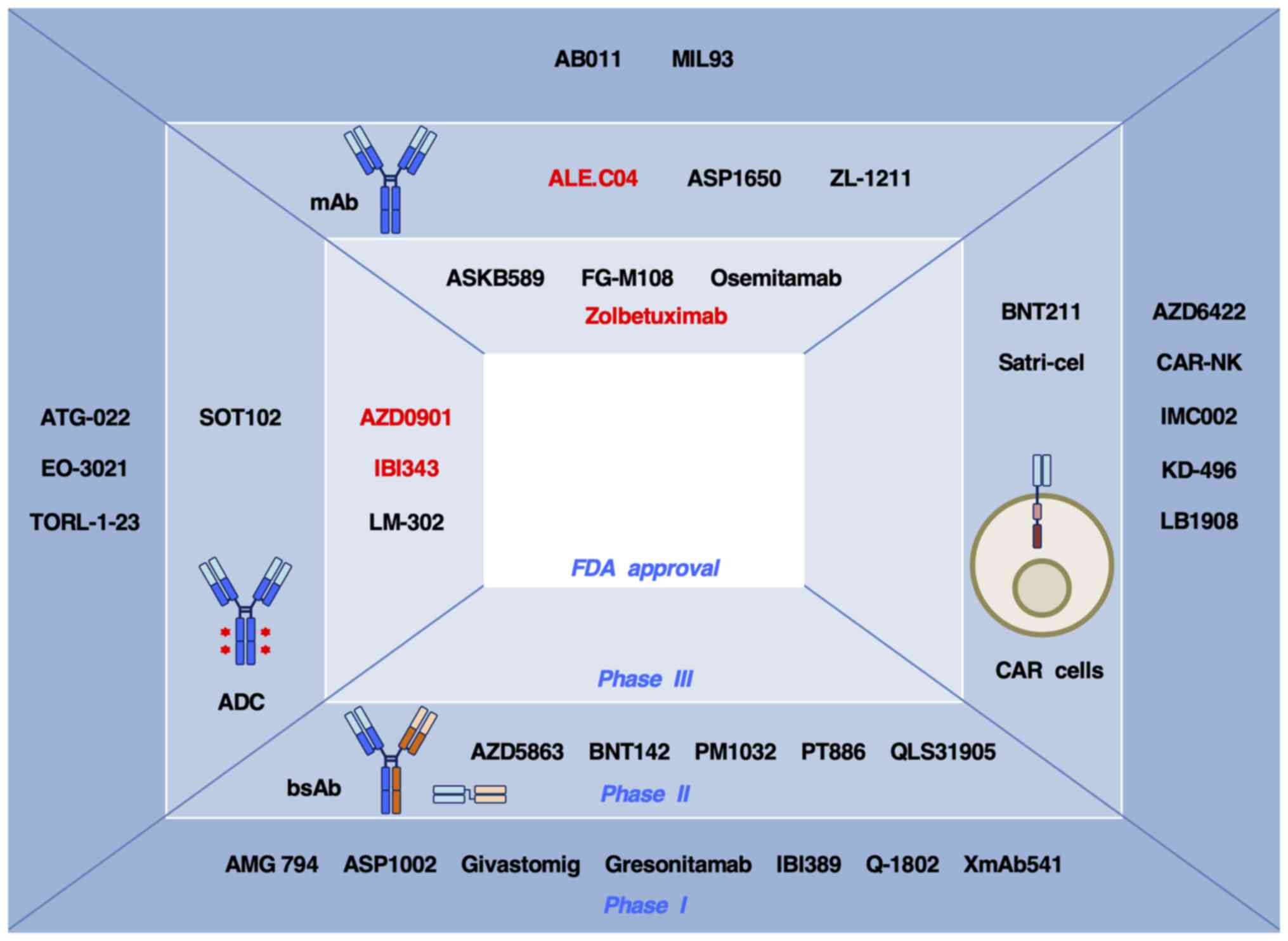
developed (29-34). Drugs targeting CLDN1, 4, 6 and
18.2 have entered clinical trials for the treatment of cancer
(Table II) and some of them
have proceeded to later-phase trials (Fig. 3). Information on CLDN-targeted
therapeutics in clinical trials is reviewed subsequently.
Humanized anti-CLDN1 mAb derived from a rat
anti-human CLDN1 mAb (OM-7D3-B3) exhibits preclinical antitumor
activity through the suppression of cancer stemness and tumor
invasion and reprogramming of the immunosuppressive tumor
microenvironment in HCC xenograft models, especially those with
WNT/β-catenin signaling activation and an epithelial-mesenchymal
transition phenotype (30,37).
The humanized anti-CLDN1 mAb ALE.C04 has also been
shown to have preclinical antitumor activity as a single agent and
in combination with immune checkpoint inhibitors in HNSCC xenograft
models via perturbation of interactions between tumor and stromal
cells to reverse extracellular matrix remodeling, tissue fibrosis
and T cell immune evasion (40,41).
ALE.C04 has received fast track designation by the
US Food and Drug Administration (FDA) for treatment of recurrent or
metastatic CLDN1-positive HNSCC (42). A phase I/II clinical trial of
ALE.C04, as a monotherapy and in combination with pembrolizumab, is
ongoing, with an estimated completion date of February 2028 [trial
no. National Clinical Trial (NCT)06054477; Table II].
CLDN4 is upregulated in solid tumors, including
triple-negative breast cancer (TNBC), colorectal and gastric cancer
(intestinal subtype), non-small cell lung cancer (NSCLC), ovarian
(serous subtype), pancreatic and prostate cancer, and urothelial
carcinoma (26,40,43). The biological roles of CLDN4 in
human carcinogenesis are dependent on the tumor type (26,39).
The bsAb ASP1002, which targets CLDN4 and 4-1BB, has
been shown to induce antitumor activity in cancer with CLDN4
upregulation through costimulatory T cell signaling activation and
subsequent T cell proliferation and cytokine production (43); however, to the best of our
knowledge, single-chain variable fragment (scFv) components
targeting CLDN4 and CD137 and their epitopes, in vitro
functions and in vivo antitumor effects of ASP1002 are
unknown. ASP1002 is in a phase I clinical trial for the treatment
of CLDN4-positive solid tumors, such as colorectal, ovarian and
prostate cancer, NSCLC, TNBC and urothelial carcinoma (trial no.
NCT05719558; Table II).
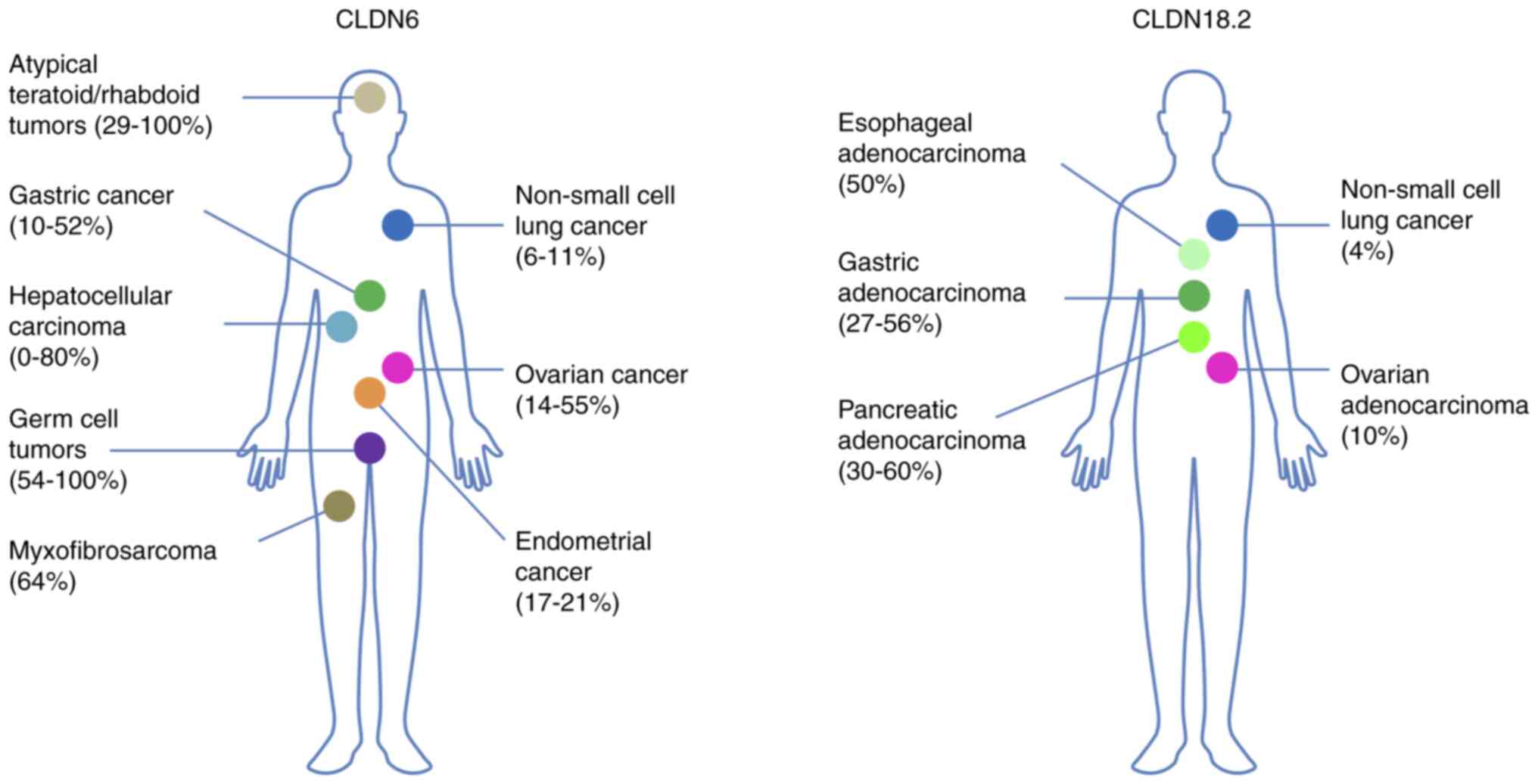
CLDN6 is preferentially expressed in pluripotent
stem cells and endodermal precursors, such as hepatic or pancreatic
progenitor cells, and in fetal tissue derived from the stomach,
pancreas and lung, but is suppressed in adult tissues (44-46). CLDN6 is then reactivated in tumor
tissue and upregulated in ovarian (14-55%), endometrial (17-21%)
and gastric cancer (10-52%), HCC (0-80%), and NSCLC (6-11%), as
well as rare malignancies, such as germ cell (54-100%) and atypical
teratoid/rhabdoid tumors (29-100%), and myxofibrosarcomas (64%)
(28,47-49).
