Introduction
Breast cancer (BC) is the leading cause of
cancer-related death in females worldwide (1). Several studies have shown that the
development of BC is associated with family history, age
advancement, mutations in BRCA1, and BRCA2 genes, prolonged
exposure to endogenous or exogenous estrogens and exposure to
ionizing radiation (2–4). Diagnosis of BC at advanced stages may
reduce the efficacy of therapeutic approaches such as surgery and
chemotherapy. Approximately 65–70% of BCs are ER+ and BC
patients with ER+ tumors respond positively to adjuvant
anti-estrogen therapy, which has produced a significant improvement
in survival and a reduction in disease relapse, especially in women
with early BC and those with ER+ tumors, who may receive
endocrine therapy (ET) alone or in combination with cytotoxic
therapy. Approximately 10–20% of BCs are ER− and BC
patients with ER− tumor do not respond to hormonal
therapy or other targeted therapies (e.g., Herceptin). Therefore,
improved prognostic outcomes for ER− BC depend on early
detection and/or development of new therapeutics with higher
efficacy for advanced stage cancer.
Signal transducer and activator of transcription 3
(STAT3) is a latent transcription factor residing in the cytoplasm
(5). STAT3 is involved in relaying
extracellular signals derived from multiple cytokines, hormones and
growth factors to the nucleus in order to transcribe the genes
involved in cell proliferation, apoptotic resistance, angiogenesis
and immune evasion (6–8). Janus kinases and Src kinases are the
upstream tyrosine kinases which phosphorylate STAT3 on Tyr-705. In
turn, STAT3 undergoes dimerization to translocate into the nucleus
to stimulate the transcription of genes involved in the
aforementioned functions (9).
STAT3 is reported to be constitutively activated in more than 20
types of cancer, including breast cancer, thereby contributing to
cancer progression and poor prognosis (7,10).
Hence, the critical role of STAT3 in breast cancer makes it an
attractive therapeutic target for cancer treatment and potentially
ER− BC.
Azaspirane derivatives are known for their tyrosine
kinase inhibitory activity and many are in clinical trials for the
treatment of various cancers (11,12).
Midostaurin, atiprimod, lestaurtinib and K252a are some of the
major azaspirane based multi-tyrosine kinase inhibitors (13). Midostaurin is an analogue of
azaspirane and a derivative of staurosporine which have been tested
in phase-II clinical trials for the treatment of acute myeloid
leukemia and it potently inhibits protein kinase C, VEGFR2, PDGFR,
KIT and FLT3 tyro-sine kinases (14,15).
Atiprimod proved to be a potent JAK2/JAK3 inhibitor in preclinical
studies (16). Lestaurtinib is an
inhibitor of JAK2, FLT3 and TrkA and undergoing phase-III clinical
trials in combination chemotherapy to treat acute lymphoblastic
leukemia (17–19). K252a is a cell-permeable
staurosporine based fungal alkaloid with inhibitory activity
against protein kinase C and trk family kinases (20). K252a has also been reported to
block leukemia inhibitory factor-induced STAT3 activation in
olfactory receptor neurons (21).
We have recently reported the synthesis and anticancer effect of
various azaspirane derivatives and demonstrated their mechanism of
action in several types of cancers (7,22,23).
In our previous study, we reported the development of azaspirane
based small molecule,
2-(1-(4-(2-cyanophenyl)1-benzyl-1H-indol-3-yl)-5-(4-methoxy-phenyl)-1-oxa-3-azaspiro(5,5)undecane
(CIMO) and demonstrated inhibition of the JAK-STAT pathway in
hepatocellular carcinoma (7). In
continuation of effort in demonstrating the pharmacological
properties of various heterocyclic compounds (24–30),
in this investigation, we evaluated the effect of CIMO in both
ER+ and ER− BC cell lines.
Materials and methods
Cell culture and reagents
BC cell lines MCF-7, T47D, BT-474, MDA-MB-231, and
BT-549 were obtained from the American Type Culture Collection
(ATCC, Rockville, MD, USA) and were cultured as per ATCC
propagation instructions. MDA-MB-231 and BT549 cell lines were
cultured in Dulbecco’s modified Eagle’s medium while MCF-7 and
BT-474 cell lines were cultured in Roswell Park Memorial Institute.
All growth media were supplemented with 10% heat-inactivated fetal
bovine serum (FBS) and 1% penicillin-streptomycin.
Cell viability assay
The BC cell lines (MCF-7, T47D, BT-474, MDA-MB-231,
BT-549) were seeded at 2.5×104/ml in a 96-well plate.
After an overnight incubation of cells, the medium was changed to
the indicated concentration of CIMO ranging from 0.01 to 10 μM.
Following 72 h incubation, alamarBlue® dye was added and
incubated for 4 h in the dark, followed by measuring fluorescence
activity at an excitation wavelength of 540 nm and an emission
wavelength of 590 nm.
ApoTox-GloTM Triplex
assay
Cells (2×104) were seeded with complete
medium in each well of a 96-well plate, 5 μM of CIMO and vehicle
control (DMSO) was added to the respective well and incubated for
24 h. Thereafter, 20 μl of viability/cytotoxicity reagent
containing both GF-AFC substrate and bis-AAF-R110 substrate was
added to all the wells, and mixed by orbital shaking (300–500 rpm
for ~30 sec) and incubated for 30 min at 37°C. After which
fluorescence measurement was obtained at the two wavelength sets:
400Ex/505Em (viability)
485Ex/520Em (cytotoxicity). After
measurement, 100 μl of Caspase-Glo® 3/7 reagent was
added to all the wells, and mixed by orbital shaking (300–500 rpm
for ~30 sec) and incubated for 30 min at room temperature followed
by luminescence measurement with an integration time between 0.5–1
sec.
3D Matrigel proliferation assay
3D Matrigel (100%) (BD BioCoat™ Matrigel™) was
coated on 48-well plates and given time to solidify. A 2% Matrigel
containing 5,000 MDA-MB-231 cells were cast above the 100% Matrigel
layer and given time to solidify. Thereafter, cells were allowed to
grow till they formed a 3D morphology before subjecting them to
treatment with different concentration of CIMO at 5, 2.5 and 1.25
μM in 2% FBS + 1% P/S containing high glucose DMEM. The media in
the wells were changed every 2 days, with microscopy images
obtained every day to observe the drug-induced effects on cells
present in a 3D culture.
