Introduction
Papillary thyroid carcinoma (PTC) is the most common
thyroid carcinoma, accounting for approximately 80% of all thyroid
malignant tumors (1–4). Despite highly curable and presenting
a 10-year survival rate more than 90%, lymph node metastasis,
especially in the neck, occurs in 20–50% of all tumor patients and
regional recurrence is found in 5–20% of patients who have
undergone total thyroidectomy (3,5,6).
Moreover, PTC is prone to spread through lymphatic ducts, resulting
in recurrence, metastases and even death (3). Recurrent PTC mainly refers to local
and distant recurrence, including recurrence of primary tumor,
lymph node metastases, invasion of the esophagus and trachea,
invasion of muscles, nerves and distant metastases. Many factors
could affect the recurrence of thyroid cancer, and 20% PTC patients
relapse after treatment and require reoperation (7–9).
Consequently, analyzing the molecular characteristics of PTC and
exploring new targets for therapy have been major clinical
concerns.
Rho-associated protein kinase 1 (ROCK1), a
serine/threonine kinase, affects cell invasion by changing the
status of the cytoskeleton (10).
Recent studies have shown that ROCK1 overexpression was found in a
variety of tumors. ROCK1 plays an important role in the regulation
of cell morphology, adhesion and motility. Its inhibitors are
capable of reducing cancer cell migration, proliferation and
invasion (11–13). ROCK1 overexpression is related to
tumor metastasis, and its inhibition was suggested to be a novel
approach for treating breast cancer (14). However, the function of ROCK1 in
PTC remains elusive.
Tumor recurrence and distant metastasis are two
major factors that are responsible for poor survival of cancer
patients (15,16). The degradation of the extracellular
matrix (ECM) is necessary for the initiation and development of
tumor metastasis (17). This
process is mainly influenced by the activity of matrix
metalloproteinases (MMPs), which are enzymes that degrade
structural components of the ECM, molecules of cell-cell and
cell-ECM interactions. MMPs can release and activate growth factors
and cytokines, facilitating tumor invasion and the metastatic
processes, with many human tumors characterized by increased
concentrations of MMPs (18–20).
MMP-9, as one of the major MMPs, is present at high levels in
malignant tumors and related to high numbers of distant metastases
and poor prognosis (21,22). However, the relationship between
ROCK1 and MMP-9 in PTC has not been reported.
In the present study, we examined the ROCK1
expression in human PTC and investigated the biological role of
ROCK1 in promoting the progression of PTC. The results showed that
ROCK1 was overexpressed in both PTC tissues and cell lines, and was
correlated inversely with the clinical outcomes. Furthermore, we
found that ROCK1 promoted the xenograft tumor growth and the
invasion of thyroid cancer cells through activating the expression
of MMP-9. In addition, levels of MMP-9 positively correlated with
the ROCK1 levels in PTC tissues. Taken together, our data suggest
that ROCK1 might be a potential prognostic marker, and play an
important role in the progression of PTC by upregulating MMP-9,
which may be a potential therapeutic target for the treatment of
PTC.
Materials and methods
Cell cultures and siRNA transfection
TPC-1 and K1 cell lines were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and
maintained in 90% RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA)
supplementing with 10% fetal bovine serum (FBS) at 37°C with 5%
CO2 according to the standard protocols. The cells were
transfected with Lipofectamine 2000 (Invitrogen) using siRNA
(Shanghai GenePharma, Co., Ltd., Shanghai, China) according to the
manufacturer's instructions. siRNA sequences of sense strands are
as follows: siROCK1-1, 5′-GAAGCGAAUGACUUAUUATT-3′ and siROCK1-2,
5′-UAAGUAAGUCAUUCGCUUCTT-3′. In addition, MMP-9 inhibitor (Abcam,
Cambridge, MA, USA) was used for invasion assays.
Patients and tissue specimens
Paraffin-embedded PTC specimens (=356) including 137
male patients and 219 female patients, who were histopathologically
diagnosed at Sun Yat-sen Memorial Hospital of Sun Yat-sen
University from January 2003 to July 2007, were evaluated in the
present study. The male to female ratio was 1:1.60. Ages ranged
from 16 to 79 years, and the median age was 43.7 years. Fifty-six
patients (15.73%) showed Hashimoto's thyroiditis (HT). The diameter
of most tumors was <4 cm, the proportion of which was 70.51%
(153/217) in the weak ROCK1 expression group and 29.49% (64/217) in
the strong ROCK1 expression group; 216 patients (60.67%) suffered
lymph node metastasis and 98 patients (27.53%) had distant organ
metastasis. Moreover, 50.56% (180 cases) of the patients presented
extrathyroid invasion, and 29.21% (104 cases) of the patients were
found with vascular invasion, 228 patients (64.04%) had stage I–II
disease based on the tumor, node, metastasis (TNM) staging system
(23), and the remaining 128
patients (35.96%) had stage III–IV disease. The primary tumors of
PTC and their corresponding adjacent non-cancerous tissues were
collected for assays. Clinical information of the samples is
presented in detail in the Table
I. The study was performed in accordance with the policies of
the Institutional Research Ethics Committee of Sun Yat-sen Memorial
Hospital. Informed consents were obtained from the patients by
BioBank, and the patients' personally identifiable information such
as names, addresses and contact information were removed.
 | Table IClinicopathological features of
patients with weak and strong ROCK1 expression in PTC. |
Table I
Clinicopathological features of
patients with weak and strong ROCK1 expression in PTC.