ASP1650 is a mouse/human chimeric anti-CLDN6 mAb
that exhibited antitumor effects on ovarian cancer and testicular
tumor cells via antibody-dependent cellular cytotoxicity (ADCC) and
complement-dependent cytotoxicity in a preclinical study (50) and has entered a phase I clinical
trial for ovarian cancer (trial no. NCT02054351) and phase II
clinical trial for male patients with germ cell tumors (trial no.
NCT03760081). The objective response rates (ORRs) of ASP1650 were 2
(1/41) and 0% (0/13) in the NCT02054351 and NCT03760081 clinical
trials, respectively (51,52).
TORL-1-23 (CLDN6-23-ADC) is an anti-CLDN6 ADC that
consists of humanized CLDN6-23-mAb that recognizes the second
extracellular loop of CLDN6, a protease cleavable linker and a
monomethyl auristatin E (MMAE) cytotoxic payload with a
drug/antibody ratio (DAR) of 4.1 (53). In a preclinical study, TORL-1-23
was >10-fold more potent than CLDN6-23-mAb and exerted in
vivo antitumor effects on bladder, endometrial and ovarian
cancer (53). Notably, in a
phase I clinical trial for the treatment of advanced cancer,
including endometrial, ovarian and testicular cancer (trial no.
NCT05103683), the ORR of TORL-1-23 in CLDN6-positive cancer was 32%
(7/22) (54).
AMG 794, BNT142 and XmAb541 are bsAbs that
simultaneously bind CLDN6 on tumor cells and CD3 on T cells for the
elimination of tumor cells through recruitment, activation and
proliferation of CLDN6-targeting cytotoxic T cells (55-57). Mouse model experiments have
revealed that AMG 794 exerted antitumor effects on epithelial
ovarian cancer and NSCLC (55)
and that BNT142 exerted antitumor effects on serous ovarian cancer
and ovarian teratocarcinoma (56). A phase I clinical trial of AMG
794 for patients with NSCLC, epithelial ovarian cancer and other
types of solid tumor (trial no. NCT05317078), a phase I/II clinical
trial of BNT142 for CLDN6-positive solid tumors (trial no.
NCT05262530) and a phase I clinical trial of XmAb541 for solid
tumors (trial no. NCT06276491) are ongoing.
SAIL66 is an anti-CLDN6 tsAb that binds CLDN6 on
tumor cells and CD3, as well as 4-1BB on T cells to induce more
robust immune reactions in CLDN6-positive tumor cells compared with
conventional anti-CLDN6 bsAbs (58). In a preclinical study, SAIL66
successfully enhanced T cell infiltration and mitigated T cell
exhaustion via potentiation of 4-1BB-mediated costimulatory
signaling in syngeneic mouse model experiments (58). In a phase I clinical trial of
SAIL66 for CLDN6-positive solid tumors, the anti-interleukin-6
receptor mAb tocilizumab was added to ameliorate cytokine release
syndrome (CRS) caused by enhanced antitumor immunity (trial no.
NCT05735366).
BNT211 consists of CLDN6-targeting and
4-1BB-stimulating CAR T cells and a CAR T cell-amplifying RNA
vaccine (CARVac) (59). CARVac
is a liposomal CLDN6-expressing RNA that induces ectopic CLDN6
expression on antigen-presenting cells, such as dendritic cells and
macrophages, for stimulation and expansion of CLDN6-targeting CAR T
cells (59). BNT211 exerted
antitumor effects on ovarian and lung tumors in a preclinical study
(45) and was assessed in a
phase I/II clinical trial for the treatment of patients with
CLDN6-positive solid tumors (trial no. NCT04503278). Although signs
of CRS appeared in ~50% of patients due to enhanced immunity, the
ORR of BNT211 was 33% (7/21) (59).
CLDN18.2 is upregulated in gastric (27-56%),
esophageal (50%) and ovarian adenocarcinoma (10%), PDAC (30-60%),
and NSCLC (4%) (27,61-64). Due to the upregulation of
non-junctional CLDN18.2 in tumor tissues and the limited expression
of tight junction protein CLDN18.2 in gastric epithelial cells,
CLDN18.2 is a quasicancer-specific antigen (34). Currently, >20 drugs directed
against CLDN18.2 are in clinical trials for treatment of GEA and
other types of cancer (Table
II).
ASKB589, FG-M108 and osemitamab are humanized/human
mAbs that had manageable safety profiles and were well-tolerated in
early-phase clinical trials (trial nos. NCT04632108/NCT05632939,
NCT04894825 and NCT04396821/NCT04495296, respectively). Notably,
the single-agent ORR of ASKB589 in solid tumors was 22% (2/9)
(66) and that of osemitamab was
58% (23/40) (70). The ORRs of
ASKB589 + capecitabine and oxaliplatin (CAPOX) without or with
anti-programmed cell death protein 1 (PD-1) mAb sintilimab in GEA
were 75 (9/12) and 80% (12/15), respectively (66,67). The ORR of FG-M108 +
nab-paclitaxel + gemcitabine in PDAC was 50% (7/14) (68) and the ORR of osemitamab + CAPOX +
anti-PD-1 mAb nivolumab was 57% (45/79) in GEA (71). Currently, randomized,
double-blind phase III clinical trials of ASKB589 + CAPOX +
sintilimab vs. placebo + CAPOX + sintilimab (trial no. NCT06206733)
and FG-M108 + CAPOX vs. placebo + CAPOX (trial no. NCT06177041) in
first-line settings for CLDN18.2-positive GEA are ongoing.
Osemitamab was granted orphan drug designation by the FDA and
combination therapy of osemitamab + chemotherapy + nivolumab is
advancing toward a phase III clinical trial for the treatment of
GEA (trial no. NCT06093425).
Zolbetuximab is a mouse/human chimeric mAb with a
single-agent ORR of 9% (4/43) in CLDN18.2-positive GEA (73). The combination of zolbetuximab +
modified 5-fluorouracil, leucovorin and oxaliplatin chemotherapy
(mFOLFOX6) resulted in greater clinical activity than placebo +
mFOLFOX6 in the SPOTLIGHT study (trial no. NCT03504397) for GEA
with CLDN18.2 upregulation. The median progression-free survival
(mPFS) time was 10.6 vs. 8.7 months [hazard ratio (HR), 0.75; 95%
CI, 0.60-0.94] and the median overall survival (mOS) time was 18.2
vs. 15.5 months (HR, 0.75; 95% CI, 0.60-0.94) (74). The combination of zolbetuximab +
CAPOX improved clinical activity in comparison with placebo + CAPOX
in the GLOW study (trial no. NCT03653507) (75). Zolbetuximab was granted priority
review designation by the FDA in July 2023 but rejected due to
unspecified deficiencies in a third-party manufacturing facility in
January 2024 (76). The data
were resubmitted to the FDA in May 2024 and decision is expected in
November 2024 under the Prescription Drug User Fee Act (77).