Flow cytometric analysis
To determine the effect of CIMO on the cell cycle,
cells were treated with CIMO at the indicated concentrations ≤5 μM.
Thereafter, cells were washed, fixed with 70% ethanol, and
incubated for 30 min at 37°C with 0.1% RNase A in PBS. Cells were
then washed again, resuspended, and stained in PBS containing 25
μg/ml propidium iodide for 30 min at room temperature. Cell
distribution across the cell cycle was examined with a Beckman
Coulter flow cytometer.
Western blotting
Western blot analysis was performed as previously
described (31,32). Briefly, CIMO treated MDA-MB-231
whole-cell extracts were lysed in lysis buffer (20 mM Tris, pH
7.4), 250 mM NaCl, 2 mM EDTA (pH 8.0), 0.1% Triton X-100, 0.01
mg/ml aprotinin, 0.005 mg/ml leupeptin, 0.4 mM PMSF, and 4 mM
NaVO4). Lysates were then spun at 14,000 rpm for 10 min
to remove insoluble material and protein concentration was
quantified. Thereafter, proteins were resolved on SDS gel. After
electrophoresis, the proteins were electrotransferred to a
nitrocellulose membrane, blocked with 5% non-fat milk, and probed
with various antibodies overnight at 4°C. The blot was washed,
exposed to HRP-conjugated secondary antibodies for 1 h, and finally
examined by chemiluminescence (ECL; GE Healthcare).
Real-time PCR
Quantitative analysis of mRNA expression by
real-time PCR was performed using ABI 7700 real-time PCR system
(Applied Biosystems) as previously described (33). Briefly, total cDNA (5 ng) from each
stable cell line was added to a 20 μl reaction containing SYBR
GreenER qPCR SuperMix and forward and reverse primer mix. All
reactions were performed in triplicate in a 384-well plate using a
two-step amplification program with 24 initial denaturation at 95°C
for 10 min, followed by 40 cycles of 95°C for 20 sec and 6°C for 30
sec. Relative mRNA expression between cDNA samples was calculated
using comparative Ct method and normalized against a panel of
housekeeping genes including β-actin, HPRT, and GAPDH. Relative
expression was computed as: Fold expression = 2−ΔCt
where ΔCt = Ct difference of sample relative to control
(ΔCtsample-control). Positive and negative relative
expression indicates increase and decrease in mRNA levels,
respectively. A P-value <0.05 was considered as statistically
significant.
Wound healing assay
The migration of cells was investigated using a
wound healing assay. MDA-MB-231 cells were seeded in a 6-cm culture
dish with complete medium and allowed to grow until ~80% confluent.
A wound was created using a pipette tip and rinsed with PBS to
