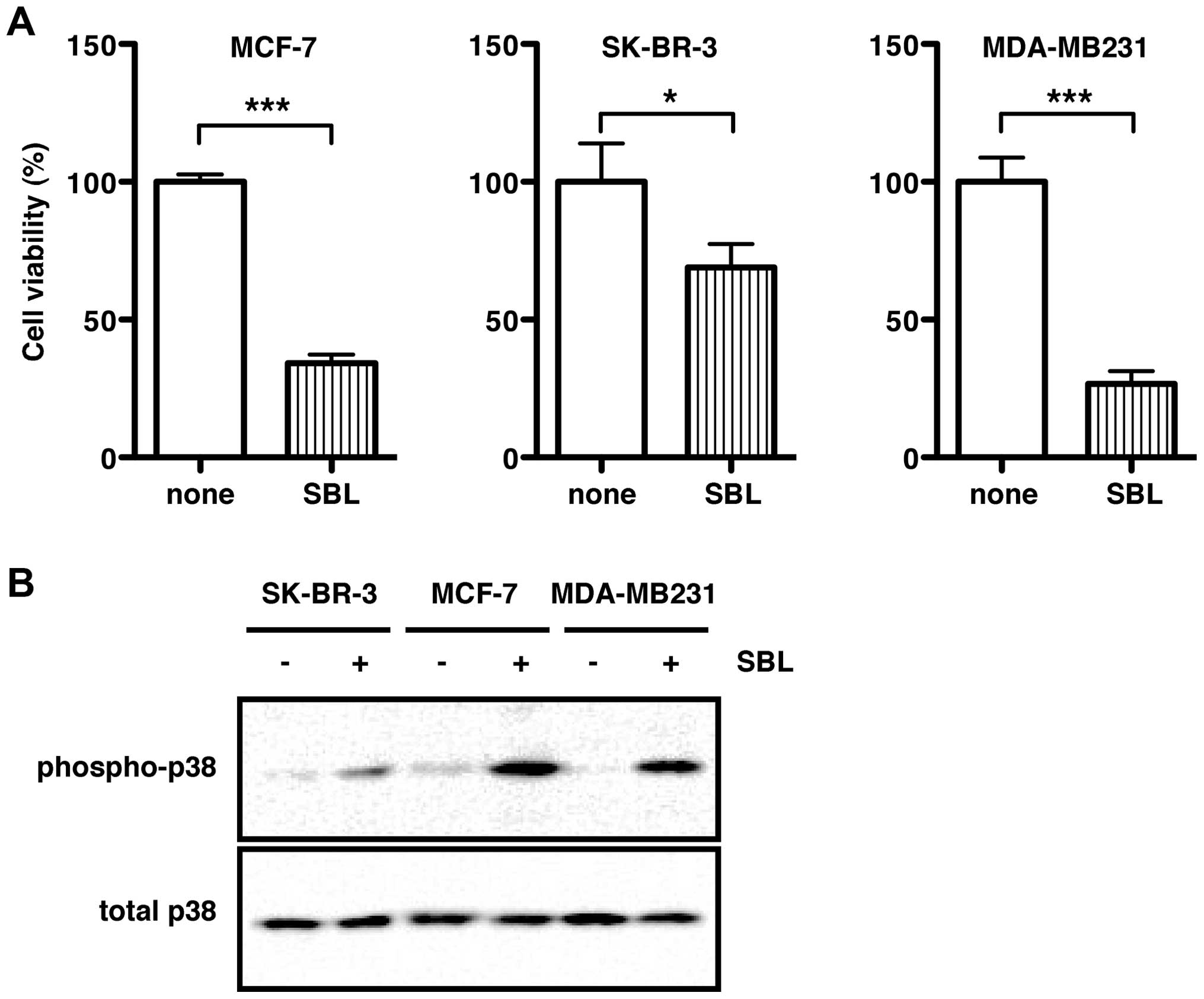
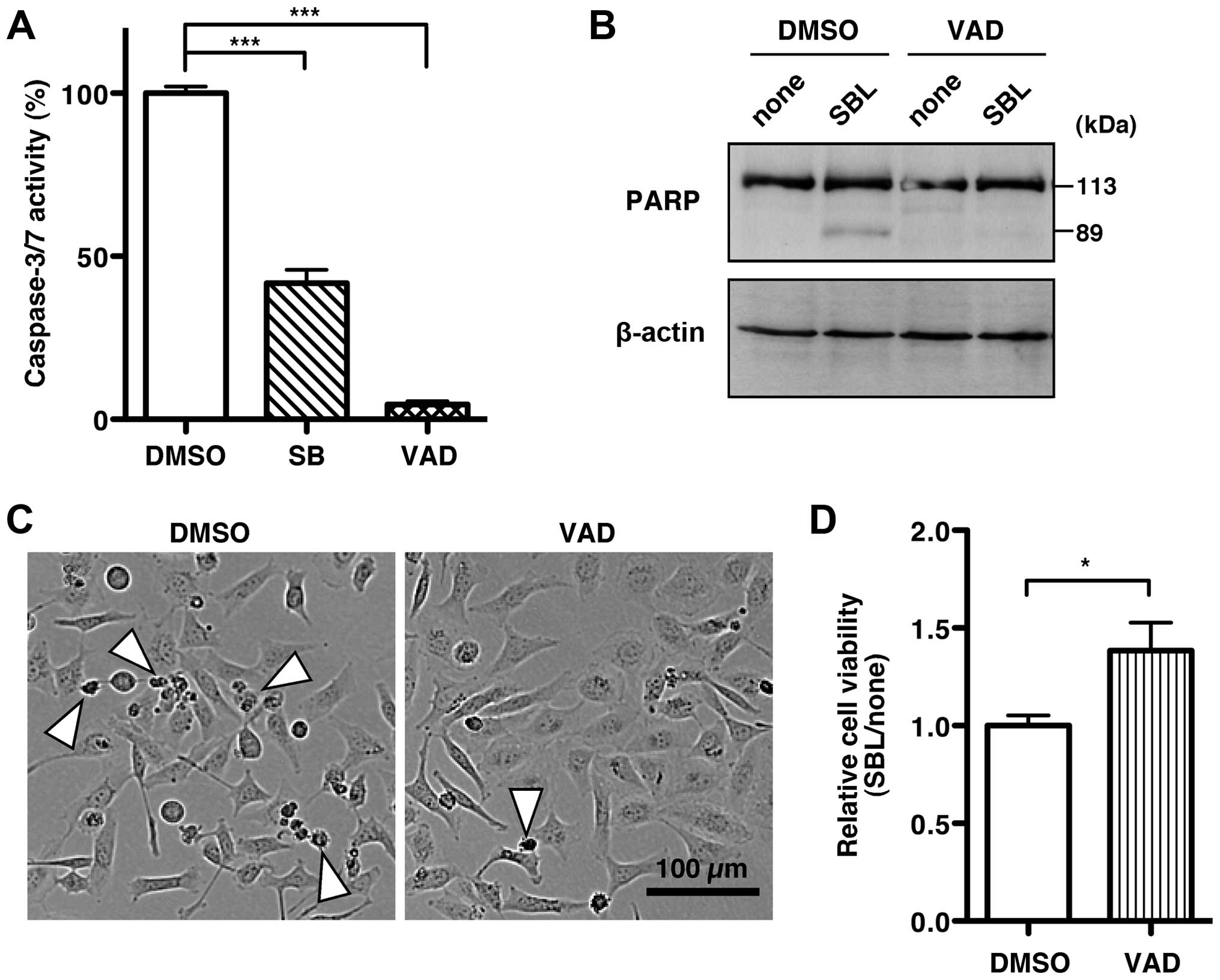
|
1
|
Nitta K, Takayanagi G, Kawauchi H and
Hakomori S: Isolation and characterization of Rana catesbeiana
lectin and demonstration of the lectin-binding glycoprotein of
rodent and human tumor cell membranes. Cancer Res. 47:4877–4883.