AZD0901, with a DAR of 4, has been shown to exert
direct cytotoxic effects on CLDN18.2-overexpressing tumor cells and
bystander killing effects on surrounding tumor cells in preclinical
studies, and had a single-agent ORR of 44% (39/89) in GEA with
CLDN18.2 upregulation in the KYM901 phase I clinical trial (trial
no. NCT04805307) (79,80). AZD0901 also exhibited manageable
safety profiles in the clinic. Anemia (62.8%), vomiting (57.5%) and
hypoalbuminemia (57.5%) were common treatment-emergent adverse
events (TEAEs), and decreased neutrophil count (18.6%) and anemia
(13.3%) were the most frequent grade ≥3 TEAEs (79). AZD0901 monotherapy for treatment
of CLDN18.2-overexpressing GEA was granted fast track designation
by the FDA in April 2022 (86)
and is currently in a phase II clinical trial with an expected
completion date of May 2025 (trial no. NCT06219941); another phase
III randomized clinical trial of AZD0901 monotherapy vs. apatinib,
docetaxel, irinotecan, paclitaxel or TAS-102, has an expected
completion date of April 2026 (trial no. NCT06346392).
IBI343 had single-agent ORRs of 28 [7/25 patients
with PDAC and biliary tract cancer (BTC)] and 40% (4/10 patients
with CLDN18.2-positive PDAC) in a phase I clinical trial (trial no.
NCT05458219) and a manageable safety profile with any-grade [anemia
(37%), nausea (26%), vomiting (26%) and decreased white blood cell
count (20%)] and grade ≥3 treatment-related adverse events (TRAEs)
[anemia (6%) and decreased white blood cell counts (3%)] (82). IBI343 monotherapy was granted
fast track designation by the FDA for the treatment of PDAC
(87). By contrast, for the
treatment of GEA, a phase II clinical trial of combination therapy
of IBI343 + sintilimab (trial no. NCT06321913) and a phase III
randomized clinical trial of IBI343 vs. irinotecan or paclitaxel,
(trial no. NCT06238843) are planned but not yet recruiting.
LM-302 had superior antitumor efficacy to
zolbetuximab in a preclinical study using gastric cancer model
(83), and a single-agent ORR of
31% (11/36) in CLDN18.2-positive GEA and a manageable safety
profile in a phase I/II clinical trial (trial no. NCT05161390)
(84). LM-302 is proceeding to a
phase III randomized clinical trial of LM-302 vs. the
investigator's choice of therapy, apatinib or irinotecan, for
treatment of CLDN18.2-positive GEA (trial no. NCT06351020).
AZD5863, gresonitamab, IBI389 and QLS31905
simultaneously binds CLDN18.2 on tumor cells and CD3 on T cells
(88,90,91,96), which activates CD3 signaling in T
cells and redirects cytotoxic T cells toward tumor killing through
lytic synapse formation and release of granzymes and perforins
(97,98).
Givastomig, PM1032, PT886 and Q-1802 simultaneously
bind CLDN18.2 and non-CD3 immune antigens; givastomig and PM1032
enhance antitumor immunity via 4-1BB-induced activation of
costimulatory signaling (89,92); PT886 reactivates phagocytosis via
CD47-dependent inhibition of 'do not eat me' signaling (94); and Q-1802 inhibits immune
checkpoints via programmed death-ligand 1 (PD-L1)/PD-1 signaling
blockade (95). Givastomig and
PT886 have been granted orphan drug designation by the FDA
(94,99).
Compared with anti-CLDN18.2 mAbs and ADCs,
anti-CLDN18.2 bsAbs are in relatively early phases of clinical
trials (88-96): Givastomig, IBI389 and Q-1802 are
in phase I clinical trials; AZD5863, PM1032 and PT886 are in phase
I/II clinical trials; and QLS31905 is proceeding to phase I/II
clinical trials (Fig. 3).
In a phase I clinical trial, IBI389 caused any-grade
TRAEs in 97.4% (111/114) and grade ≥3 TRAEs in 55.3%, including
any-grade CRS (57.0%) and grade 3 CRS (0.9%), of patients with
solid tumors, such as GEA (n=37) and PDAC (n=66) (trial no.
NCT05164458). The single-agent ORR of IBI389 was 31% (8/26) in GEA
with CLDN18.2 overexpression (91).
In a phase I/II clinical trial, PM1032 caused
any-grade TRAEs in 73% (22/30) and grade ≥3 TRAEs in 10% of
gastrointestinal cancer cases (trial no. NCT05839106). The
single-agent ORR of PM1032 was 20% (2/10) in CLDN18.2-positive GEA
(93).
In a phase I clinical trial (trial no. NCT04856150)
of Q-1802, any-grade TRAEs, including nausea (62%; 18/29), vomiting
(62%) and abdominal pain (28%), immune-related adverse events (AEs;
such as abnormal thyroid function, rash and arthritis) (24%), and
grade 3 TRAEs such as nausea and vomiting (24%) and grade 4 TRAE
hyponatremia (3%) were observed. The single-agent ORR of Q-1802 was
22% (2/9) in gastrointestinal cancer (95).
QLS31905 treatment was associated with any-grade
TRAEs in 98% (51/52) and grade ≥3 TRAEs in 40% of patients treated
with 0.5-500.0 mg/kg once/week (qW) or once every 2 weeks (q2W)
QLS31905, including grade 3 CRS in 2 patients in the 350 mg/kg qW
QLS31905 cohort, in a phase I clinical trial (trial no.
NCT05278832). The single-agent ORR of QLS31905 was 11% (3/27) in
the phase I study (96), and a
phase I/II clinical trial of QLS31905 plus chemotherapy is planned
(trial no. NCT06041035).
Satricabtagene autoleucel (Satri-cel) refers to
autologous CAR T cells targeting CLDN18.2: CAR T cells were
generated from peripheral blood mononuclear cells via lentiviral
transduction of a second-generation CAR construct consisting of an
extracellular humanized anti-CLDN18.2 scFv and a CD8α hinge region,
CD28 transmembrane domain and cytoplasmic CD28 costimulatory and
CD3ζ signaling domains. CAR T cells were formulated and infused
back following preconditioning combination therapy with
cyclophosphamide, fludarabine and nab-paclitaxel or gemcitabine
(100,101).
Satri-cel was assessed in a phase I clinical trial
(trial no. NCT03874897) for the treatment of solid tumors on the
basis of the results of a preclinical study that revealed antitumor
effects with persistent infiltration of CAR T cells into
CLDN18.2-positive gastric cancer patient-derived xenograft models
(100). A total of ~75% of
patients in the Satri-cel clinical trial received bridging therapy,
such as folinic acid, fluorouracil and irinotecan, nab-paclitaxel
or irinotecan, during autologous CAR T cell production (median, 27
days; range, 22-187 days) (101). Satri-cel exhibited tolerability
and safety profiles with manageable AEs, such as
preconditioning-associated transient hematological toxicity (grade
≥3, 100%), CRS (any grade, 97%; grade ≥3, 0%), nausea (any grade,
67%; grade ≥3, 1%), vomiting (any grade, 53%; grade ≥3, 3%) and
gastric mucosal injury (grade 1/2, 7%; grade 3, 1%) and without
immune effector cell-associated neurotoxicity syndrome, and
clinical activity, as indicated by an ORR of 39% (38/98), mPFS time
of 4.4 months (95% CI, 3.7-6.6) and mOS time of 8.8 months (95% CI,
7.1-10.2) (101). Currently, a
randomized phase I/II clinical trial of Satri-cel vs. apatinib,
irinotecan or paclitaxel, for the treatment of CLDN18.2-positive
GEA and PDAC is ongoing (trial no. NCT04581473).
CLDN18.2-targeting CAR T cells, such as LB1908
expressing a CAR with a 4-1BB costimulatory domain (102), IMC002 harboring a CAR with
anti-CLDN18.2 variable heavy domain of heavy chain antibody instead
of a scFv (103), AZD6422-armed
CAR T cells with a dominant-negative TGF-β type II receptor
(dnTGFBR2) to overcome the TGF-β-induced immunosuppressive tumor
microenvironment (104) and
KD-496 bispecific CAR T cells that simultaneously recognize
CLDN18.2 and NK group 2 member D (NKG2D) ligands on tumor cells
(105), are also in phase I
clinical trials for the treatment of patients with
CLDN18.2-positive tumors (Table
II).
CLDN-directed therapy poses issues related to
antibody-based therapeutic modalities. Perspectives on mAbs, ADCs,
bsAbs and CAR drugs are discussed subsequently, with a focus on
CLDN targeting for cancer treatment.
Phase III SPOTLIGHT and GLOW clinical trials for
CLDN18.2-positive GEA demonstrated greater clinical activity of
zolbetuximab + chemotherapy than placebo + chemotherapy (74,75). Other anti-CLDN18.2 mAbs have been
assessed, and ASKB589, FG-M108 and osemitamab are being assessed in
randomized phase III clinical trials with expected completion dates
of December 2026, January 2027 and October 2025, respectively
(Fig. 3). The single-agent ORR
of chimeric mAb zolbetuximab is 9% (73), whereas that of humanized mAbs
ASKB589 and osemitamab, which have increased affinity and enhanced
ADCC effects, is 22 and 58%, respectively (66,70). Previous-generation mAb drugs were
chimeric antibodies that elicit host immune responses to remaining
variable regions derived from mouse antibody, while
current-generation mAb drugs are human/humanized antibodies without
mouse-derived variable regions (106). Comparison of the clinical
activity (mPFS and mOS) of zolbetuximab and other humanized/human
anti-CLDN18.2 mAbs is warranted.