remove detached cells before the treatment with varying
concentration of CIMO. The microscopic observation of the cells was
recorded as described previously (34).
Invasion assay
The invasion assay was performed with slight
modifications in a method described previously (35). A BD Biocoat MatrigelTM
invasion chamber with 8-μm pores in the light-tight polyethylene
terephthalate membrane and was coated with a reconstituted basement
membrane gel (BD Biosciences). MDA-MB-231 cells (1×104)
were suspended in serum-free DMEM and seeded into the Matrigel
Transwell chambers. The cells were incubated with different
concentrations of CIMO (1.25, 2.5 and 5 μM). After 24-h incubation,
the wells were gently removed with cotton swabs. The Transwell
insert was fixed in 4% PFA for 15 min at 4°C. Thereafter, the
insert was washed twice in PBS and stained with Hoechst. The
invading cells were then counted in randomly selected areas under
microscopic observation.
Data analysis
All data analysis was done using the GraphPad Prism
(V.60f) software. The data given in this study are the mean ± SD
with n=3. An unpaired t-test was used with Welch’s correction for
statistical analysis between treatment and control, with
*P<0.05 and **P<0.01 in the
figures.
Results
CIMO suppresses proliferation of
ER+ and ER− BC cells
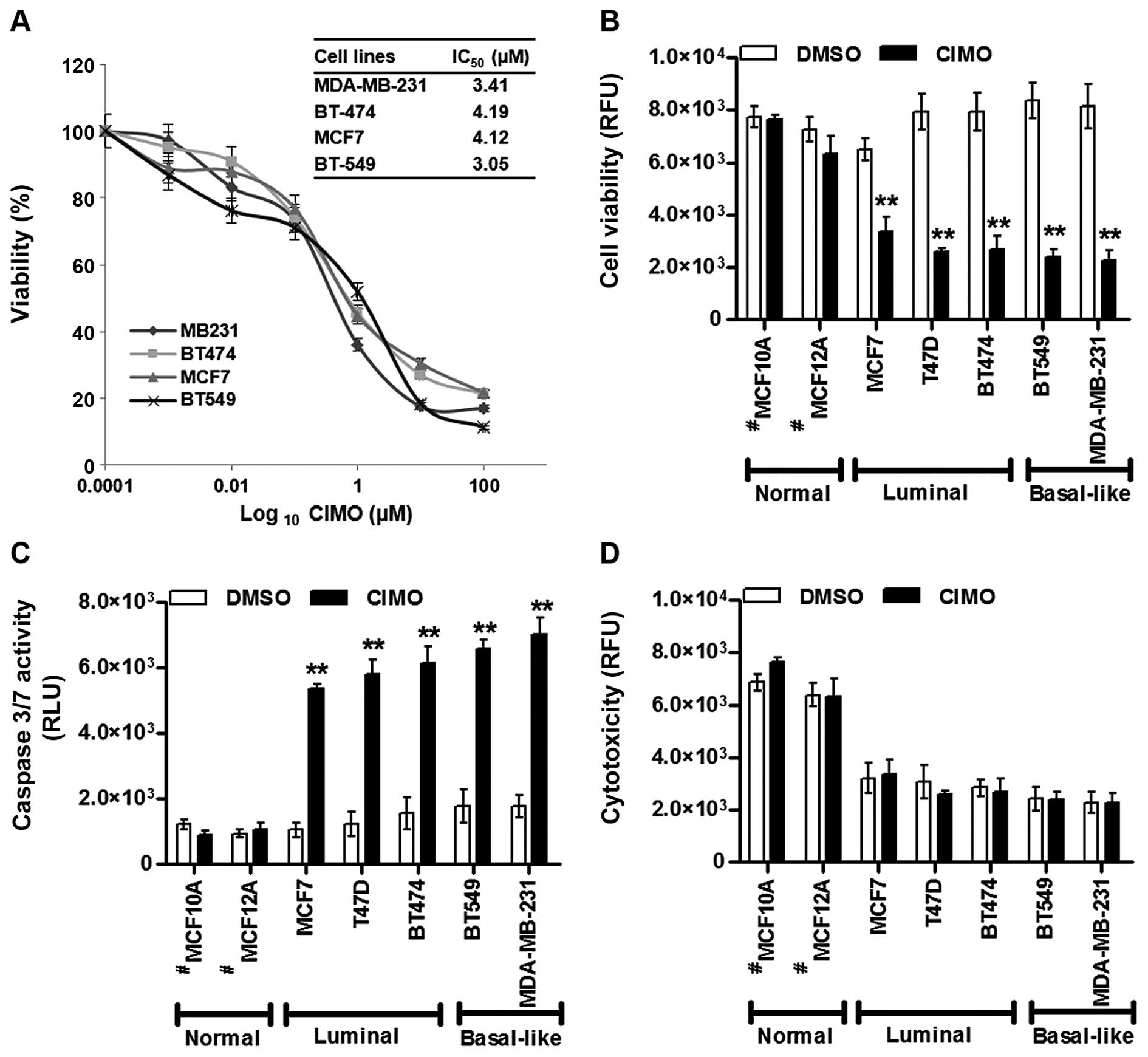
Initially, we evaluated the effect of CIMO on a
panel of five BC cell lines (ER+: MCF-7, T47D, and
BT-474 and ER−: MDA-MB-231 and BT-549) using an
alamarBlue cell viability assay. The dose-response curve indicated
that CIMO was able to produce a substantial decrease in cell
viability in all cell lines with BT-549 exhibiting relatively the
lowest IC50 value of 3.05 μM followed by MDA-MB-231,
MCF-7 and BT-474 with the IC50 values 3.41, 4.12 and
4.19 μM, respectively (Fig. 1A).
The ApoTox-Glo™ triplex assay results indicated a decreased
cellular viability with higher apoptotic activity in both
MDA-MB-231 cells than BT-549 cells (Fig. 1B–D). Nevertheless, across all BC
cell lines, significantly higher apoptotic levels were detected
with a corresponding decrease in cellular viability. CIMO exhibited
no substantial cytotoxicity against normal immortalized mammary
epithelial cells and/or against BC cell lines.
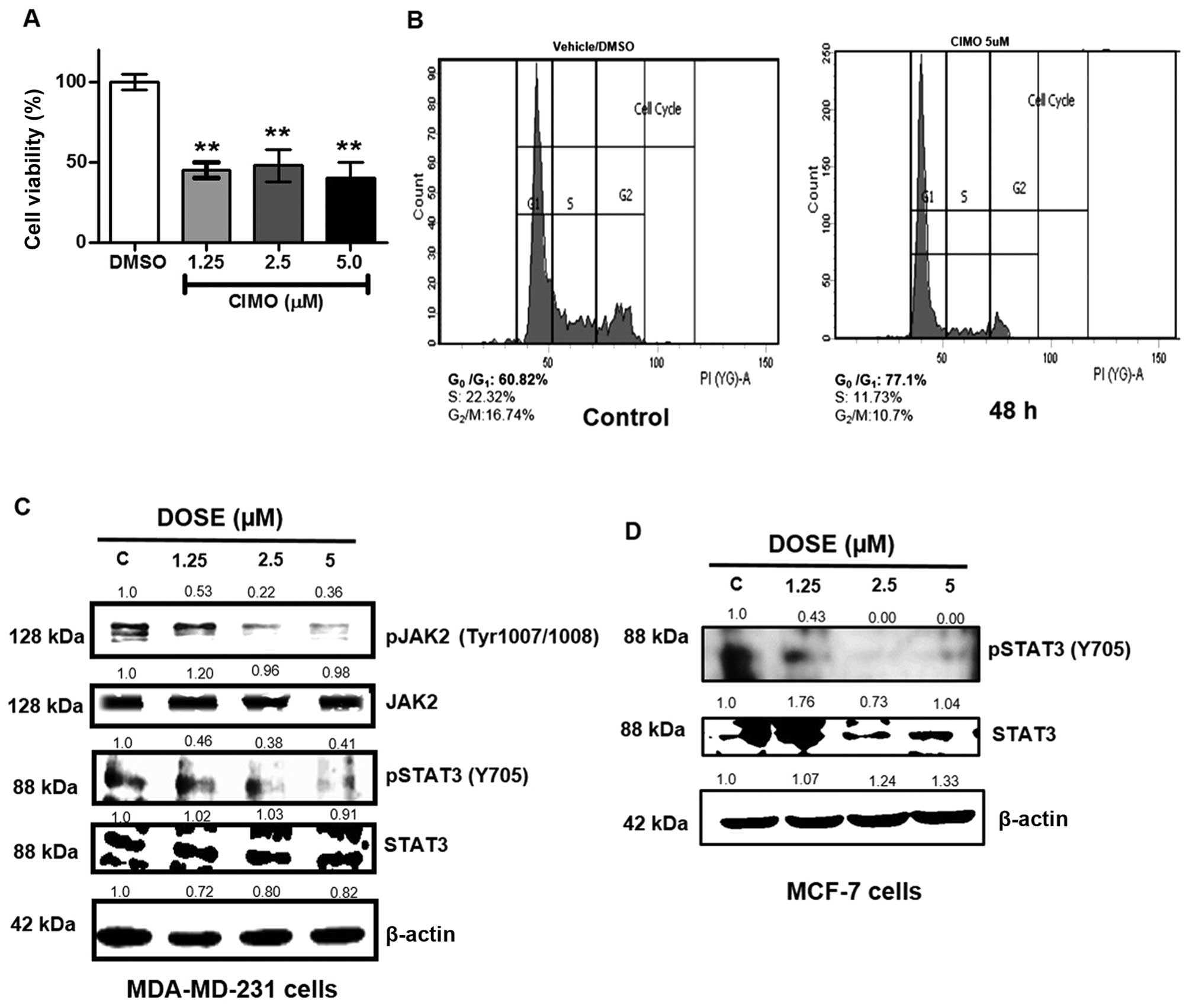
CIMO decreases proliferation of
MDA-MB-231 cells in 3D culture
Tumor cells are more resistant to anticancer agents
in three-dimensional multicellular spheroidal conformation compared
to monolayer culture (36).