| Clinical
features | No. of
patients
(356) | ROCK1 expression
| χ2 | P-value |
|---|
Weak
(175) | Strong
(181) |
|---|
| Age (years) | | | | | |
| <45 | 194 | 101 | 93 | 1.439 | 0.2303 |
| ≥45 | 162 | 74 | 88 | | |
| Sex | | | | | |
| Male | 137 | 67 | 70 | 0.006 | 0.9400 |
| Female | 219 | 108 | 111 | | |
| Hashimoto's
thyroiditis | | | | | |
| No | 300 | 154 | 146 | 3.613 | 0.0573 |
| Yes | 56 | 21 | 35 | | |
| Tumor size
(cm) | | | | | |
| ≤4 | 217 | 153 | 64 | 101.4 |
<0.0001a |
| ≥4 | 139 | 22 | 117 | | |
| Lymphatic
metastasis | | | | | |
| No | 140 | 126 | 14 | 154.0 |
<0.0001a |
| Yes | 216 | 49 | 167 | | |
| Distant organ
metastasis | | | | | |
| No | 258 | 149 | 109 | 27.70 |
<0.0001a |
| Yes | 98 | 26 | 72 | | |
| Extrathyroid
invasion | | | | | |
| No | 176 | 126 | 50 | 70.09 |
<0.0001a |
| Yes | 180 | 49 | 131 | | |
| Vascular
invasion | | | | | |
| No | 252 | 147 | 105 | 29.06 |
<0.0001a |
| Yes | 104 | 28 | 76 | | |
| TNM stage | | | | | |
| I+II | 228 | 133 | 95 | 21.36 |
<0.0001a |
| III+IV | 128 | 42 | 86 | | |
Immunohistochemistry
Immunohistochemistry (IHC) staining for ROCK1 was
performed on 356 human PTC and the matched adjacent non-cancerous
tissues. Briefly, paraffin-embedded sections (4 μm thick)
were deparaffinized, rehydrated, and microwave-heated for 15 min in
0.01 mol/l citric buffer (pH 6.0) for antigen retrieval. Then, 3%
hydrogen peroxide was applied to block endogenous peroxidase
activity. After 30 min of blocking with normal serum (Invitrogen),
the primary rabbit anti-ROCK1 antibody (1:100 dilution; Abcam), or
the corresponding control isotype IgG for antibody used were
applied and incubated overnight at 4°C. Slides were washed three
times for 5 min each with phosphate-buffered saline (PBS). The
biotinylated secondary antibody and the streptavidin-biotin complex
were each incubated for 60 min at room temperature. After rinsing
with PBS, the slides were immersed for 10 min in DAB
(3,3-diaminobenzidine) (Sigma-Aldrich, St. Louis, MO, USA) solution
(0.4 mg/ml, with 0.003% hydrogen peroxide), then monitored under a
microscope. The reaction was terminated by immersing the slides in
distilled water. Slides were then counterstained with hematoxylin,
dehydrated and coverslipped.
Scoring of IHC staining
ROCK1-positive staining is localized in the
cytoplasm of thyrocyte. The quantitative analysis of IHC staining
analysis was performed for ROCK1 protein expression level in the
tissue specimens. Two experienced investigators scored
independently all the slides by the method previously described
(24). Scores considered both the
proportion of positive staining tumor cells and the staining
intensity. The proportion of staining is graded as: 0, no positive
tumor cells; 1, <10% positive; 2, 10–50% positive, and 3,
>50% positive. The intensity of staining is determined as: 0, no
staining; 1, weak staining; 2, moderate staining and 3, strong
staining. The staining index (SI) is calculated as the product of
staining intensity and percentage of positive tumor cells,
resulting in scores of 0, 1, 2, 3, 4, 6 and 9. Cut-off values for
ROCK1 are chosen based on a measurement of heterogeneity using the
log-rank test with respect to disease-free survival (DFS). We
identified the optimal cut-off as: the SI score of ≥4 was
considered as strong ROCK1 expression, and ≤3 as weak ROCK1
expression.
IHC staining for protein expression in tumor and
adjacent non-cancerous tissues was quantitatively analyzed with the
AxioVision Rel.4.6 computerized image analysis system assisted with
the automatic measurement program (Carl Zeiss, Oberkochen,
Germany). Briefly, to assess the mean optical density (MOD), which
represents the strength of staining signals by measuring per
positive pixels, we evaluated the stained sections at ×200
magnification and ten representative staining fields of each
section were analyzed.
RNA extraction and PCR assays
The fresh tissue specimens were collected with
liquid nitrogen, and RNA was extracted using the TRIzol reagent and
a reverse transcription kit (Invitrogen). The primers for
quantitative real-time PCR assay were ROCK1-F,
5′-CAAATGAAGGTGAATGTAGAAA-3′ and ROCK1-R:
5′-GCAGGAAAGTGGTAGAGTGT-3′; MMP-9-F, 5′-GCCTGGCACATAGTAGGCCC-3′ and
MMP-9-R, 5′-TCTCTCAGCCGGCATC-3′; GAPDH-F, 5′-GA
CTCATGACCACAGTCCATGC-3′ and GAPDH-R, 5′-AGAGGCAGGGATGATGTTCTG-3′.
All primers were synthesized by Shanghai Generay Biotech, Co., Ltd,
(Shanghai, China). The PCR reaction conditions were as follows:
initial denaturation for 2 min at 94°C, 35 cycles of denaturation
for 30 sec, annealing for 30 sec at 94°C, and extension for 1 min
at 72°C, followed by 10 min at 72°C. Expression data were
normalized to the housekeeping gene GAPDH as a loading control.
Western blot analysis
Protein extracts were separated through 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis, transferred to
nitrocellulose membranes, probed with rabbit polyclonal antibodies
against MMP-9 (1:1,000 dilution; Abcam), then incubated with
peroxidase-conjugated goat anti-rabbit Ig secondary antibody
(Oncogene Research Products, Cambridge, MA, USA) and visualized
using chemiluminescence (Amersham, Arlington Heights, IL, USA).
Transduction with retroviral vectors
Ectopic expression of ROCK1 in thyroid cancer cells
was achieved using retroviral vectors. Briefly, ROCK1 cDNA was
cloned into retroviral transfer plasmid pMSCV to generate ROCK1
expression vector, co-transfecting in 293FT cells by using standard
calcium phosphate transfection method as previously described
(25). Thirty-six hours after the
cotransfection, supernatants were collected and incubated with
TPC-1 and K1 cells for 24 h for following assay.
Invasion assay
Cell invasion assays were performed in vitro
as the method previously described (26). Transwell inserts for 24-well plates
(Corning Costar, Cambridge, MA, USA) were coated with prediluted
Matrigel (BD Biosciences, Bedford, MA, USA) and allowed to gel at
37°C for 30 min. Cells were seeded at a density of 3×105
per insert and the lower chamber of the Transwell was filled with
500 μl Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS). After 24 h of
incubation, cells remaining on the upper surface of the Transwell
membrane were removed by a cotton bud. Cells were quantified as the
number of cells found in 6 random microscope fields in three
independent inserts.
Tumor implantation
Thyroid cancer K1 cells were inoculated into the
mammary fat pads of female nude mice. Mice were examined by
palpation for tumor formation for more than 60 days. After tumors
were detected, tumor size was measured every 7 days by calipers,
and tumor volume was calculated as: Volume (mm3) =
Length × Width2 × 0.5 every 7 days for 8 weeks. The
animals were sacrificed when xenografts reached ~1.5 cm in diameter
and tumor engrafts were harvested, weighed and used for IHC
staining. Tumor formation were determined by microscopic
examination.