CLDN-targeting ADCs have exhibited improved clinical
outcomes over CLDN-targeting chimeric mAbs in early-phase clinical
trials; the single-agent ORRs of ATG-022 (78), AZD0901 (79), EO-3021 (81), IBI343 (82), LM-302 (84) and TORL-1-23 (54) were 20, 44, 38, 28, 31 and 32%,
respectively, compared with 0-9% for the chimeric anti-CLDN mAbs
ASP1650 (51,52) and zolbetuximab (73).
ORRs of the aforementioned anti-CLDN ADCs are
similar to those of FDA-approved ADCs targeting other
tumor-associated antigens, such as the ORR of 31.5% of
anti-trophoblast cell surface antigen 2 ADC sacituzumab govitecan
(109) and the ORR of 42.9% of
the anti-nectin cell adhesion molecule 4 ADC enfortumab vedotin
(110). AZD0901, IBI343 and
LM-302 have entered or are entering phase III clinical trials,
which will yield mPFS and mOS data in the future.
Satri-cel, an autologous CAR T cell therapy with a
second-generation CLDN18.2-directed CAR, has an ORR of 39%
(101), which is similar to the
ORRs of BNT211 (CLDN6-targeting CAR-T cell + RNA vaccine; 33%)
(59) and other solid
tumor-targeting CAR T cell therapies directed at carcinoembryonic
antigen (15%; 6/40) (120),
EGFR (18%; 2/11) (121),
glypican 3 (50%; 11/22) (122)
and guanylyl cyclase 2C (26%; 5/19) (123), but less effective than
FDA-approved CAR T cell therapies targeting hematological
malignancies, such as the CD19-directed CAR T cells axicabtagene
ciloleucel (82%; 83/101) (124)
and B cell maturation antigen-directed CAR T cells ciltacabtagene
autoleucel (98%; 95/97) (125).
Numerous challenges hinder the activities of CAR T
cells, especially those in solid tumors. These include
epitope-losing subclonal replacement on the basis of pretreatment
heterogeneity and/or posttreatment evolution (126-128), decreased infiltration of CAR T
cells into an immunosuppressive tumor microenvironment harboring
angiogenic endothelial cells, extracellular matrix-remodeling
cancer-associated fibroblasts, regulatory T cells, M2-type
macrophages and monocytic myeloid-derived suppressor cells
(129-131), and decreased persistence of CAR
T cells due to mechanisms similar to the exhaustion of effector T
cells with diminished anti-tumor activity dependent on TGF-β
signaling as well as PD-L1/PD-1 and other coinhibitory signaling
(132-134).
bsCAR T cells dually targeting CLDN18.2 and NKG2D
ligands have been developed to address intratumor heterogeneity
(105). CLDN6-directed CAR-NK
cells, which incorporate the NKG2D transmembrane domain, CD244
costimulatory domain and the DNAX-activation protein 10 signaling
domain have been developed to enhance antitumor immunity (60). CLDN18.2-directed CAR T cells
armed with dnTGFBR2 have been developed to eliminate
immunosuppressive effects of TGF-β (104) and preclinical CAR T cells armed
with forkhead-box O1 transcription factor have been developed to
maintain the stem/memory cell subpopulation, retain effector
functions and prevent the exhaustion phenotype (133,134). These strategies might further
improve the clinical activity of CLDN-targeted CAR therapy.
Drugs directed at CLDN1, 4, 6 and 18.2
tumor-associated antigens have been developed on the basis of mAb,
ADC, bsAb or CAR modalities. A total of >30 CLDN-targeting
therapeutics have entered clinical trials, and anti-CLDN18.2 mAbs
(ASKB589, FG-M108, osemitamab and zolbetuximab) and ADCs (AZD0901,
IBI343 and LM-302) have been assessed in phase III clinical trials.
AZD0901, IBI343, zolbetuximab and anti-CLDN1 mAb ALE.C04 have been
granted fast track or priority review designation by the FDA but
have not yet been approved as of August 2024. However, there is a
lack of results from phase III clinical trials other than those
involving zolbetuximab. Monotherapies with human/humanized ADCs and
armed CAR T cells may be promising choices for CLDN-directed cancer
therapy.
Not applicable.
MasukoK and MasaruK wrote the manuscript. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by the Katoh Fund for
Knowledge-Base and the Global Network Projects.
|
1
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katoh M and Katoh M: CLDN23 gene,
frequently down-regulated in intestinal-type gastric cancer, is a
novel member of CLAUDIN gene family. Int J Mol Med. 11:683–689.
2003.PubMed/NCBI
|
|
3
|
Krause G, Winkler L, Mueller SL, Haseloff
RF, Piontek J and Blasig IE: Structure and function of claudins.
Biochim Biophys Acta. 1778:631–645. 2008. View Article : Google Scholar
|
|
4
|
Baltzegar DA, Reading BJ, Brune ES and
Borski RJ: Phylogenetic revision of the claudin gene family. Mar
Genomics. 11:17–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Günzel D, Stuiver M, Kausalya PJ, Haisch
L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M and Müller D:
Claudin-10 exists in six alternatively spliced isoforms that
exhibit distinct localization and function. J Cell Sci.
122:1507–1517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niimi T, Nagashima K, Ward JM, Minoo P,
Zimonjic DB, Popescu NC and Kimura S: Claudin-18, a novel
downstream target gene for the T/EBP/NKX2.1 homeodomain
transcription factor, encodes lung- and stomach-specific isoforms
through alternative splicing. Mol Cell Biol. 21:7380–7390. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zihni C, Mills C, Matter K and Balda MS:
Tight junctions: From simple barriers to multifunctional molecular
gates. Nat Rev Mol Cell Biol. 17:564–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vecchio AJ, Rathnayake SS and Stroud RM:
Structural basis for Clostridium perfringens enterotoxin targeting
of claudins at tight junctions in mammalian gut. Proc Natl Acad Sci
USA. 118:e20246511182021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Günzel D and Yu AS: Claudins and the
modulation of tight junction permeability. Physiol Rev. 93:525–569.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
France MM and Turner JR: The mucosal
barrier at a glance. J Cell Sci. 130:307–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stamatovic SM, Johnson AM, Sladojevic N,
Keep RF and Andjelkovic AV: Endocytosis of tight junction proteins
and the regulation of degradation and recycling. Ann N Y Acad Sci.
1397:54–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Horowitz A, Chanez-Paredes SD, Haest X and
Turner JR: Paracellular permeability and tight junction regulation
in gut health and disease. Nat Rev Gastroenterol Hepatol.
20:417–432. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meoli L and Günzel D: The role of claudins
in homeostasis. Nat Rev Nephrol. 19:587–603. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka H, Yamamoto Y, Kashihara H,
Yamazaki Y, Tani K, Fujiyoshi Y, Mineta K, Takeuchi K, Tamura A and
Tsukita S: Claudin-21 has a paracellular channel role at tight
junctions. Mol Cell Biol. 36:954–964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raya-Sandino A, Lozada-Soto KM, Rajagopal
N, Garcia-Hernandez V, Luissint AC, Brazil JC, Cui G, Koval M,
Parkos CA, Nangia S and Nusrat A: Claudin-23 reshapes epithelial
tight junction architecture to regulate barrier function. Nat
Commun. 14:62142023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hadj-Rabia S, Baala L, Vabres P,
Hamel-Teillac D, Jacquemin E, Fabre M, Lyonnet S, De Prost Y,
Munnich A, Hadchouel M and Smahi A: Claudin-1 gene mutations in
neonatal sclerosing cholangitis associated with ichthyosis: A tight
junction disease. Gastroenterology. 127:1386–1390. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Askari M, Karamzadeh R, Ansari-Pour N,
Karimi-Jafari MH, Almadani N, Sadighi Gilani MA, Gourabi H, Vosough
Taghi Dizaj A, Mohseni Meybodi A, Sadeghi M, et al: Identification
of a missense variant in CLDN2 in obstructive azoospermia. J Hum
Genet. 64:1023–1032. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klar J, Piontek J, Milatz S, Tariq M,
Jameel M, Breiderhoff T, Schuster J, Fatima A, Asif M, Sher M, et
al: Altered paracellular cation permeability due to a rare CLDN10B
variant causes anhidrosis and kidney damage. PLoS Genet.
13:e10068972017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sineni CJ, Yildirim-Baylan M, Guo S,
Camarena V, Wang G, Tokgoz-Yilmaz S, Duman D, Bademci G and Tekin
M: A truncating CLDN9 variant is associated with autosomal
recessive nonsyndromic hearing loss. Hum Genet. 138:1071–1075.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wilcox ER, Burton QL, Naz S, Riazuddin S,
Smith TN, Ploplis B, Belyatseva I, Ben-Yosef T, Liburd NA, Morell
RJ, et al: Mutations in the gene encoding tight junction claudin-14
cause autosomal recessive deafness DFNB29. Cell. 104:165–172. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simon DB, Lu Y, Choate KA, Velazquez H,
Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G,
Rodriguez-Soriano J, et al: Paracellin-1, a renal tight junction
protein required for paracellular Mg2+ resorption. Science.