1987.PubMed/NCBI
|
|
2
|
Dall'Olio F and Chiricolo M:
Sialyltransferases in cancer. Glycoconj J. 18:841–850. 2001.
View Article : Google Scholar
|
|
3
|
Hu CC, Tang CH and Wang JJ: Caspase
activation in response to cytotoxic Rana catesbeiana ribonuclease
in MCF-7 cells. FEBS Lett. 503:65–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liao YD, Huang HC, Chan HJ and Kuo SJ:
Large-scale preparation of a ribonuclease from Rana catesbeiana
(bullfrog) oocytes and characterization of its specific cytotoxic
activity against tumor cells. Protein Expr Purif. 7:194–202. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen JN, Yiang GT, Lin YF, Chou PL, Wu TK,
Chang WJ, Chen C and Yu YL: Rana catesbeiana ribonuclease induces
cell apoptosis via the caspase-9/-3 signaling pathway in human
glioblastoma DBTRG, GBM8901 and GBM8401 cell lines. Oncol Lett.
9:2471–2476. 2015.PubMed/NCBI
|
|
6
|
Ogawa Y, Sugawara S, Tatsuta T, Hosono M,
Nitta K, Fujii Y, Kobayashi H, Fujimura T, Taka H, Koide Y, et al:
Sialylglycoconjugates in cholesterol-rich microdomains of P388
cells are the triggers for apoptosis induced by Rana catesbeiana
oocyte ribonuclease. Glycoconj J. 31:171–184. 2014. View Article : Google Scholar
|
|
7
|
Tatsuta T, Sugawara S, Takahashi K, Ogawa
Y, Hosono M and Nitta K: Cancer-selective induction of apoptosis by
leczyme. Front Oncol. 4:1392014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nitta K, Ozaki K, Ishikawa M, Furusawa S,
Hosono M, Kawauchi H, Sasaki K, Takayanagi Y, Tsuiki S and Hakomori
S: Inhibition of cell proliferation by Rana catesbeiana and Rana
japonica lectins belonging to the ribonuclease superfamily. Cancer
Res. 54:920–927. 1994.PubMed/NCBI
|
|
9
|
Liao YD: A pyrimidine-guanine
sequence-specific ribonuclease from Rana catesbeiana (bullfrog)
oocytes. Nucleic Acids Res. 20:1371–1377. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Titani K, Takio K, Kuwada M, Nitta K,
Sakakibara F, Kawauchi H, Takayanagi G and Hakomori S: Amino acid
sequence of sialic acid binding lectin from frog (Rana catesbeiana)
eggs. Biochemistry. 26:2189–2194. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang HC, Wang SC, Leu YJ, Lu SC and Liao
YD: The Rana catesbeiana rcr gene encoding a cytotoxic
ribonuclease. Tissue distribution, cloning, purification,
cytotoxicity, and active residues for RNase activity. J Biol Chem.
273:6395–6401. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nitta K, Ozaki K, Tsukamoto Y, Furusawa S,
Ohkubo Y, Takimoto H, Murata R, Hosono M, Hikichi N, Sasaki K, et
al: Characterization of a Rana catesbeiana lectin-resistant mutant
of leukemia P388 cells. Cancer Res. 54:928–934. 1994.PubMed/NCBI
|
|
13
|
Nitta K, Ozaki K, Tsukamoto Y, Hosono M,
Ogawakonno Y, Kawauchi H, Takayanagi Y, Tsuiki S and Hakomori S:
Catalytic lectin (leczyme) from bullfrog (Rana catesbeiana) eggs.
Int J Oncol. 9:19–23. 1996.PubMed/NCBI
|
|
14
|
Tatsuta T, Hosono M, Sugawara S, Kariya Y,
Ogawa Y, Hakomori S and Nitta K: Sialic acid-binding lectin
(leczyme) induces caspase-dependent apoptosis-mediated
mitochondrial perturbation in Jurkat cells. Int J Oncol.