Statistical analysis
The statistical analyses were performed using the
Statistical Package for Social Sciences software for Windows
Version 13.0 (SPSS, Inc., Chicago, IL, USA). The Chi-squared test
was used to analyze the associations between ROCK1 expression and
the clinicopathological features of PTC. Kaplan-Meier analysis was
used for overall survival (OS), disease-free survival (DFS), lymph
node recurrence-free survival (LNRFS) and distant recurrence-free
survival (DRFS) calculations. OS was calculated as the time from
the date of diagnosis to the date of death or the date of the last
follow-up (if death did not occur). DFS was calculated as the time
from the date of surgery to the date of the first recurrence or
metastasis after surgery (in patients with recurrence or
metastasis) or to the date of the last follow-up (in patients
without recurrence and metastasis). LNRFS was defined as the time
from the date of surgery to the date of lymph node relapse, and
DRFS was defined as the time from the date of surgery to the date
of distant recurrence. The prognostic significance of clinical and
pathologic characteristics was determined using the univariate Cox
regression analysis. Cox proportional hazards models were fitted
for multivariate analysis. The correlation between ROCK1 and MMP-9
mRNA expression was determined using the Pearson's correlation
test. Continuous data were compared using the Student's t-test. All
statistical tests were two-tailed. Errors were SD of averaged
results and P-values <0.05 were accepted as a significant
difference.
Results
ROCK1 expression is correlated with PTC
metastasis
Among 356 tumor samples, 50.84% (181/356) of cases
show strong ROCK1 expression, and 49.16% (175/356) show weak
expression (Table I). The diameter
of most tumors is <4 cm, the proportion of which is 70.51%
(153/217) in the weak ROCK1 expression group and 29.49% (64/217) in
the strong ROCK1 expression group. There is a significant
association between strong ROCK1 expression and tumor size
(P<0.0001), lymphatic metastasis (P<0.0001), distant organ
metastasis (P<0.0001), extrathyroid invasion (P<0.0001),
vascular invasion (P<0.0001) and TNM stage (P<0.0001).
However, no association was found between the ROCK1 expression and
other clinicopathological features. According to the data, ROCK1
expression in tumors might be useful for identifying the malignancy
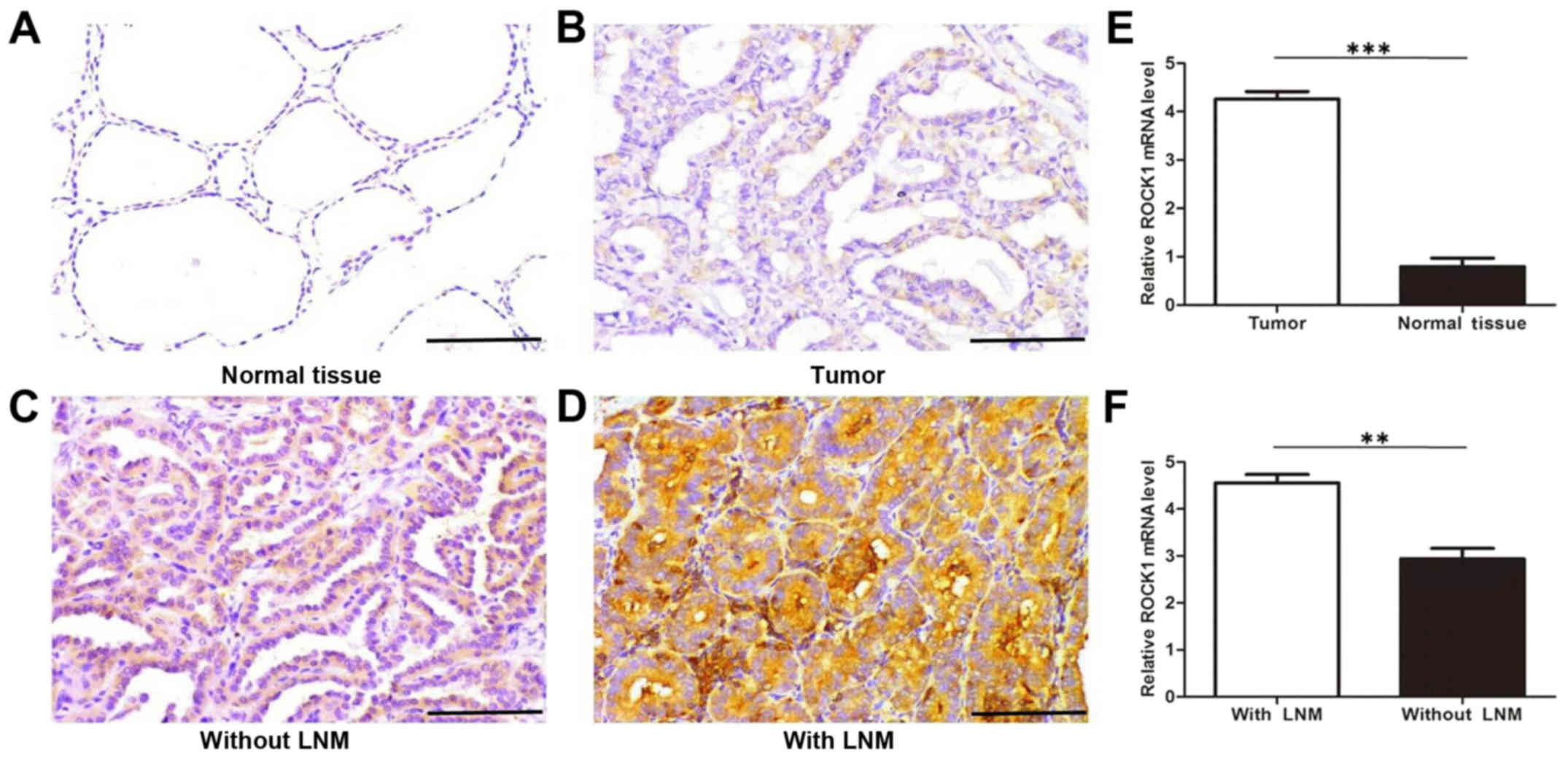
of PTC. Immunostaining results also show a significantly higher
level of ROCK1 expression in PTC tissues compared with adjacent
tissues (Fig. 1A and B). Moreover,
these data further indicate that the ROCK1 protein expression is
remarkably stronger in PTC with lymphatic metastasis than in those
without lymphatic metastasis (Fig. 1C
and D). To confirm these observations, we investigated the mRNA
expression of ROCK1 in 125 cases of PTC tissues, and their normal
adjacent tissues, 128 cases of PTC with lymph node metastasis, and
97 cases of PTC without lymph node metastasis using qRT-PCR
analysis. The results indicate that ROCK1 mRNA expression is
significantly higher in PTC tissues than normal adjacent tissues,
and greatly higher in PTC tissues with lymph node metastasis than
in those without metastasis (Fig. 1E
and F). These findings suggest that elevated ROCK1 expression
contributes to the invasiveness and metastasis of PTC.