285:103–106. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konrad M, Schaller A, Seelow D, Pandey AV,
Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C,
et al: Mutations in the tight-junction gene claudin 19 (CLDN19) are
associated with renal magnesium wasting, renal failure, and severe
ocular involvement. Am J Hum Genet. 79:949–957. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riedhammer KM, Stockler S, Ploski R,
Wenzel M, Adis-Dutschmann B, Ahting U, Alhaddad B, Blaschek A,
Haack TB, Kopajtich R, et al: De novo stop-loss variants in CLDN11
cause hypomyelinating leukodystrophy. Brain. 144:411–419. 2021.
View Article : Google Scholar :
|
|
24
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakayama I, Shinozaki E, Sakata S,
Yamamoto N, Fujisaki J, Muramatsu Y, Hirota T, Takeuchi K,
Takahashi S, Yamaguchi K and Noda T: Enrichment of CLDN18-ARHGAP
fusion gene in gastric cancers in young adults. Cancer Sci.
110:1352–1363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morin PJ: Claudin proteins in human
cancer: Promising new targets for diagnosis and therapy. Cancer
Res. 65:9603–9606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sahin U, Koslowski M, Dhaene K, Usener D,
Brandenburg G, Seitz G, Huber C and Türeci O: Claudin-18 splice
variant 2 is a pan-cancer target suitable for therapeutic antibody
development. Clin Cancer Res. 14:7624–7634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Turksen K and Troy TC: Junctions gone bad:
Claudins and loss of the barrier in cancer. Biochim Biophys Acta.
1816:73–79. 2011.PubMed/NCBI
|
|
29
|
Qu H, Jin Q and Quan C: CLDN6: From
traditional barrier function to emerging roles in cancers. Int J
Mol Sci. 22:134162021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roehlen N, Muller M, Nehme Z, Crouchet E,
Jühling F, Del Zompo F, Cherradi S, Duong FHT, Almeida N, Saviano
A, et al: Treatment of HCC with claudin-1-specific antibodies
suppresses carcinogenic signaling and reprograms the tumor
microenvironment. J Hepatol. 78:343–355. 2023. View Article : Google Scholar
|
|
31
|
Katoh M and Katoh M: Precision medicine
for human cancers with Notch signaling dysregulation (Review). Int
J Mol Med. 45:279–297. 2020.PubMed/NCBI
|
|
32
|
Cao W, Xing H, Li Y, Tian W, Song Y, Jiang
Z and Yu J: Claudin18.2 is a novel molecular biomarker for
tumor-targeted immunotherapy. Biomark Res. 10:382022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vonniessen B, Tabariès S and Siegel PM:
Antibody-mediated targeting of Claudins in cancer. Front Oncol.
14:13207662024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakayama I, Qi C, Chen Y, Nakamura Y, Shen
L and Shitara K: Claudin 18.2 as a novel therapeutic target. Nat
Rev Clin Oncol. 21:354–369. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miwa N, Furuse M, Tsukita S, Niikawa N,
Nakamura Y and Furukawa Y: Involvement of claudin-1 in the
beta-catenin/Tcf signaling pathway and its frequent upregulation in
human colorectal cancers. Oncol Res. 12:469–476. 2001. View Article : Google Scholar
|
|
36
|
Katoh M: Multi-layered prevention and
treatment of chronic inflammation, organ fibrosis and cancer
associated with canonical WNT/β-catenin signaling activation
(Review). Int J Mol Med. 42:713–725. 2018.PubMed/NCBI
|
|
37
|
Zeisel MB, Dhawan P and Baumert TF: Tight
junction proteins in gastrointestinal and liver disease. Gut.
68:547–561. 2019. View Article : Google Scholar
|
|
38
|
Bhat AA, Syed N, Therachiyil L, Nisar S,
Hashem S, Macha MA, Yadav SK, Krishnankutty R, Muralitharan S,
Al-Naemi H, et al: Claudin-1, a double-edged sword in cancer. Int J
Mol Sci. 21:5692020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hana C, Thaw Dar NN, Galo Venegas M and
Vulfovich M: Claudins in cancer: A current and future therapeutic
target. Int J Mol Sci. 25:46342024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Toso A, Teixiera G, Zimmermann T,
Schmitter D, Meyer M, Muller M, Mailly L, Baumert T and Iacone R:
193P CLAUDIN-1 targeting antibodies in solid tumors: From ALE.C04
to CLAUDIN-1 oncology platform. Immunooncol Technol. 16(Suppl 1):
S1003052022. View Article : Google Scholar
|
|
41
|
Toso A, Teixeira G, Zimmermann T, Gill SG,
Schmitter D, Meyer M, Muller M, Mailly L, Baumert T, Manenti L and
Iacone R: Abstract LB284: CLAUDIN-1 targeting antibody ALE.C04
drives single activity and restores anti-PD1 efficacy in solid
tumors. Cancer Res. 83(Suppl 8): LB2842023. View Article : Google Scholar
|
|
42
|
Rosa K: FDA grantsfast track status to
ALE.C04 for recurrent or metastatic CLDN1+ HNSCC. OncLive. 2023,
https://www.onclive.com/view/fda-grants-fast-track-status-to-ale-c04-for-recurrent-or-metastatic-cldn1-hnscc.
|
|
43
|
Pelster M, Marron TU, Friend BD, Fan A,
Yang J and Spira AI: Phase 1 study of ASP1002, a bispecific
antibody targeting claudin 4 (CLDN4) and CD137, in patients with
locally advanced (LA) or metastatic solid tumors that express
CLDN4. J Clin Oncol. 42(Suppl 16): TPS26702024. View Article : Google Scholar
|
|
44
|
Ben-David U, Nudel N and Benvenisty N:
Immunologic and chemical targeting of the tight-junction protein
Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat
Commun. 4:19922013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reinhard K, Rengstl B, Oehm P, Michel K,
Billmeier A, Hayduk N, Klein O, Kuna K, Ouchan Y, Wöll S, et al: An
RNA vaccine drives expansion and efficacy of claudin-CAR-T cells
against solid tumors. Science. 367:446–453. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kong FE, Li GM, Tang YQ, Xi SY, Loong JHC,
Li MM, Li HL, Cheng W, Zhu WJ, Mo JQ, et al: Targeting tumor
lineage plasticity in hepatocellular carcinoma using an anti-CLDN6
antibody-drug conjugate. Sci Transl Med. 13:eabb62822021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang C, Guo C, Li Y, Liu K, Zhao Q and
Ouyang L: Identification of claudin-6 as a molecular biomarker in
pan-cancer through multiple omics integrative analysis. Front Cell
Dev Biol. 9:7266562021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Du H, Yang X, Fan J and Du X: Claudin 6:
Therapeutic prospects for tumours, and mechanisms of expression and
regulation (Review). Mol Med Rep. 24:6772021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tsang N, Veillard N, Horsley E, Havenith
K, Janghra N, Zeitseva K, Oblette C, Kirby I, Hogg PW, Zammarchi F,
et al: Abstract 3122: Preclinical development of a novel
camptothecin-based antibody-drug conjugate targeting solid tumors
expressing Claudin-6. Cancer Res. 84(76 Suppl): S31222024.
View Article : Google Scholar
|
|
50
|
Türeci Ö, Kreuzberg M, Walter K, Wöll S,
Schmitt R, Mitnacht-Kraus R, Nakajo I, Yamada T and Sahin U:
Abstract 882: The anti-claudin 6 antibody, IMAB027, induces
antibody-dependent cellular and complement-dependent cytotoxicity
in claudin 6-expressing cancer cells. Cancer Res. 78(Suppl 13):
S8822018. View Article : Google Scholar
|
|
51
|
Sahin U, Jaeger D, Marme F, Mavratzas A,
Krauss J, De Greve J, Vergote I and Tureci O: First-in-human phase
I/II dose-escalation study of IMAB027 in patients with recurrent
advanced ovarian cancer (OVAR): Preliminary data of phase I part. J
Clin Oncol. 33(15 Suppl): S55372015. View Article : Google Scholar
|
|
52
|
Adra N, Vaughn DJ, Einhorn LH, Hanna NH,
Funt SA, Rosales M, Arozullah A and Feldman DR: A phase II study
assessing the safety and efficacy of ASP1650 in male patients with
relapsed refractory germ cell tumors. Invest New Drugs.
40:1087–1094. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
McDermott MSJ, O'Brien NA, Hoffstrom B,
Gong K, Lu M, Zhang J, Luo T, Liang M, Jia W, Hong JJ, et al:
Preclinical efficacy of the antibody-drug conjugate CLDN6-23-ADC
for the treatment of CLDN6-positive solid tumors. Clin Cancer Res.