Therefore, we analyzed the effect of CIMO on 3D culture of
MDA-MB-231 cells. BC cells were cultured in Matrigel, treated with
CIMO at indicated doses and cellular viability was measured with
alamarBlue on day 10. Treatment with CIMO decreased the cell
viability by >50% compared to vehicle control in 3D culture
(Fig. 2A).
CIMO arrests MDA-MB-231 cells at G0/G1
phase
In order to evaluate the effect of CIMO on the
distribution of the cell cycle in MDA-MB-231 cells, we performed
flow cytometric analysis. MDA-MB-231 cells were treated with CIMO
at different time intervals up to 48 h and stained with propidium
iodide to analyze the cell cycle distribution. We observed that
CIMO increased the accumulation of cells in G0/G1 phase of the cell
cycle (Fig. 2B). The treatment of
BC cells with 5 μM of CIMO for 48 h resulted in an increased G0/G1
population of 77.1% compared to 60.82% in vehicle control.
CIMO suppresses the basal activation of
STAT3 in ER+ and ER− cells
Azaspiranes have been reported to possess inhibitory
activity against the JAK-STAT pathway. Therefore, we further
evaluated the inhibitory potential of CIMO towards the activity of
JAK2 and STAT3 in ER− (MDA-MB-231) and STAT3 in
ER+ cells by western blotting via antibodies recognizing
phospho-JAK2 (Tyr-1007/1008) and phospho-STAT3 (Tyr-705). We
observed that, CIMO significantly inhibited the phosphorylation of
JAK2 and STAT3 in a dose-dependent manner, with a maximum
inhibition identified at 5 μM and 6 h. At the same time, the
expression of total JAK2 and STAT3 proteins remained unaltered
(Fig. 2C and D).
CIMO downregulates the expression of
STAT3 targeted genes in MDA-MB-231 cells
Activated STAT3 has been reported to modulate the
expression of antiapoptotic proteins (37,38).
Therefore, we evaluated whether CIMO modulates the expression of
various STAT3-regulated genes. Real-time PCR analysis demonstrated
that exposure of MDA-MB-231 cells to CIMO decreased mRNA
levels of CCND1, CCNE1, CDK2 and CDK4 required for
cell cycle progression (39). In
addition, the mRNA levels of CDKN2A an inhibitor of
CDK4 was increased in MDA-MB-231 cells treated with CIMO
relative to vehicle exposed cells (40). CIMO treated MDA-MB-231 cells
exhibited decreased mRNA levels of the pro-survival gene,
BCL-xL. Concordantly, the mRNA levels of genes
encoding pro-apoptotic MDM2, S100A4, BAX and CDKN1B
were increased after CIMO exposure in MDA-MB-231 cells (Fig. 3A and B). Furthermore, western blot
analysis demonstrated that protein levels of CCND1 and BCL2 and
BCL-xL were decreased in MDA-MB-231 cells after treatment with CIMO
in a dose-dependent manner (Fig.
3C).
CIMO promotes apoptosis via the
mitochondrial pathway in MDA-MB-231 cells
Cleavage of pro-caspase 9 serves as a marker of
cells undergoing apoptosis via the mitochondrial pathway with
subsequent activation of the executioner caspase 3 and 7 (41). We therefore investigated whether
CIMO promoted apoptosis through the intrinsic pathway in MDA-MB-231
cells. We observed that, CIMO treatment produced a decrease in the
level of pro-caspase 9 and increased levels of cleaved caspase 3
and 7 as direct evidence of mitochondrial mediated apoptosis
(Fig. 4). Dephosphorylation of BAD
protein at Ser-136 results in dimerization with BCL2 and BCL-xL to
induce the release of cytochrome c to promote apoptosis via
the intrinsic pathway (42).
Treatment with CIMO decreased BAD phosphorylation in a
dose-dependent manner. In addition, we also observed an increased
expression of TP53 (Fig. 4).
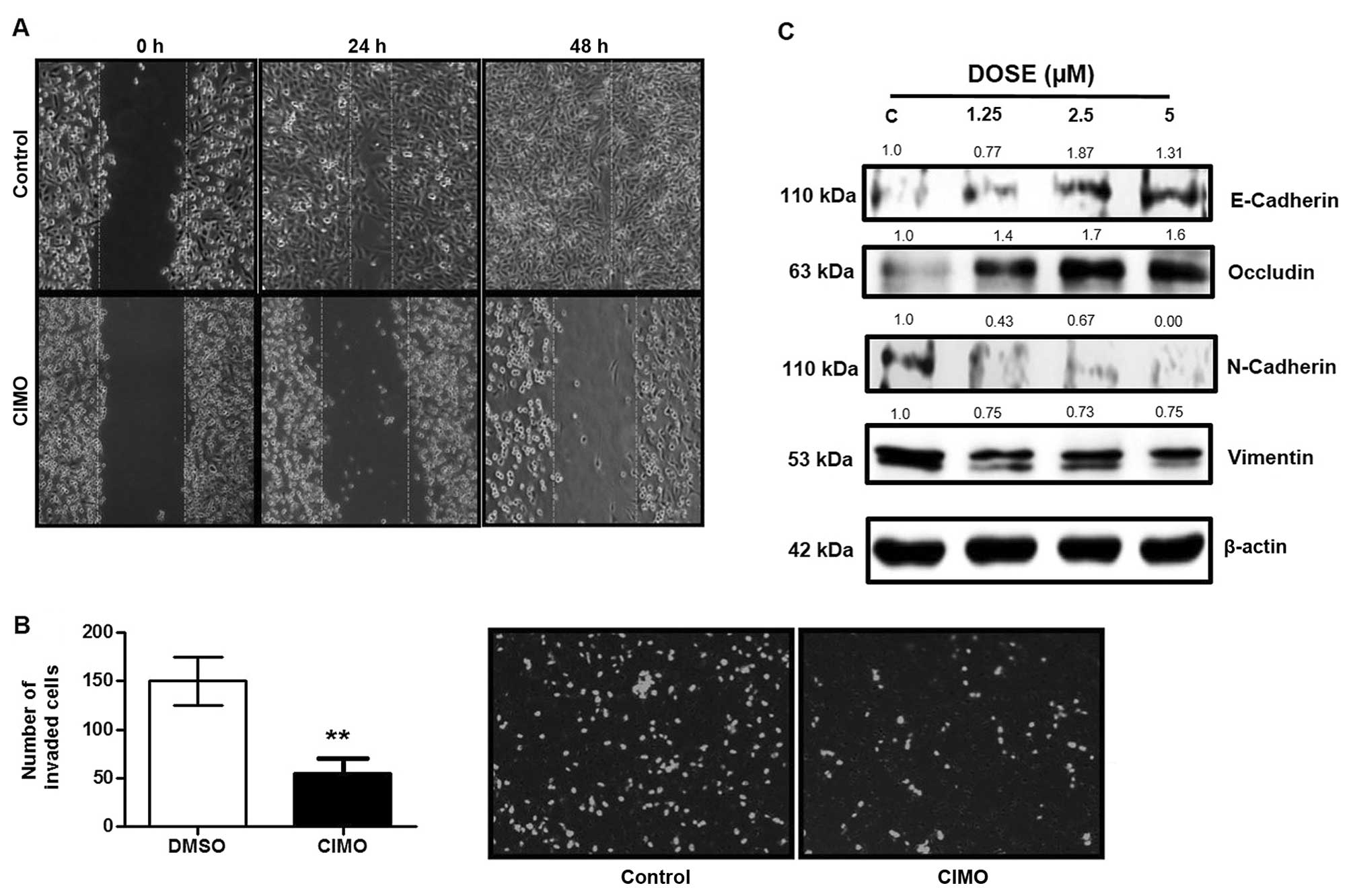
CIMO suppresses cell invasion and
migration in MDA-MB231 cells
STAT3 regulated gene products are also reported to
be associated with migration and invasion of cancer cells (6,7). To
evaluate whether CIMO repressed the motility of cancer cells, we
performed a wound healing assay. CIMO significantly inhibited cell
migration at 5 μM (Fig. 5A).