ROCK1 expression is associated with PTC
stages
The expression pattern of ROCK1 in 356 cases of
paraffin-embedded PTC tissues includes 61 stage I, 167 stage II, 88
stage III, and 40 stage IV. As shown in Fig. 2A, ROCK1 expression is upregulated
in all stages of PTC comparing with that in normal tissues.
Notably, the ROCK1 expression is localized mainly in the cytoplasm
of cancer cells. Moreover, comparative quantification of the MOD of
ROCK1 staining among normal tissues and PTC specimens of different
stages are summarized in Fig. 2B.
The MOD of ROCK1 staining increases while PTC progresses from lower
stages to higher (P<0.05). Furthermore, quantitative analysis
verified that mRNA expression of ROCK1 in stages I–IV tumors was
significantly higher than that in normal tissue, and also increases
from lower stages to higher, by qRT-PCR assays (P<0.05; Fig. 2C). Taken as a whole, these data
support the notion that the progression of PTC is associated with
increased ROCK1 expression.
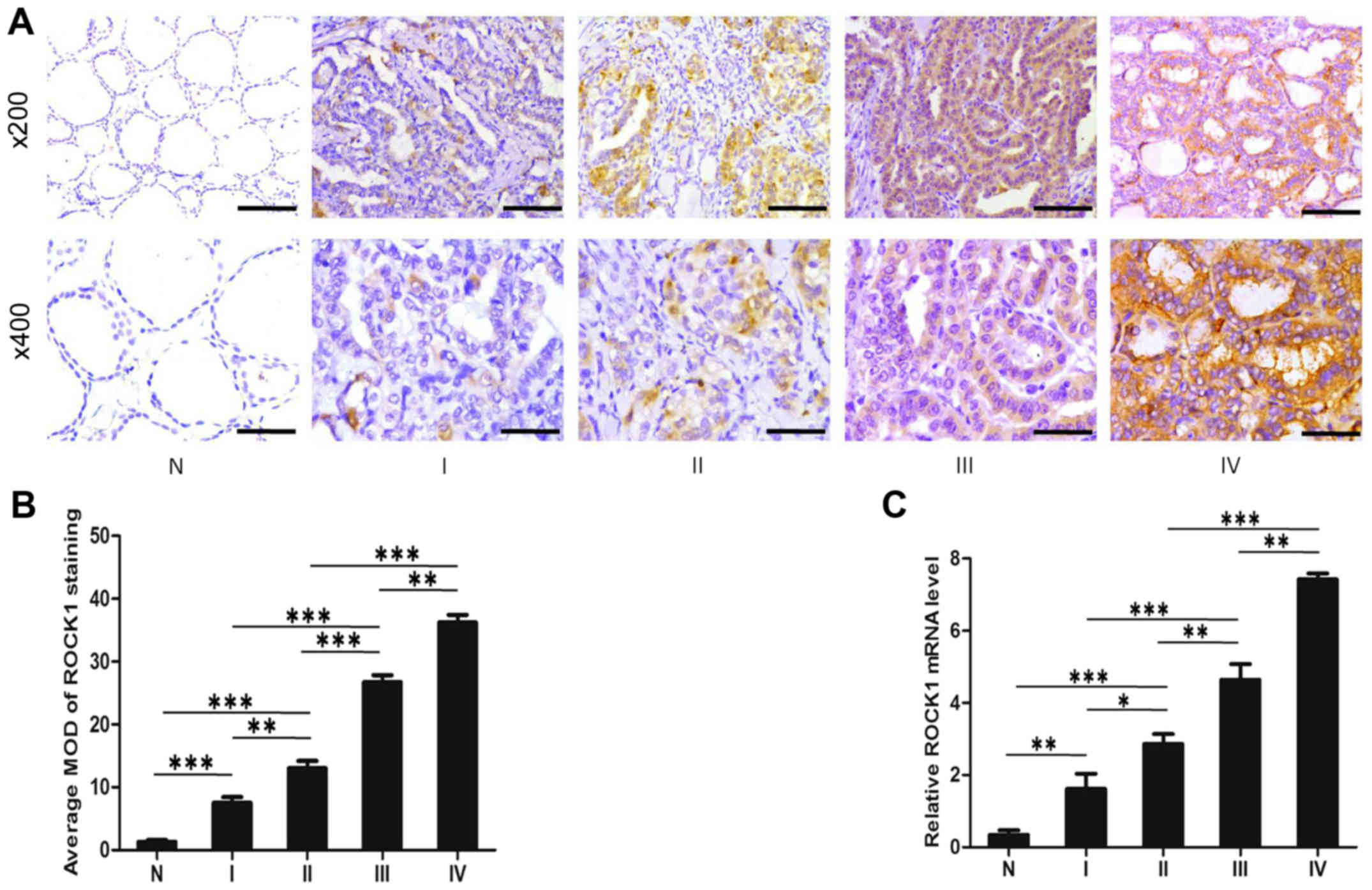 | Figure 2ROCK1 expression is associated with
PTC stages. (A) Representative images from IHC assays of
paraffin-embedded specimens of different stages of PTC tissue
specimens and their adjacent non-cancerous tissues. Stage I (n=61),
stage II (n=167), stage III (n=88) and stage IV (n=40). (IHC,
magnification, ×200 and ×400; scale bars, 100 μm and 50
μm). (B) Comparative quantification of the MOD of ROCK1
staining among normal tissues and PTC specimens of different
stages. (C) Verification of mRNA expression of ROCK1 in stages I–IV
PTC tumors using qRT-PCR assay. *P<0.05,
**P<0.01, ***P<0.001 from the Student's
t-test; IHC, immunohistochemistry; PTC, papillary thyroid
carcinoma; ROCK1, Rho-associated protein kinase 1; MOD, mean
optical density. |
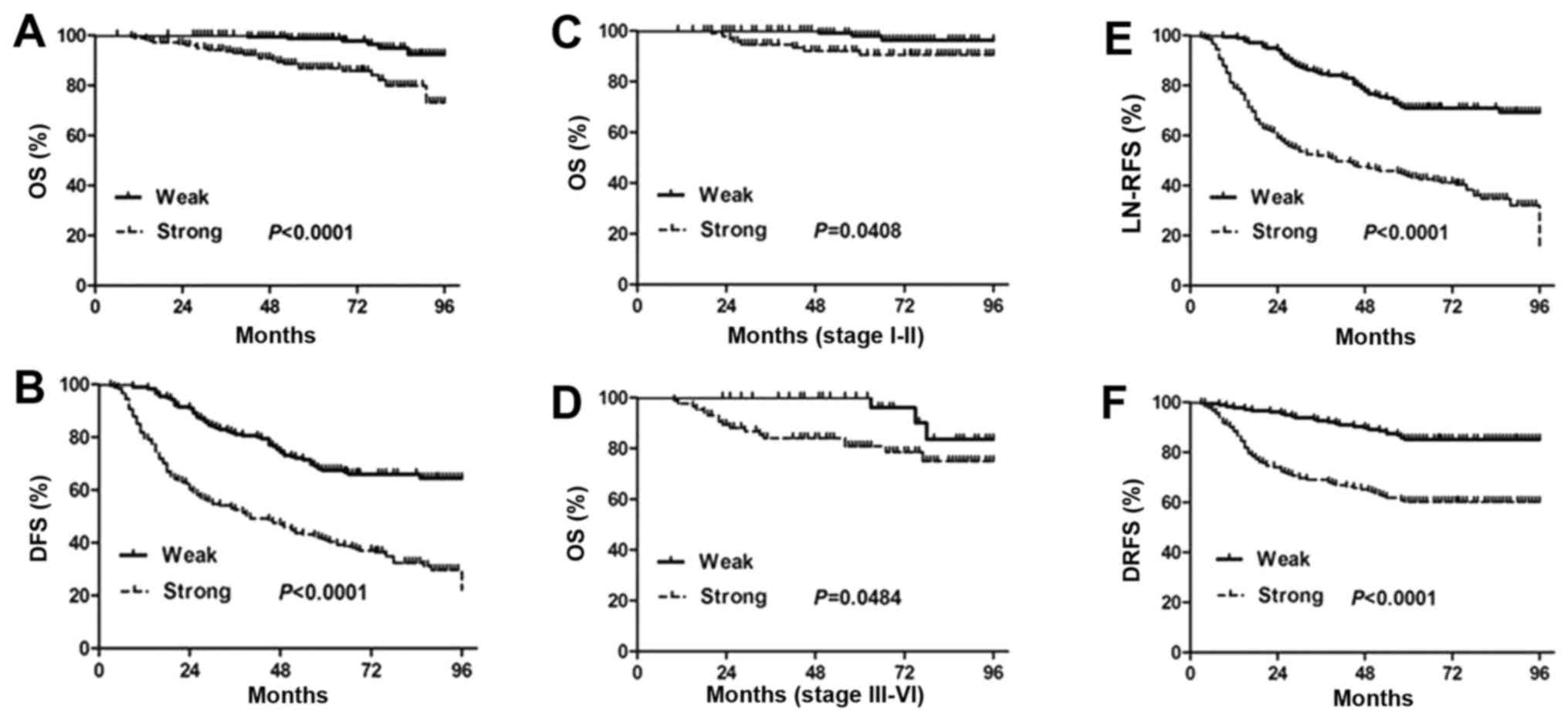
ROCK1 overexpression reduces patient's
survival
Kaplan-Meier analysis using the log-rank test was
performed to calculate the relationship between the ROCK1
expression and the survival rate in PTC patients. The results show
that strong expression of ROCK1 is markedly associated with reduced
overall survival and disease-free survival (P<0.0001; Fig. 3A and B). The median survival time
is significantly shorter in the patients with strong ROCK1
expression than those with weak ROCK1 expression. Moreover, the
cumulative 8-year survival rate is only 86.18% (156/181) in the
strong ROCK1 expression group, whereas it is 96.57% (169/175) in
the weak ROCK1 expression group.
To further validate these findings, we next assessed
the prognostic significance of ROCK1 expression in different
subgroups of PTC patients stratified according to the TNM stage.
Notably, strong ROCK1 expression is significantly correlated with
shorter overall survival time in different PTC subgroups. Overall