29:2131–2143. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Konecny GE, Wahner Hendrickson AE,
Winterhoff B, Machado A, Chander C, Davenport S, Bilic S, Miller
LL, Chung A, Press MF, et al: 756P First-in-human phase I study of
a novel claudin 6 (CLDN6) targeted antibody drug conjugate (ADC)
TORL-1-23. Ann Oncol. 34(Suppl 2): S5172023. View Article : Google Scholar
|
|
55
|
Pham E, Henn A, Sable B, Wahl J, Conner K,
Matthes K, Gupta S, Yabut R, Aeffner F, Wilson KL, et al: Abstract
5202: AMG 794, a Claudin 6-targeted half-life extended (HLE)
bispecific T cell engager (BITE®) molecule for non-small
cell lung cancer and epithelial ovarian cancer. Cancer Res.
82(Suppl 12): S52022022. View Article : Google Scholar
|
|
56
|
Stadler CR, Ellinghaus U, Fischer L,
Bähr-Mahmud H, Rao M, Lindemann C, Chaturvedi A, Scharf C, Biermann
I, Hebich B, et al: Preclinical efficacy and pharmacokinetics of an
RNA-encoded T cell-engaging bispecific antibody targeting human
claudin 6. Sci Transl Med. 16:eadl27202024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Faber MS, Lee SH, Kim YK, Qi J, Avery KN,
Nguyen DHT, Rashid R, Eivazi A, Chu SY, Diaz JE, et al: Abstract
1860: Bispecific claudin-6 x CD3 antibodies in a 2 + 1 format
demonstrate selectivity and activity on human ovarian cancer cells.
Cancer Res. 81(Suppl 13): S18602021. View Article : Google Scholar
|
|
58
|
Kamikawa T, Kimura N, Ishii S, Muraoka M,
Taniguchi K, Uchikawa R, Yoshimoto M, Okuda-Miura M, Akai S, Kodama
T, et al: 1172 SAIL66, a next generation of T cell engager
targeting CLDN6, potentiates efficacy. J Immunother Cancer.
11(Suppl 1): S11722023.
|
|
59
|
Mackensen A, Haanen JBAG, Koenecke C,
Alsdorf W, Wagner-Drouet E, Borchmann P, Heudobler D, Ferstl B,
Klobuch S, Bokemeyer C, et al: CLDN6-specific CAR-T cells plus
amplifying RNA vaccine in relapsed or refractory solid tumors: The
phase 1 BNT211-01 trial. Nat Med. 29:2844–2853. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li J, Hu H, Lian H, Yang S, Liu M, He J,
Cao B, Chen D, Hu Y, Zhi C, et al: NK-92MI cells engineered with
anti-claudin-6 chimeric antigen receptors in immunotherapy for
ovarian cancer. Int J Biol Sci. 20:1578–1601. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Micke P, Mattsson JSM, Edlund K, Lohr M,
Jirström K, Berglund A, Botling J, Rahnenfuehrer J, Marincevic M,
Pontén F, et al: Aberrantly activated claudin 6 and 18.2 as
potential therapy targets in non-small-cell lung cancer. Int J
Cancer. 135:2206–2214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dottermusch M, Krüger S, Behrens HM,
Halske C and Röcken C: Expression of the potential therapeutic
target claudin-18.2 is frequently decreased in gastric cancer:
Results from a large Caucasian cohort study. Virchows Arch.
475:563–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen J, Xu Z, Hu C, Zhang S, Zi M, Yuan L
and Cheng X: Targeting CLDN18.2 in cancers of the gastrointestinal
tract: New drugs and new indications. Front Oncol. 13:11323192023.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lyu SI, Fretter C, Simon AG, Spielmann SM,
Damanakis AI, Zhao Y, Bruns CJ, Schmidt T, Popp FC, Waldschmidt D,
et al: Extent and clinical significance of the therapy-relevant
tight junction protein Claudin 18.2 in pancreatic ductal
adenocarcinoma-real-world evidence. Transl Oncol. 47:1020442024.
View Article : Google Scholar
|
|
65
|
Li J, Pan H, Liu T, Xu N, Zhang Y, Qin Y,
Shi J, Liao D, Shen L, Luo S, et al: A multicenter, phase 1 study
of AB011, a recombinant humanized anti-CLDN18.2 monoclonal
antibody, as monotherapy and combined with capecitabine and
oxaliplatin (CAPOX) in patients with advanced solid tumors. J Clin
Oncol. 41(Suppl 4): S3912023. View Article : Google Scholar
|
|
66
|
Zhang M, Gong J, Wang J, Shi J, Zhu H,
Wang Y, Chen Y, Wang F, Qu X, Yu J, et al: A phase I/II study of
ASKB589 [anti-claudin 18.2 (CLDN18.2) monoclonal antibody] in
patients with solid tumors. J Clin Oncol. 41(Suppl 4): S3972023.
View Article : Google Scholar
|
|
67
|
Peng Z, Shen L, He Y, Chen J,
Hickingbottom B and Lu J: A phase Ib/II study of ASKB589
[anti-Claudin 18.2 (CLDN18.2) monoclonal antibody] combined with
CAPOX and PD-1 inhibitor as first-line treatment for locally
advanced, relapsed and metastatic gastric/gastro-esophageal
junction (G/GEJ) adenocarcinoma. J Clin Oncol. 42(Suppl 3):
S3172024. View Article : Google Scholar
|
|
68
|
Jin Z, Zhang Y, Liu F, Zhang S, Gong J,
Zhang M, Liang X, Wang J, Li Y, Yang X, et al: FG-M108 plus
nab-paclitaxel and gemcitabine (AG) as first-line (1L) treatment
for patients with Claudin-18.2 (CLDN18.2) positive locally advanced
unresectable or metastatic pancreatic cancer (PC): Preliminary
results from the phase 1b study. J Clin Oncol. 42(Suppl 16):
S41422024. View Article : Google Scholar
|
|
69
|
Huang J, Zhang B, Wang Y, Wang F, Yu Z, Wu
S, Zheng Y, Cao Y, Xu J, Lan D, et al: Safety and preliminary
efficacy of MIL93 in patients with advanced solid tumors: The
monotherapy part of a phase 1 trial. J Clin Oncol. 41(Suppl 4):
S7982023. View Article : Google Scholar
|
|
70
|
Janjigian Y, Tolcher A, Mehta R, Cecchini
M, Van Tine B, Kundranda M, Olatunji A, Patel MR, Berlin J,
Rocha-Lima CMSP, et al: Abstract CT132: A Phase I/IIa clinical
trial (TranStar101) to evaluate the safety, tolerability and
pharmacokinetics of OSEMITAMAB administered as monotherapy or in
combination with nivolumab or standard of care in patients with
locally advanced or metastatic solid tumors. Cancer Res. 84(Suppl
7): CT1322024. View Article : Google Scholar
|
|
71
|
Zhang X, Guo Z, Zhang J, Guo W, Sun M, Xu
N, Qi C, Zhu X, Zhang L, Qian X, et al: First-line osemitamab
(TST001) plus nivolumab and capox for advanced g/GEJ cancer
(TranStar102): Results of cohort G from a phase I/IIa study. J Clin
Oncol. 42(Suppl 16): S40482024. View Article : Google Scholar
|
|
72
|
Sharma S, Starodub A, Xu N, Chaudhry A,
Sun M, Pelster M, Fu Y, Zhang X, Huang Z, Liu W and Hsu K:
Preliminary results of a phase 1/2, first-in-human, open-label,
dose escalation study of ZL-1211 (anti-Claudin 18.2 mAb) in
patients with unresectable or metastatic solid tumors. J Clin
Oncol. 41(Suppl 16): S25372023. View Article : Google Scholar
|
|
73
|
Türeci O, Sahin U, Schulze-Bergkamen H,
Zvirbule Z, Lordick F, Koeberle D, Thuss-Patience P, Ettrich T,
Arnold D, Bassermann F, et al: A multicentre, phase IIa study of
zolbetuximab as a single agent in patients with recurrent or
refractory advanced adenocarcinoma of the stomach or lower
oesophagus: The MONO study. Ann Oncol. 30:1487–1495. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Shitara K, Lordick F, Bang YJ, Enzinger P,
Ilson D, Shah MA, Van Cutsem E, Xu RH, Aprile G, Xu J, et al:
Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive,
HER2-negative, untreated, locally advanced unresectable or
metastatic gastric or gastro-oesophageal junction adenocarcinoma
(SPOTLIGHT): A multicentre, randomised, double-blind, phase 3
trial. Lancet. 401:1655–1668. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shah MA, Shitara K, Ajani JA, Bang YJ,
Enzinger P, Ilson D, Lordick F, Van Cutsem E, Gallego Plazas J,
Huang J, et al: Zolbetuximab plus CAPOX in CLDN18.2-positive
gastric or gastroesophageal junction adenocarcinoma: The
randomized, phase 3 GLOW trial. Nat Med. 29:2133–2141. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ra J: FDA denies approval for Astellas'
investigational gastric cancer drug. Pharmaceutical Technology.