Further investigation using Transwell invasion chamber demonstrated
that CIMO inhibited the invasion of BC cells (Fig. 5B). Loss of CDH1 and OCLN promotes
invasiveness, and increased expression of CDH2 and VIM is
correlated with metastasis and poor prognosis in human cancers
(43–46). Given the anti-invasive property of
CIMO, we further analyzed the expression of epithelial-mesenchymal
transition proteins including CDH1, CDH2, OCLN and
VIM. Fig. 5C demonstrates
the upregulation of CDH1 and OCLN and downregulation
of CDH2 and VIM in a dose-dependent manner.
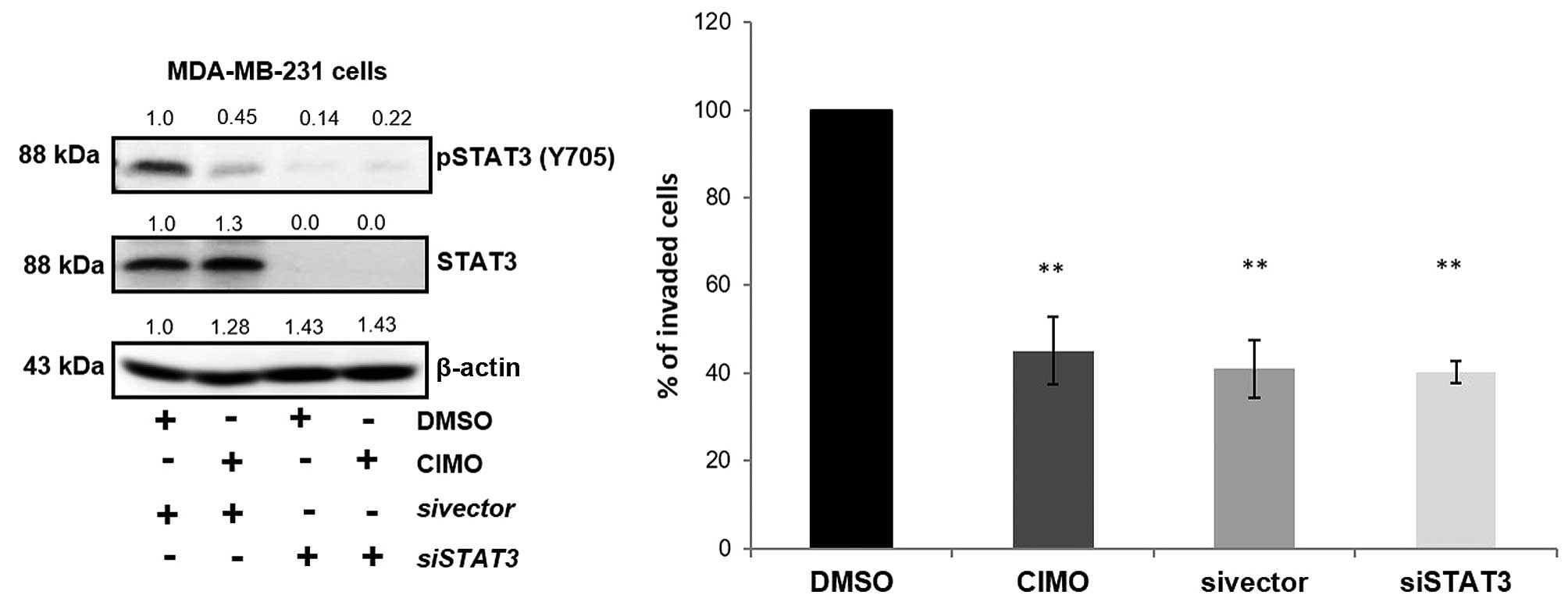
We next evaluated the effect of siRNA-mediated
deletion of STAT3 transcripts on cell invasion. Transient
transfection of STAT3-directed siRNA in MDA-MB-231 cells showed
decreased levels of phospho-STAT3 and STAT3 protein compared with
their respective controls which was confirmed using western blot
analysis. We also observed the decreased phosphorylation of STAT3
on treatment with CIMO without altering the levels of total STAT3
(Fig. 6). Cells transfected and/or
exposure to CIMO exhibited reduced invasion and migratory
properties when compared to DMSO treated cells indicating that
deletion/inhibition of STAT3 plays a critical role in motility of
cancer cells.
Discussion
STAT3, upon phosphorylation, dimerizes and
translocate to the nucleus where it relays its oncogenic signals
via regulating genes involved in cell growth, survival,
angiogenesis, and cell migration (22,39,40,42).