survival of patients with strong ROCK1 expression is significantly
decreased compared to those with weak ROCK1 expression in either
stages I+II subgroup (N=228; P=0.0408, log-rank; Fig. 3C) or stages III+IV subgroup (N=128;
P=0.0484, log-rank; Fig. 3D).
Collectively, the data suggest that the level of ROCK1 expression
strongly and significantly correlates with the prognosis of PTC and
the disease outcome.
We also observed that ROCK1 expression in PTC
patients is significantly correlated with LN-RFS (P<0.0001) and
DRFS (P<0.0001; Fig. 3E and F).
Lymph node recurrence and distant metastasis are responsible for
poor survival of PTC patients. PTC displays an indolent course and
shows a 10-year survival rate of ~90% (27). Our results may suggest a potential
prognostic role of ROCK1 for PTC patients.
We next performed a univariate Cox regression
analysis for disease-free survival (DFS). As shown in Table II, DFS is strongly correlated with
ROCK1 expression (P<0.001) besides with tumor size (P=0.028),
lymphatic metastasis (P<0.001), distant organ metastasis
(P=0.027), extrathyroid invasion (P=0.003), vascular invasion
(P<0.001) and TNM stage (P<0.001).
 | Table IIUnivariate Cox regression analysis of
disease-free survival in relation to clinicopathological
features. |
Table II
Univariate Cox regression analysis of
disease-free survival in relation to clinicopathological
features.
| Clinical
features | PTC (N=356)
|
|---|
| HR (95% CI) | P-value |
|---|
| Age (years) | | 0.457 |
| <45 | 1 | |
| ≥45 | 0.463
(0.125–2.835) | |
| Tumor size
(cm) | |
0.028a |
| ≤4 | 1 | |
| >4 | 2.605
(1.764–3.879) | |
| Lymphatic
metastasis | |
<0.001a |
| No | 1 | |
| Yes | 6.223
(0.834–9.431) | |
| Distant organ
metastasis | |
0.027a |
| No | 1 | |
| Yes | 2.856
(1.210–12.081) | |
| Extrathyroid
invasion | |
0.003a |
| No | 1 | |
| Yes | 1.472
(1.414–1.898) | |
| Vascular
invasion | |
<0.001a |
| No | 1 | |
| Yes | 2.235
(1.114–11.797) | |
| ROCK1
expression | |
<0.001a |
| Weak | 1 | |
| Strong | 4.541
(1.349–8.782) | |
| TNM stage | |
0.001a |
| I+II | 1 | |
| III+IV | 4.893
(1.806–13.785) | |
Furthermore, ROCK1 expression was also demonstrated
as a useful prognostic biomarker for PTC patients by multivariate
analysis (Table III) including
tumor size (HR, 1.275; 95% CI, 1.021–1.592; P=0.032), lymphatic
metastasis (HR, 2.380; 95% CI, 1.361–4.142; P<0.001), distant
organ metastasis (HR, 4.284; 95% CI, 1.697–10.931; P=0.002),
extrathyroid invasion (HR, 1.557; 95% CI, 1.229–2.204; P<0.001),
vascular invasion (HR, 2.237; 95% CI, 1.209–4.516; P=0.009), TNM
stage (HR, 1.553; 95% CI, 1.275–1.903; P<0.001). Collectively,
these data suggest that ROCK1 might serve as a previously
unappreciated prognostic predictor of the long-term survival of PTC
patients.