2014, https://www.pharmaceutical-technology.com/news/fda-denies-approval-for-astellas-investigational-gastric-cancer-drug/.
|
|
77
|
Conroy R: FDA acknowledges zolbetuximab
BLA resubmission for CLDN18.2+ gastric cancer. CancerNetwork. 2024,
https://www.cancernetwork.com/view/fda-acknowledges-zolb-etuximab-bla-resubmission-for-cldn18-2-gastric-cancer/.
|
|
78
|
Bishnoi S, Cao D, Mendis SR, Coward J,
Zhao J, Xie H and Zheng L: An open-label, multicenter, phase I
study of ATG-022 in patients with advanced/metastatic solid tumors
(CLINCH). J Clin Oncol. 42(Suppl 16): S30322024. View Article : Google Scholar
|
|
79
|
Xu RH, Ruan DY, Zhang DS, Liu FR, Luo SX,
Zhuang ZX, Wang ZN, Liu FN, Zhang YQ, Yang JW, et al: A phase 1
trial of claudin 18.2-specific antibody-drug conjugate CMG901 in
patients with advanced gastric/gastroesophageal junction cancer. J
Clin Oncol. 41(Suppl 36): S4344202023. View Article : Google Scholar
|
|
80
|
Raufi AG, Goyal L, Smyth E, Szekeres P,
Petrone M, Hobson R, Thress K, Origuchi M, Nehra J, Brown JS, et
al: CLARITY-PanTumor01: A phase 2 trial of the claudin
18.2-specific antibody-drug conjugate AZD0901 (CMG901) in patients
with CLDN18.2-expressing advanced solid tumors. J Clin Oncol.
42(Suppl 16): TPS31632024. View Article : Google Scholar
|
|
81
|
Wang Y, Gong J, Lin R, Zhao S, Wang J,
Wang Q, Zhang Y, Su D, Zhang J, Dong Q, et al: First-in-human dose
escalation and expansion study of SYSA1801, an antibody-drug
conjugate targeting claudin 18.2 in patients with
resistant/refractory solid tumors. J Clin Oncol. 41(Suppl 16):
S30162023. View Article : Google Scholar
|
|
82
|
Yu X, Zhang J, Tazbirkova A, Yang J, Yue
J, Sun Y, Pan Y, Sun M, Qin Y, Shen L, et al: Safety and efficacy
of IBI343 (anti-claudin18.2 antibody-drug conjugate) in patients
with advanced pancreatic ductal adenocarcinoma or biliary tract
cancer: Preliminary results from a phase 1 study. J Clin Oncol.
42(Suppl 16): S30372024. View Article : Google Scholar
|
|
83
|
Huang W, Li Y, Liu Z, Rodon L, Correia S,
Li Y and Li R: Preclinical activity for TPX-4589 (LM-302), an
antibody-drug conjugate targeting tight junction protein CLDN18.2
in solid tumors. Eur J Cancer. 174(Suppl 1): S41–S42. 2022.
View Article : Google Scholar
|
|
84
|
Bai C, Xue J, Zheng Y, Sun M, Ying J, Zhou
F, Yu Y, Sun Y, Xing L, Zhang Y, et al: A phase 1/2 study of
LM-302, an anti-claudin 18.2 (CLDN18.2) antibody-drug conjugate in
patients with advanced gastric/gastroesophageal junction cancer. J
Clin Oncol. 42(Suppl 16): S30282024. View Article : Google Scholar
|
|
85
|
Spisek R: 2P SOT102, a novel
CLDN18.2-targeting antibody-drug conjugate for gastric and
pancreatic cancer with a wide range of the tumor target expression.
ESMO Open. 8(1 Suppl 2): S1011962023. View Article : Google Scholar
|
|
86
|
Rosa K: CMG901 elicits responses in
CLDN18.2-expressing gastric/GEJ cancer. OncLive. 2023, https://www.onclive.com/view/cmg901-elicits-responses-in-cldn18-2-expressing-gastric-gej-cancer/.
|
|
87
|
Wahner A: IBI343 receives FDA fast track
designation for advanced/metastatic PDAC. OncLive. 2024, https://www.onclive.com/view/ibi343-receives-fda-fast-track-designation-for-advanced-metastatic-pdac/.
|
|
88
|
Gaspar M, Natoli M, Castan L, Rahmy S,
Kelton C, Mulgrew K, Korade M, Huhn O, Rees DG, Sigurdardottir A,
et al: 1169 AZD5863: A specific, potent, affinity-optimized claudin
18.2 and CD3 binding T cell-engager that elicits low cytokine
release and is capable of bystander killing. J Immunother Cancer.
11(Suppl 1): S11692023.
|
|
89
|
Gao J, Wang Z, Jiang W, Zhang Y, Meng Z,
Niu Y, Sheng Z, Chen C, Liu X, Chen X, et al: CLDN18.2 and 4-1BB
bispecific antibody givastomig exerts antitumor activity through
CLDN18.2-expressing tumor-directed T-cell activation. J Immunother
Cancer. 11:e0067042023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xu Y, Fu J, Henderson M, Lee F, Jurcak N,
Henn A, Wahl J, Shao Y, Wang J, Lyman M, et al: CLDN18.2 BiTE
engages effector and regulatory T cells for antitumor immune
response in preclinical models of pancreatic cancer.
Gastroenterology. 165:1219–1232. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zheng L, Ruihong D, Jieer Y, Xu Q, Guo Z,
Hu C, Sun Y, Niu Z, Hao J, Zhang M, et al: Safety and preliminary
efficacy results of IBI389, an anti-CLDN18.2/CD3 bispecific
antibody, in patients with solid tumors and gastric or
gastro-esophageal tumors: A phase 1 dose escalation and expansion
study. J Clin Oncol. 42(Suppl 16): S25192024. View Article : Google Scholar
|
|
92
|
Wang J, Dong T, Gong X, Li D, Sun J, Luo Y
and Wu H: Safety and pharmacokinetic assessment of the FIC
CLDN18.2/4-1BB bispecific antibody in rhesus monkeys. Int J
Toxicol. 43:291–300. 2024. View Article : Google Scholar
|
|
93
|
Guo Y, Wu L, Li Y, Wen J, Xue J, Wang Z,
Li P, Zhao W, Liu J, Rao X, et al: First-in-human phase I/II safety
and preliminary efficacy of PM1032, a bispecific antibody targeting
CLDN18.2 and 4-1BB, in patients with advanced solid tumors. J Clin
Oncol. 42(Suppl 16): S26622024. View Article : Google Scholar
|
|
94
|
Overman MJ, Melhem R, Blum-Murphy MA,
Ramos C, Petrosyan L, Li J, Perer JK, Zou H, Wang M and Wright HM:
A phase I, first-in-human, open-label, dose escalation and
expansion study of PT886 in adult patients with advanced gastric,
gastroesophageal junction, and pancreatic adenocarcinomas. J Clin
Oncol. 41(Suppl 4): TPS7652023. View Article : Google Scholar
|
|
95
|
Yk W, Gong J, Sun Y, Zhang J, Ni S, Hou J,
Chen X, Wang Y, Yu Q, Qu X, et al: Interim results of a
first-in-human phase 1 study of Q-1802, a CLDN18.2/PD-L1 bsABs, in
patients with relapsed or refractory solid tumors. J Clin Oncol.
41(Suppl 4): S3822023. View Article : Google Scholar
|
|
96
|
Wang Y, Gong J, Sun Y, Yang S, Zhang M,
Cui J, Lv J, Su H, Wang J, Lu J, et al: 132P A phase I clinical
trial of QLS31905 in advanced solid tumors. Immunooncol Technol.
20(Suppl): S1006042023. View Article : Google Scholar
|
|
97
|
Klein C, Brinkmann U, Reichert JM and
Kontermann RE: The present and future of bispecific antibodies for
cancer therapy. Nat Rev Drug Discov. 23:301–319. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Goebeler ME, Stuhler G and Bargou R:
Bispecific and multispecific antibodies in oncology: Opportunities
and challenges. Nat Rev Clin Oncol. 21:539–560. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tucker N: FDA grants orphan drug
designation to TJ-CD4B for gastric cancer. Targeted Oncology. 2022,
https://www.targetedonc.com/view/fda-grants-orphan-drug-designation-to-tj-cd4b-for-gastric-cancer/.
|
|
100
|
Jiang H, Shi Z, Wang P, Wang C, Yang L, Du
G, Zhang H, Shi B, Jia J, Li Q, et al: Claudin18.2-specific
chimeric antigen receptor engineered T cells for the treatment of
gastric cancer. J Natl Cancer Inst. 111:409–418. 2019. View Article : Google Scholar
|
|
101
|
Qi C, Liu C, Gong J, Liu D, Wang X, Zhang
P, Qin Y, Ge S, Zhang M, Peng Z, et al: Claudin18.2-specific CAR T
cells in gastrointestinal cancers: phase 1 trial final results. Nat
Med. 30:2224–2234. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhen DB, Thota R, del Corral C, Geng D,
Yang T, Wang C, Amato G, Akram M, Miller DS, Bubuteishvili-Pacaud L
and Gibson M: A phase 1, open-label, dose escalation and expansion,
multicenter study of claudin 18.2-targeted chimeric antigen
receptor T-cells in patients with unresectable, locally advanced,
or metastatic gastric, gastroesophageal junction, esophageal, or
pancreatic adenocarcinoma. J Clin Oncol. 41(Suppl 4): TSP4802023.