Hence, it is no surprise that a mutation in this gene alone can
support oncogenic activity and give rise to uncontrolled cell
proliferation (31). JAK2, a
non-receptor tyrosine kinase promoting STAT3 activation was
observed to be constitutively activated in >50–60% of primary
breast tumors and tumor-derived cell lines with drug resistance
(32). Hence, inhibition of
JAK2/STAT3 signaling is an attractive approach in disrupting
aggressive subtypes of breast cancer including ER− BC.
The aim of this study was to further investigate the effects of the
oxazine-based compound CIMO, that has been recently reported to
disrupt the JAK-STAT pathway in hepatocellular carcinoma. In
ER+ and ER− BC, CIMO inhibits the kinase
activation of JAK2 and hence subsequently reduces the JAK2 mediated
activation of STAT3 specifically at Y705. As previous studies have
demonstrated that CIMO has no effect on S727 phosphorylation of
STAT3 in hepatocellular carcinoma cells, the phosphorylation
activity at S727 of STAT3 was not investigated.
Constitutive activation of STAT3 by receptor
tyrosine kinases EGFR, HER2, fibroblast growth factor receptor
(FGFR), IGFR, HGFR and platelet-derived growth factor receptor
(PDGFR), growth hormone, prolactin, receptor-associated kinases
(JAK) and non-receptor kinases (Src and ABL) through
phosphorylation has been documented in BC cells (47–49).
This constitutive STAT3 activation leads to increased expression of
proteins such as MMP-2, MMP1, MEK5, c-Fos and VEGF and promote
invasion (25). As such, the
ability of CIMO to suppress invasion was evident and due to its
ability to disrupt the JAK2/STAT3 pathway. Constitutive STAT3
activation is able to promote EMT via STAT3 promoted SNAIL1
expression including increased expression of mesenchymal proteins
VIM and CDH2 (26,27). In particular, MDA-MB-231 cells
exhibiting higher STAT3 activity have shown to have a higher VIM
expression (50). Hence, CIMO was
able to decrease the expression of EMT proteins via the
inactivation of STAT3. This demonstrates the effect of CIMO on
suppressing STAT3 mediated migration and invasion in ER−
BC cells.
Inhibition of JAK2 and STAT3 activity by CIMO
directly correlated with decreased expression of STAT3-regulated
proteins BCL2, BCL-xL and CCND1. In association with other
proteins, BCL2 and BCL-xL protein maintain the integrity of outer
membrane of mitochondria in the cells. Herein, we demonstrated that
CIMO exposure to ER− BC cells increased cleavage of
caspase 9 by subsequently increased cleaved caspase 3 and 7, which
signify the induction of late-phase intrinsic apoptosis.
Concordantly, CIMO exposure to ER− BC cells also
increased expression of BAD protein that indicated the induction of
mitochondrial outer membrane permeabilization (MOMP) that leads to
intrinsic apoptosis, as interaction between dephosphorylated-BAD
and BCL2/BCL-xL protein consequent to permeabilization of the
mitochondrial outer membrane (51). Moreover, CIMO exposure to BC cells
also decreases expression of CCND1 and stimulates a proliferative
arrest in G0/G1 phase that indicated the disruption of the cell
cycle progression. Together, CIMO exposure to ER− BC
cells inhibits cell cycle progression and survival.
In conclusion, the design of therapeutic agents
against ER− BC remains as a prime challenge in clinical
management of BC. Herein, we report that the azaspirane based small
molecule, CIMO as an inhibitor of the JAK2-STAT3 pathway in
ER− BC cells with no or very low cytotoxicity towards
normal cells. CIMO promotes apoptosis through the repression of
STAT3 activity on target genes. In addition, CIMO suppressed
cellular migration and invasion mediated via STAT3 regulated EMT
related proteins. Therefore, CIMO emerges as a potential inhibitor
targeting the ER− BC cells whose growth is dependent on
the constitutive activation of the JAK2/STAT3 signaling pathway
(52).
Acknowledgements
This study was supported by University Grants
Commission (41-257-2012-SR), Vision Group Science and Technology,
Department of Science and Technology (no. SR/FT/LS-142/2012) to
Basappa. K.S.R. would like to thank Department of Science and
Technology Indo-Korea (INT/Indo-Korea/122/2011-12) and Institution
of Excellence, University of Mysore for financial support. This
study was also supported by grants from the National Medical
Research Council of Singapore (R-713-000-177-511), and by the NCIS
Yong Siew Yoon Research Grant through donations from the Yong Loo
Lin Trust to A.P.K. P.E.L. and A.P.K. were supported by grants from
the NMRC Clinician Scientist IRG (R-713-000-163-511) and the
National Research Foundation Singapore and the Singapore Ministry
of Education under its Research Centers of Excellence initiative to
Cancer Science Institute of Singapore, National University of
Singapore. C.D.M. would like to thank the University of Mysore for
Department of Science and Technology-Promotion of University
Research and Scientific Excellence (DST-PURSE) Research Associate
fellowship.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narod SA, Dubé M-P, Klijn J, Lubinski J,
Lynch HT, Ghadirian P, Provencher D, Heimdal K, Moller P, Robson M,
et al: Oral contraceptives and the risk of breast cancer in BRCA1
and BRCA2 mutation carriers. J Natl Cancer Inst. 94:1773–1779.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Donovan M, Tiwary CM, Axelrod D, Sasco AJ,
Jones L, Hajek R, Sauber E, Kuo J and Davis DL: Personal care
products that contain estrogens or xenoestrogens may increase
breast cancer risk. Med Hypotheses. 68:756–766. 2007. View Article : Google Scholar
|
|
4
|
Ronckers CM, Erdmann CA and Land CE:
Radiation and breast cancer: A review of current evidence. Breast
Cancer Res. 7:21–32. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Srinivasa V, Li F, Siveen KS, et al:
Synthesis and biological evaluation of tetrahydropyridinepyrazoles
(‘PFPs’) as inhibitors of STAT3 phosphorylation. MedChemComm.