 | Table IIIMultivariate Cox regression analysis
of disease-free survival in relation to clinicopathological
features. |
Table III
Multivariate Cox regression analysis
of disease-free survival in relation to clinicopathological
features.
| Clinical
features | PTC (N=356)
|
|---|
| HR (95% CI) | P-value |
|---|
| Tumor size
(cm) | |
0.032a |
| ≤4 | 1 | |
| >4 | 1.275
(1.021–1.592) | |
| Lymphatic
metastasis | |
<0.001a |
| No | 1 | |
| Yes | 2.380
(1.361–4.142) | |
| Distant organ
metastasis | |
0.002a |
| No | 1 | |
| Yes | 4.284
(1.697–10.931) | |
| Extrathyroid
invasion | |
<0.001a |
| No | 1 | |
| Yes | 1.557
(1.229–2.204) | |
| Vascular
invasion | |
0.009a |
| No | 1 | |
| Yes | 2.237
(1.209–4.516) | |
| ROCK1
expression | |
<0.001a |
| Weak | 1 | |
| Strong | 2.895
(1.697–5.126) | |
| TNM stage | |
<0.001a |
| I+II | 1 | |
| III+IV | 1.553
(1.275–1.903) | |
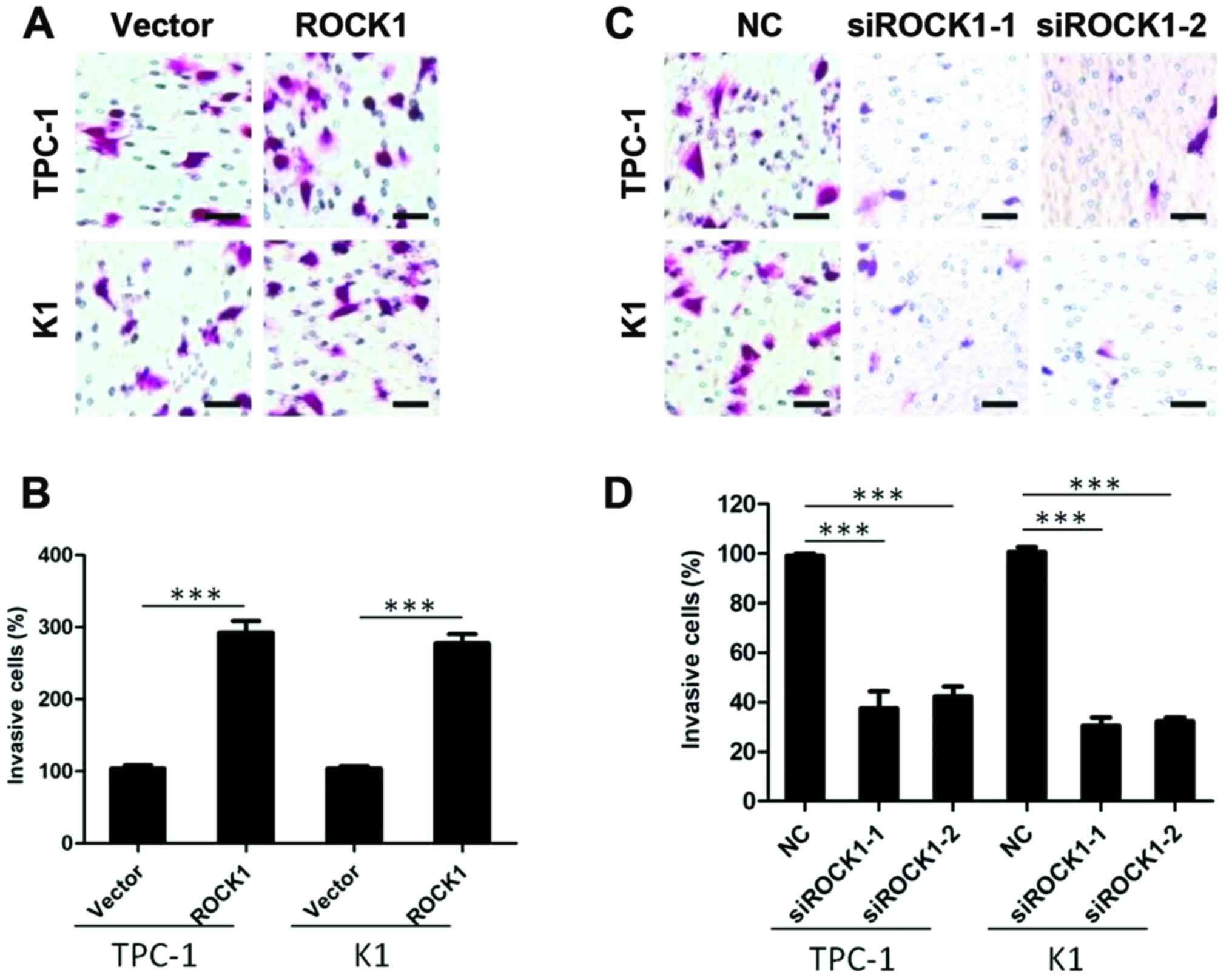
ROCK1 promotes invasiveness on PTC cell
lines
To investigate whether and how ROCK1 impacts on the
aggressive nature of PTC cells, TPC-1 and K1 thyroid cancer cells
were engineered to overexpress ROCK1 and tested for their ability
of invasion. As shown in Fig. 4A and
B, the invasion assay demonstrated that ROCK1 overexpression
efficiently promoted PTC cells invasion (P<0.001). To further
confirm the role of ROCK1 in cancer cell invasion, we knocked down
ROCK1 expression by specific siRNA, and the invasive capability of
TPC-1 and K1 thyroid cancer cells was markedly inhibited
(P<0.001) (Fig. 4C and D),
suggesting that ROCK1 plays a role in PTC invasion.
ROCK1 promotes tumor growth in vivo
To determine if ROCK1 is involved in tumor growth
in vivo, we used tumor implantation model to assess tumor
growth using thyroid cancer K1 cells. The nude mice were
subcutaneously injected with 1×106 thyroid cancer K1
cells with lenti-ROCK1, ROCK1 shRNA or negative control,
respectively. Representative images of the xenograft tumors on week
8 are shown in Fig. 5A. Our data
revealed that knockdown of ROCK1 decreased the volume of the
xenograft tumors, while overexpression of ROCK1 showed a
proliferative tendency with significantly greater tumor volume
(P<0.05; Fig. 5B). We also
observed similar results on tumor weight in mice injected with
thyroid cancer K1 cells with lenti-ROCK1 and ROCK1 shRNA compared
with control mice (P<0.05; Fig.