View Article : Google Scholar
|
|
103
|
Luo T, Lu Z, Zheng R, Zhou J, Wang S, Hao
R and Sun M: Outstanding safety and efficacy data of IMC002, an
autologous CLDN18.2-targeting CAR-T, in CLDN18.2+ advanced solid
tumors. J Clin Oncol. 42(Suppl 16): e160122024. View Article : Google Scholar
|
|
104
|
Britton Z, Breen S, Carrasco R, Clark B,
Broggi MAS, Lapointe JM, Giraldo N, Rao Attili BMN, Hatke A,
Grigoriadou C, et al: 235 Preclinical evaluation and anti-tumor
activity of AZD6422, a CLDN18.2 targeting armored CAR-T for
gastric, esophageal and pancreatic cancers. J Immunother Cancer.
11(Suppl 1): A1–A1731. 2023.
|
|
105
|
Xu H, Li W, Lv H, Gu D, Wei X and Dai H:
Tandem CAR-T cells targeting CLDN18.2 and NKG2DL for treatment of
gastric cancer. J Clin Oncol. 40(Suppl 16): S40302022. View Article : Google Scholar
|
|
106
|
Paul S, Konig MF, Pardoll DM, Bettegowda
C, Papadopoulos N, Wright KM, Gabelli SB, Ho M, van Elsas A and
Zhou S: Cancer therapy with antibodies. Nat Rev Cancer. 24:399–426.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fu Z, Li S, Han S, Shi C and Zhang Y:
Antibody drug conjugate: the 'biological missile' for targeted
cancer therapy. Signal Transduct Target Ther. 7:932022. View Article : Google Scholar
|
|
108
|
Fuentes-Antrás J, Genta S, Vijenthira A
and Siu LL: Antibody-drug conjugates: In search of partners of
choice. Trends Cancer. 9:339–354. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kalinsky K, Diamond JR, Vahdat LT, Tolaney
SM, Juric D, O'Shaughnessy J, Moroose RL, Mayer IA, Abramson VG,
Goldenberg DM, et al: Sacituzumab govitecan in previously treated
hormone receptor-positive/HER2-negative metastatic breast cancer:
Final results from a phase I/II, single-arm, basket trial. Ann
Oncol. 31:1709–1718. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Rosenberg J, Sridhar SS, Zhang J, Smith D,
Ruether D, Flaig TW, Baranda J, Lang J, Plimack ER, Sangha R, et
al: EV-101: A phase I study of single-agent enfortumab vedotin in
patients with nectin-4-positive solid tumors, including metastatic
urothelial carcinoma. J Clin Oncol. 38:1041–1049. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Park K, Haura EB, Leighl NB, Mitchell P,
Shu CA, Girard N, Viteri S, Han JY, Kim SW, Lee CK, et al:
Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung
cancer progressing on platinum chemotherapy: Initial results from
the CHRYSALIS phase I study. J Clin Oncol. 39:3391–3402. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Fayette J, Clatot F, Brana I, Saada E, van
Herpen CML, Mazard T, Perez CA, Tabernero J, Le Tourneau C,
Hollebecque A, et al: Petosemtamab (MCLA-158) with pembrolizumab as
first-line (1L) treatment of recurrent/metastatic (r/m) head and
neck squamous cell carcinoma (HNSCC): Phase 2 study. J Clin Oncol.
42(Suppl 16): S60142024. View Article : Google Scholar
|
|
113
|
Schram AM, Goto K, Kim DW, Martin-Romano
P, Ou SHI, O'Kane GM, O'Reilly EM, Umemoto K, Duruisseaux M,
Neuzillet C, et al: Efficacy and safety of zenocutuzumab, a HER2 x
HER3 bispecific antibody, across advanced NRG1 fusion (NRG1+)
cancers. J Clin Oncol. 40(Suppl 16): S1052022. View Article : Google Scholar
|
|
114
|
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK
and Kim WH: EGFR in gastric carcinomas: Prognostic significance of
protein overexpression and high gene copy number. Histopathology.
52:738–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM,
Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, et
al: HER2 screening data from ToGA: Targeting HER2 in gastric and
gastroesophageal junction cancer. Gastric Cancer. 18:476–484. 2015.
View Article : Google Scholar :
|
|
116
|
Ahn S, Lee J, Hong M, Kim ST, Park SH,
Choi MG, Lee JH, Sohn TS, Bae JM, Kim S, et al: FGFR2 in gastric
cancer: Protein overexpression predicts gene amplification and high
H-index predicts poor survival. Mod Pathol. 29:1095–1103. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yashiro M, Kuroda K, Masuda G, Okuno T,
Miki Y, Yamamoto Y, Sera T, Sugimoto A, Kushiyama S, Nishimura S,
et al: Clinical difference between fibroblast growth factor
receptor 2 subclass, type IIIb and type IIIc, in gastric cancer.
Sci Rep. 11:46982021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK,
Lee BL, Bang YJ and Kim WH: MET in gastric carcinomas: Comparison
between protein expression and gene copy number and impact on
clinical outcome. Br J Cancer. 107:325–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Katoh M, Loriot Y, Brandi G, Tavolari S,
Wainberg ZA and Katoh M: FGFR-targeted therapeutics: Clinical
activity, mechanisms of resistance and new directions. Nat Rev Clin
Oncol. 21:312–329. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang H, Yang Z, Zhu X, Li J, Gao Y, Zhang
Y, Tong Z, Fu Q, Bao X, Li B, et al: Phase I trial of
hypoxia-responsive CEA CAR-T cell therapy in patients with heavily
pretreated solid tumor via intraperitoneal or intravenous
transfusion. J Clin Oncol. 42(Suppl 16): S35142024. View Article : Google Scholar
|
|
121
|
Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H
and Han W: Chimeric antigen receptor-modified T cells for the
immunotherapy of patients with EGFR-expressing advanced
relapsed/refractory non-small cell lung cancer. Sci China Life Sci.
59:468–479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang Q, Fu Q, Cao W, Wang H, Xu X, Huang
J, Zou A, Zhu J, Wan H, Yao Y, et al: Phase I study of C-CAR031, a
GPC3-specific TGFβRIIDN armored autologous CAR-T, in patients with
advanced hepatocellular carcinoma (HCC). J Clin Oncol. 42(Suppl
16): S40192024. View Article : Google Scholar
|
|
123
|
Qi C, Liu C, Li J, Gong J, Wang X, Wang Z,
Lu X, He T, Ding Y, Wu F, et al: Phase I study of GUCY2C CAR-T
therapy IM96 in patients with metastatic colorectal cancer. J Clin
Oncol. 42(Suppl 16): S25182024. View Article : Google Scholar
|
|
124
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene ciloleucel CAR T-cell therapy in
refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Martin T, Usmani SZ, Berdeja JG, Agha M,
Cohen AD, Hari P, Avigan D, Deol A, Htut M, Lesokhin A, et al:
Ciltacabtagene autoleucel, an anti-B-cell maturation antigen
chimeric antigen receptor T-cell therapy, for relapsed/refractory
multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol.
41:1265–1274. 2023. View Article : Google Scholar
|
|
126
|
Majzner RG and Mackall CL: Tumor antigen
escape from CAR T-cell therapy. Cancer Discov. 8:1219–1226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Shah NN and Fry TJ: Mechanisms of
resistance to CAR T cell therapy. Nat Rev Clin Oncol. 16:372–385.
2019.PubMed/NCBI
|
|
128
|
Larson RC and Maus MV: Recent advances and
discoveries in the mechanisms and functions of CAR T cells. Nat Rev
Cancer. 21:145–161. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hou AJ, Chen LC and Chen YY: Navigating
CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug
Discov. 20:531–550. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Tauriello DVF, Sancho E and Batlle E:
Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer.
22:25–44. 2022. View Article : Google Scholar
|
|
131
|
Katoh M and Katoh M: WNT signaling and
cancer stemness. Essays Biochem. 66:319–331. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Gumber D and Wang LD: Improving CAR-T
immunotherapy: Overcoming the challenges of T cell exhaustion.
EBioMedicine. 77:1039412022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Chan JD, Scheffler CM, Munoz I, Sek K, Lee
JN, Huang YK, Yap KM, Saw NYL, Li J, Chen AXY, et al: FOXO1
enhances CAR T cell stemness, metabolic fitness and efficacy.
Nature. 629:201–210. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Doan AE, Mueller KP, Chen AY, Rouin GT,
Chen Y, Daniel B, Lattin J, Markovska M, Mozarsky B, Arias-Umana J,
et al: FOXO1 is a master regulator of memory programming in CAR T
cells. Nature. 629:211–218. 2024. View Article : Google Scholar : PubMed/NCBI
|