5:32–40. 2014. View Article : Google Scholar
|
|
6
|
Huang S: Regulation of metastases by
signal transducer and activator of transcription 3 signaling
pathway: Clinical implications. Clin Cancer Res. 13:1362–1366.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohan CD, Bharathkumar H, Bulusu KC,
Pandey V, Rangappa S, Fuchs JE, Shanmugam MK, Dai X, Li F,
Deivasigamani A, et al: Development of a novel azaspirane that
targets the Janus kinase-signal transducer and activator of
transcription (STAT) pathway in hepatocellular carcinoma in vitro
and in vivo. J Biol Chem. 289:34296–34307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
9
|
Lee JH, Kim C, Sethi G and Ahn KS:
Brassinin inhibits STAT3 signaling pathway through modulation of
PIAS-3 and SOCS-3 expression and sensitizes human lung cancer
xenograft in nude mice to paclitaxel. Oncotarget. 6:6386–6405.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strati P, Kantarjian H, Ravandi F, Nazha
A, Borthakur G, Daver N, Kadia T, Estrov Z, Garcia-Manero G,
Konopleva M, et al: Phase I/II trial of the combination of
midostaurin (PKC412) and 5-azacytidine for patients with acute
myeloid leukemia and myelodysplastic syndrome. Am J Hematol.
90:276–281. 2015. View Article : Google Scholar :
|
|
12
|
M.D. Anderson Cancer Center. Study of
atiprimod treatment for patients with advanced cancer. https://clinicaltrials.gov/ct2/show/NCT00430014.
Verified February 2012.
|
|
13
|
Aubert L, Guilbert M, Corbet C, Génot E,
Adriaenssens E, Chassat T, Bertucci F, Daubon T, Magné N, Le
Bourhis X, et al: NGF-induced TrkA/CD44 association is involved in
tumor aggressiveness and resistance to lestaurtinib. Oncotarget.
6:9807–9819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Millward MJ, House C, Bowtell D, Webster
L, Olver IN, Gore M, Copeman M, Lynch K, Yap A, Wang Y, et al: The
multikinase inhibitor midostaurin (PKC412A) lacks activity in
metastatic melanoma: A phase IIA clinical and biologic study. Br J
Cancer. 95:829–834. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stone RM, DeAngelo DJ, Klimek V, Galinsky
I, Estey E, Nimer SD, Grandin W, Lebwohl D, Wang Y, Cohen P, et al:
Patients with acute myeloid leukemia and an activating mutation in
FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor,
PKC412. Blood. 105:54–60. 2005. View Article : Google Scholar
|
|
16
|
Quintás-Cardama A, Manshouri T, Estrov Z,
Harris D, Zhang Y, Gaikwad A, Kantarjian HM and Verstovsek S:
Preclinical characterization of atiprimod, a novel JAK2 AND JAK3
inhibitor. Invest New Drugs. 29:818–826. 2011. View Article : Google Scholar
|
|
17
|
Knapper S, Burnett AK, Littlewood T, Kell
WJ, Agrawal S, Chopra R, Clark R, Levis MJ and Small D: A phase 2
trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line
treatment for older patients with acute myeloid leukemia not
considered fit for intensive chemotherapy. Blood. 108:3262–3270.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hexner EO, Serdikoff C, Jan M, Swider CR,
Robinson C, Yang S, Angeles T, Emerson SG, Carroll M, Ruggeri B, et
al: Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses
JAK2/STAT5 signaling and the proliferation of primary erythroid
cells from patients with myeloproliferative disorders. Blood.
111:5663–5671. 2008. View Article : Google Scholar
|
|
19
|
Children’s Oncology Group. Combination
chemotherapy with or without lestaurtinib in treating younger
patients with newly diagnosed acute lymphoblastic leukemia.
https://clinicaltrials.gov/ct2/show/NCT00557193.
Verified September 2015.
|
|
20
|
Tapley P, Lamballe F and Barbacid M: K252a
is a selective inhibitor of the tyrosine protein kinase activity of
the trk family of oncogenes and neurotrophin receptors. Oncogene.
7:371–381. 1992.PubMed/NCBI
|
|
21
|
Moon C, Yoo J-Y, Matarazzo V, Sung YK, Kim
EJ and Ronnett GV: Leukemia inhibitory factor inhibits neuronal
terminal differentiation through STAT3 activation. Proc Natl Acad
Sci USA. 99:9015–9020. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bharathkumar H, Mohan CD, Rangappa S, Kang
T, Keerthy HK, Fuchs JE, Kwon NH, Bender A, Kim S, Basappa, et al:
Screening of quinoline, 1,3-benzoxazine, and 1,3-oxazine-based
small molecules against isolated methionyl-tRNA synthetase and A549
and HCT116 cancer cells including an in silico binding mode
analysis. Org Biomol Chem. 13:9381–9387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basappa, Sugahara K, Thimmaiah KN, Bid HK,
Houghton PJ and Rangappa KS: Anti-tumor activity of a novel
HS-mimetic-vascular endothelial growth factor binding small
molecule. PLoS One. 7:e394442012. View Article : Google Scholar :
|
|
24
|
Rakesh KS, Jagadish S, Vinayaka AC,
Hemshekhar M, Paul M, Thushara RM, Sundaram MS, Swaroop TR, Mohan
CD, Basappa, et al: A new ibuprofen derivative inhibits platelet
aggregation and ROS mediated platelet apoptosis. PLoS One.
9:e1071822014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anusha S, Anandakumar BS, Mohan CD, et al:
Preparation and use of combustion-derived Bi2O3 for the synthesis
of heterocycles with anti-cancer properties by Suzuki-coupling
reactions. RSC Advances. 4:52181–52188. 2014.
|
|
26
|
Bharathkumar H, Mohan CD, Ananda H, Fuchs
JE, Li F, Rangappa S, Surender M, Bulusu KC, Girish KS, Sethi G, et
al: Microwave-assisted synthesis, characterization and cytotoxic
studies of novel estrogen receptor α ligands towards human breast
cancer cells. Bioorg Med Chem Lett. 25:1804–1807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anilkumar NC, Sundaram MS, Mohan CD,
Rangappa S, Bulusu KC, Fuchs JE, Girish KS, Bender A, Basappa and
Rangappa KS: A one pot synthesis of novel bioactive
tri-substitute-condensed-imidazopyridines that targets snake venom
phospholipase A2. PLoS One. 10:e01318962015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keerthy HK, Mohan CD, Sivaraman Siveen K,
Fuchs JE, Rangappa S, Sundaram MS, Li F, Girish KS, Sethi G,
Basappa, et al: Novel synthetic biscoumarins target tumor necrosis
factor-α in hepatocellular carcinoma in vitro and in vivo. J Biol
Chem. 289:31879–31890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anusha S, Mohan CD, Ananda H, Baburajeev
CP, Rangappa S, Mathai J, Fuchs JE, Li F, Shanmugam MK, Bender A,
et al: Adamantyl-tethered-biphenylic compounds induce apoptosis in
cancer cells by targeting Bcl homologs. Bioorg Med Chem Lett.