5C). Noteworthy, our data indicated that IHC detection of Ki-67
staining in K1 xenograft tumors showed similar patterns (Fig. 5D). Together, these data suggest
that inhibiting ROCK1 activity might be an effective approach for
treating PTC.
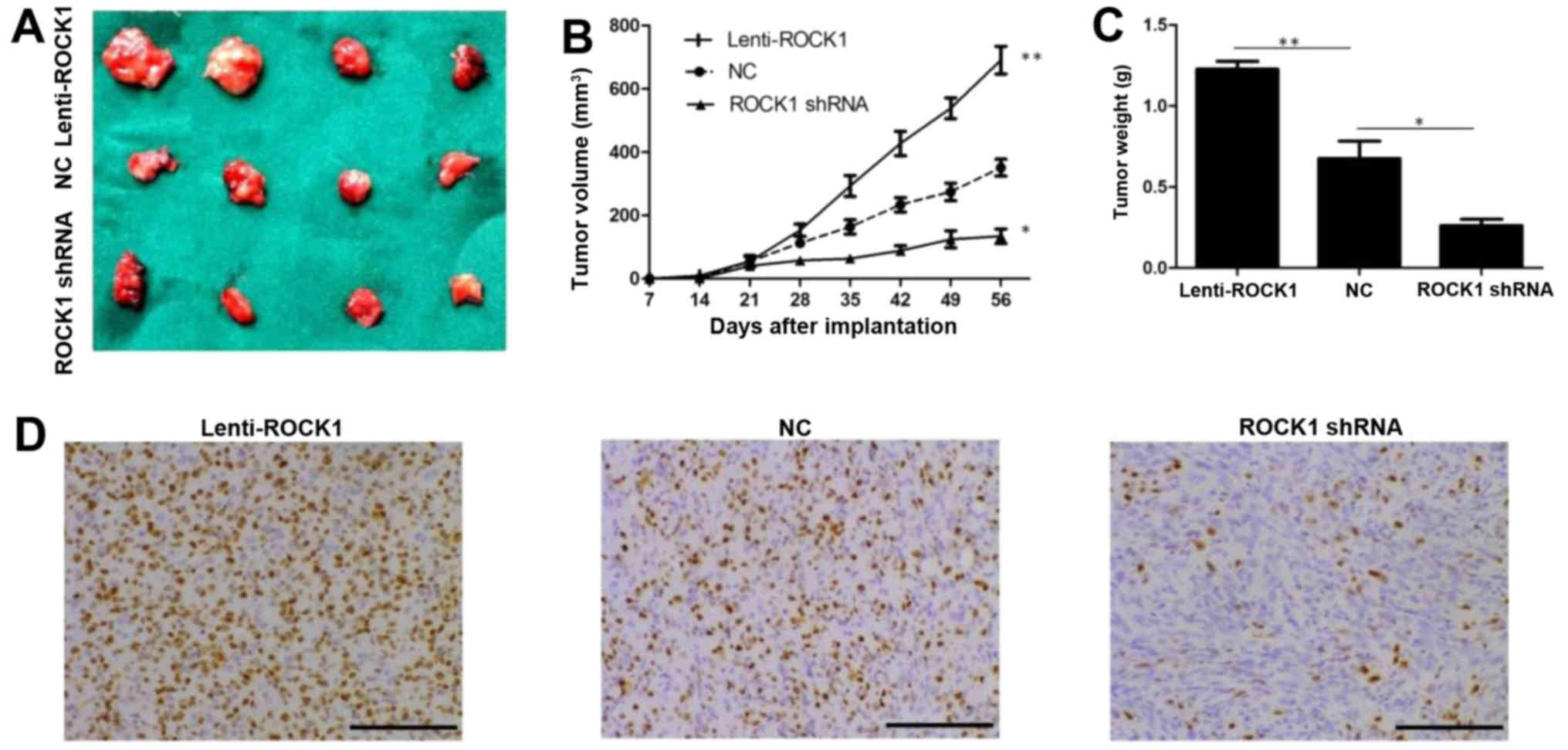 | Figure 5ROCK1 promotes thyroid cancer K1
cells growth in vivo. The nude mice were subcutaneously
injected with 1×106 thyroid cancer K1 cells with
lenti-ROCK1, ROCK1 shRNA or negative control, respectively. (A)
Representative images of the tumors on week 8 are shown. (B)
Xenograft assay revealed that knockdown of ROCK1 decreased the
volume of the xenograft tumors, while overexpression of ROCK1
showed a proliferative tendency with significantly greater tumor
volume (*P<0.05, **P<0.01). (C)
Knockdown of ROCK1 decreased the weight of the xenograft tumors,
while overexpression of ROCK1 showed a proliferative tendency with
significantly greater tumor weight (*P<0.05,
**P<0.01). (D) Representative images of
immunohistochemical detection of Ki-67 staining in K1 xenograft
tumors from control, lenti-ROCK1 or ROCK1 shRNA-treated mice,
respectively (IHC, magnification, ×200; scale bars, 100 μm).