43:1402–1412. 2013.PubMed/NCBI
|
|
15
|
Tatsuta T, Hosono M, Miura Y, Sugawara S,
Kariya Y, Hakomori S and Nitta K: Involvement of ER stress in
apoptosis induced by sialic acid-binding lectin (leczyme) from
bullfrog eggs. Int J Oncol. 43:1799–1808. 2013.PubMed/NCBI
|
|
16
|
Yiang GT, Yu YL, Chou PL, Tsai HF, Chen
LA, Chen YH, Su KJ, Wang JJ, Bau DT and Wei CW: The cytotoxic
protein can induce autophagocytosis in addition to apoptosis in
MCF-7 human breast cancer cells. In Vivo. 26:403–409.
2012.PubMed/NCBI
|
|
17
|
Tatsuta T, Hosono M, Takahashi K, Omoto T,
Kariya Y, Sugawara S, Hakomori S and Nitta K: Sialic acid-binding
lectin (leczyme) induces apoptosis to malignant mesothelioma and
exerts synergistic antitumor effects with TRAIL. Int J Oncol.
44:377–384. 2014.
|
|
18
|
Tang CH, Hu CC, Wei CW and Wang JJ:
Synergism of Rana catesbeiana ribonuclease and IFN-gamma triggers
distinct death machineries in different human cancer cells. FEBS
Lett. 579:265–270. 2005. View Article : Google Scholar
|
|
19
|
Iordanov MS, Wong J, Newton DL, Rybak SM,
Bright RK, Flavell RA, Davis RJ and Magun BE: Differential
requirement for the stress-activated protein kinase/c-Jun
NH(2)-terminal kinase in RNAdamage-induced apoptosis in primary and
in immortalized fibroblasts. Mol Cell Biol Res Commun. 4:122–128.
2000. View Article : Google Scholar
|
|
20
|
Endo Y, Tsurugi K, Yutsudo T, Takeda Y,
Ogasawara T and Igarashi K: Site of action of a Vero toxin (VT2)
from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic
ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem.
171:45–50. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saxena SK, O'Brien AD and Ackerman EJ:
Shiga toxin, Shiga-like toxin II variant, and ricin are all
single-site RNA N-glycosidases of 28 S RNA when microinjected into
Xenopus oocytes. J Biol Chem. 264:596–601. 1989.PubMed/NCBI
|
|
22
|
Smith WE, Kane AV, Campbell ST, Acheson
DW, Cochran BH and Thorpe CM: Shiga toxin 1 triggers a ribotoxic
stress response leading to p38 and JNK activation and induction of
apoptosis in intestinal epithelial cells. Infect Immun.
71:1497–1504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ikeda M, Gunji Y, Yamasaki S and Takeda Y:
Shiga toxin activates p38 MAP kinase through cellular Ca(2+)
increase in Vero cells. FEBS Lett. 485:94–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Y, Mikulski SM, Ardelt W, Rybak SM and
Youle RJ: A cytotoxic ribonuclease. Study of the mechanism of
onconase cytotoxicity. J Biol Chem. 268:10686–10693.
1993.PubMed/NCBI
|
|
25
|
Lee JE and Raines RT: Contribution of
active-site residues to the function of onconase, a ribonuclease
with antitumoral activity. Biochemistry. 42:11443–11450. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Juan G, Ardelt B, Li X, Mikulski SM,
Shogen K, Ardelt W, Mittelman A and Darzynkiewicz Z: G1 arrest of
U937 cells by onconase is associated with suppression of cyclin D3
expression, induction of p16INK4A, p21WAF1/CIP1 and p27KIP and
decreased pRb phosphorylation. Leukemia. 12:1241–1248. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rybak SM, Pearson JW, Fogler WE, Volker K,
Spence SE, Newton DL, Mikulski SM, Ardelt W, Riggs CW, Kung HF, et
al: Enhancement of vincristine cytotoxicity in drug-resistant cells
by simultaneous treatment with onconase, an antitumor
ribo-nuclease. J Natl Cancer Inst. 88:747–753. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pavlakis N and Vogelzang NJ: Ranpirnase -
an antitumour ribo-nuclease: Its potential role in malignant
mesothelioma. Expert Opin Biol Ther. 6:391–399. 2006. View Article : Google Scholar : PubMed/NCBI
|