26:1056–1060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anusha S, Cp B, Mohan CD, Mathai J,
Rangappa S, Mohan S, Chandra, Paricharak S, Mervin L, Fuchs JE, et
al: A Nano-MgO and ionic liquid-catalyzed ‘green’ synthesis
protocol for the development of adamantyl-imidazolo-thiadiazoles as
anti-tuberculosis agents targeting sterol 14α-demethylase (CYP51).
PLoS One. 10:e01397982015. View Article : Google Scholar
|
|
31
|
Baburajeev CP, Dhananjaya Mohan C, Ananda
H, Rangappa S, Fuchs JE, Jagadish S, Sivaraman Siveen K,
Chinnathambi A, Ali Alharbi S, Zayed ME, et al: Development of
novel triazolo-thiadiazoles from heterogeneous ‘Green’ catalysis as
protein tyrosine phosphatase 1B inhibitors. Sci Rep. 5:141952015.
View Article : Google Scholar
|
|
32
|
Ashwini N, Garg M, Mohan CD, Fuchs JE,
Rangappa S, Anusha S, Swaroop TR, Rakesh KS, Kanojia D, Madan V, et
al: Synthesis of 1,2-benzisoxazole tethered 1,2,3-triazoles that
exhibit anticancer activity in acute myeloid leukemia cell lines by
inhibiting histone deacetylases, and inducing p21 and tubulin
acetylation. Bioorg Med Chem. 23:6157–6165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pandey V, Perry JK, Mohankumar KM, Kong
XJ, Liu SM, Wu ZS, Mitchell MD, Zhu T and Lobie PE: Autocrine human
growth hormone stimulates oncogenicity of endometrial carcinoma
cells. Endocrinology. 149:3909–3919. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neelgundmath M, Dinesh KR, Mohan CD, Li F,
Dai X, Siveen KS, Paricharak S, Mason DJ, Fuchs JE, Sethi G, et al:
Novel synthetic coumarins that targets NF-κB in hepatocellular
carcinoma. Bioorg Med Chem Lett. 25:893–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bharathkumar H, Paricharak S, Dinesh KR,
et al: Synthesis, biological evaluation and in silico and in vitro
mode-of-action analysis of novel dihydropyrimidones targeting
PPAR-γ. RSC Advances. 4:45143–45146. 2014.
|
|
36
|
Morin PJ: Drug resistance and the
microenvironment: Nature and nurture. Drug Resist Updat. 6:169–172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aggarwal BB, Vijayalekshmi RV and Sung B:
Targeting inflammatory pathways for prevention and therapy of
cancer: Short-term friend, long-term foe. Clin Cancer Res.
15:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rajendran P, Li F, Manu KA, Shanmugam MK,
Loo SY, Kumar AP and Sethi G: γ-Tocotrienol is a novel inhibitor of
constitutive and inducible STAT3 signalling pathway in human
hepatocellular carcinoma: Potential role as an antiproliferative,
pro-apoptotic and chemosensitizing agent. Br J Pharmacol.
163:283–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Firestone GL and Sundar SN: Anticancer
activities of artemisinin and its bioactive derivatives. Expert Rev
Mol Med. 11:e322009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Castellano M, Pollock PM, Walters MK,
Sparrow LE, Down LM, Gabrielli BG, Parsons PG and Hayward NK:
CDKN2A/p16 is inactivated in most melanoma cell lines. Cancer Res.
57:4868–4875. 1997.PubMed/NCBI
|
|
41
|
Keerthy HK, Garg M, Mohan CD, Madan V,
Kanojia D, Shobith R, Nanjundaswamy S, Mason DJ, Bender A, Basappa,
et al: Synthesis and characterization of novel
2-amino-chromene-nitriles that target Bcl-2 in acute myeloid
leukemia cell lines. PLoS One. 9:e1071182014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Singhai R, Patil VW, Jaiswal SR, Patil SD,
Tayade MB and Patil AV: E-Cadherin as a diagnostic biomarker in
breast cancer. N Am J Med Sci. 3:227–233. 2011. View Article : Google Scholar
|
|
44
|
Martin TA, Mansel RE and Jiang WG: Loss of
occludin leads to the progression of human breast cancer. Int J Mol
Med. 26:723–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhuo H, Jiang K, Dong L, Zhu Y, Lü L, Lü
Y, Zhang YB, Zhang H, Ye YJ and Wang S: Overexpression of
N-cadherin is correlated with metastasis and worse survival in
colorectal cancer patients. Chin Sci Bull. 58:3529–3534. 2013.
View Article : Google Scholar
|
|
46
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lieblein JC, Ball S, Hutzen B, Sasser AK,
Lin HJ, Huang TH, Hall BM and Lin J: STAT3 can be activated through
paracrine signaling in breast epithelial cells. BMC Cancer.
8:3022008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan ZL, Guan YJ, Wang L, Wei W, Kane AB
and Chin YE: Central role of the threonine residue within the p+1
loop of receptor tyrosine kinase in STAT3 constitutive
phosphorylation in metastatic cancer cells. Mol Cell Biol.
24:9390–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang W, Qian P, Zhang X, Zhang M, Wang H,
Wu M, Kong X, Tan S, Ding K, Perry JK, et al: Autocrine/paracrine
human growth hormone-stimulated microRNA 96-182-183 cluster
promotes epithelial-mesenchymal transition and invasion in breast
cancer. J Biol Chem. 290:13812–13829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu Y, Diab I, Zhang X, Izmailova ES and
Zehner ZE: Stat3 enhances vimentin gene expression by binding to
the antisilencer element and interacting with the repressor
protein, ZBP-89. Oncogene. 23:168–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Elkholi R, Floros KV and Chipuk JE: The
role of BH3-only proteins in tumor cell development, signaling, and
treatment. Genes Cancer. 2:523–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Crown J, O’Shaughnessy J and Gullo G:
Emerging targeted therapies in triple-negative breast cancer. Ann
Oncol. (Suppl 6): vi56–65. 2012.PubMed/NCBI
|