ROCK1, Rho-associated protein kinase 1; PTC, papillary thyroid
carcinoma; MMP-9, matrix metalloproteinase-9. |
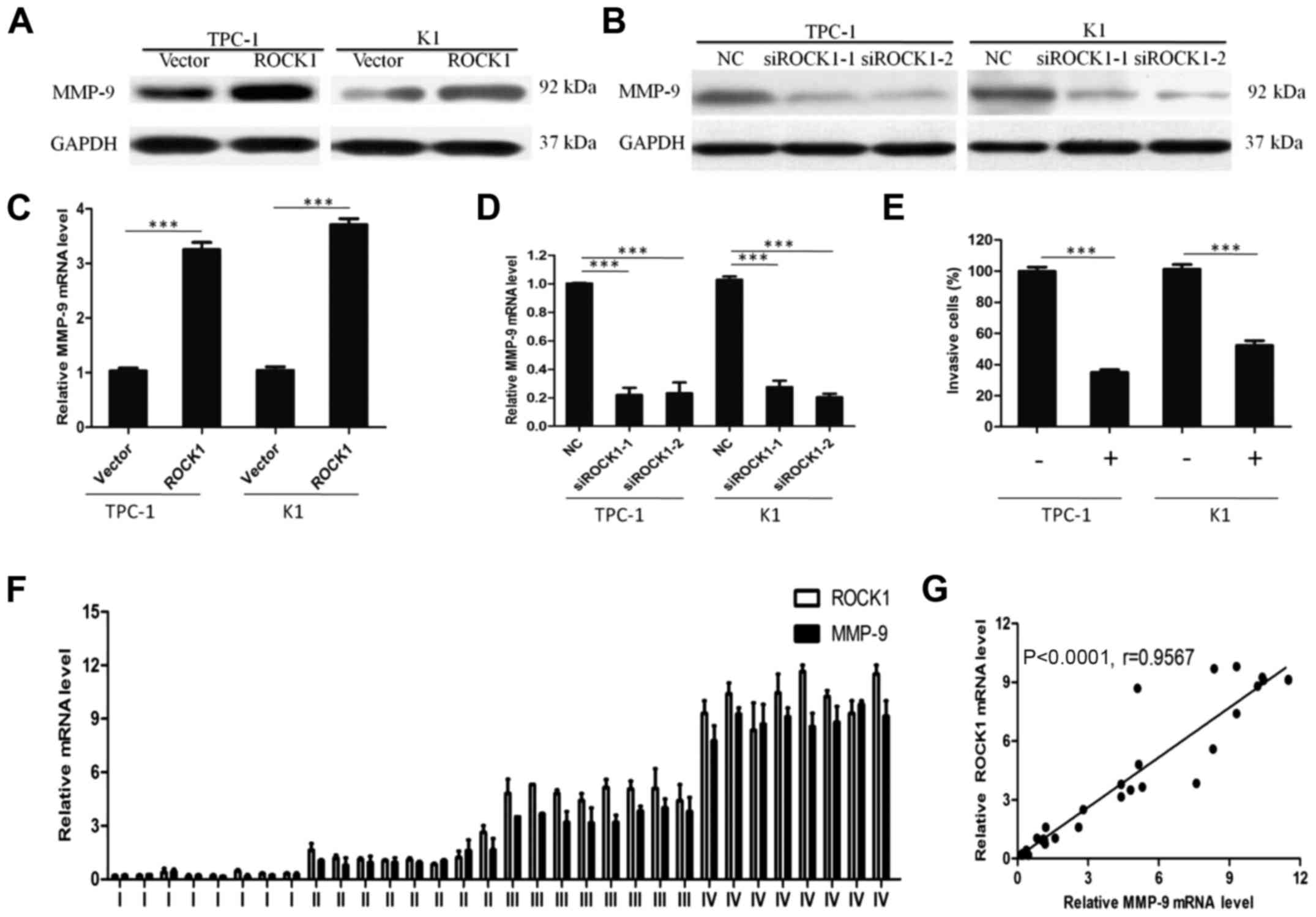
ROCK1 may promote PTC invasion through
MMP-9
To understand the mechanism by which ROCK1 enhances
cancer cell invasion, we analyzed MMP-9 expression in
ROCK1-overexpressed cells by in vitro assays. ROCK1
overexpression significantly increased the expression of MMP-9
protein (Fig. 6A), indicating a
possible correlation between MMP-9 and ROCK1. Moreover, qRT-PCR
showed that ectopic expression of ROCK1 resulted in MMP-9 mRNA
overexpression (Fig. 6C). In
contrast, the knockdown of ROCK1 expression markedly decreased both
protein and mRNA level of MMP-9 (Fig.
6B and D). When MMP-9 expression was suppressed by a specific
inhibitor, the effect of ROCK1 in enhancing PTC cell invasion was
blocked as shown in Fig. 6E,
suggesting that MMP-9 is necessary for ROCK1 promoting PTC cell
invasion.
In agreement, ROCK1 and MMP-9 mRNA expression level
studied by qRT-PCR increased from stages I–IV with similar patterns
in PTC tissues (Fig. 6F). Levels
of MMP-9 positively correlated with the ROCK1 levels in PTC tissues
(Fig. 6G; r=0.9567, P<0.0001).
Collectively, our results confirm that ROCK1 promotes cell invasion
in PTC through the upregulation of MMP-9.
Discussion
To the best of our knowledge, for the first time,
our results confirmed that ROCK1 is overexpressed in PTC, and that
the ROCK1 overexpression is significantly associated with the
clinical and pathological features, as well as the prognosis, of
PTC. Consistent with this, we have demonstrated that ROCK1 might
promote the invasiveness of cancer cells, possibly through
upregulating MMP-9. This study also demonstrated the role of ROCK1
in invasion malignant tumors. Moreover, ROCK1 may be a powerful
predictor of the outcome of papillary thyroid cancer patients.
ROCK1 enhanced the invasion of cancer cells and
direct phosphorylation of myosin light chain, which leads to
increased cell migration and invasion (28–30).
ROCK1 overexpression has been reported in several cancer types.
Based on their oncogenic activity, ROCK1 was examined as
therapeutic targets in various tumors, such as lung tumors
(13,31), glioblastoma (32), osteosarcoma (33,34),
prostate (35,36), breast (37,38),
ovarian cancer (39),
hepatocellular carcinoma (40) and
bladder cancer (41). Moreover,
ROCK1 was indicated as an independent predictor of patients
survival.
However, the influence of ROCK1 in PTC has remained
unknown. Evidence in support of a connection such as provided by
the present study, includes positive results of ROCK1 detection in
tumor tissues paired with non-tumorous tissue, and in a cohort of
356 PTC specimens and two PTC cell lines. Further support for a
possible role of ROCK1 in PTC pathogenesis derived from the
analysis that revealed a strong correlation of ROCK1 expression
with the IHC staging and inversely, with the survival of the
disease. Moreover, our results suggest that a strong ROCK1
expression might be associated with tumor size, lymphatic
metastasis, distant organ metastasis, extrathyroid invasion,
vascular invasion and TNM stage, further implying an involvement of
ROCK1 in the incidence and development of PTC. In addition, we
demonstrated an association between strong ROCK1 expression and the
prognosis of patients with PTC using Kaplan-Meier survival curves.
Our findings indicate that a strong expression of ROCK1 indicates
worse OS, DFS, LN-RFS and DRFS. At the same time, our univariate
and multivariate analysis using Cox proportional hazards regression
model showed that strong expression of ROCK1 was related to PTC
patient prognosis. Knockdown of ROCK1 decreased the volume and
weight of the xenograft tumors, while overexpression of ROCK1
showed a proliferative tendency with significantly greater tumor
volume and weight in vivo. Based on these results, our data
not only suggest that ROCK1 is likely to be biologically involved
in the progression of PTC, but also might represent a valuable
independent prognostic biomarker for the disease.
While this study has provided strong evidence for
the upregulation of ROCK1 expression in PTC, the molecular
mechanism underlying the observed ROCK1 upregulation is yet to be
elucidated. Multiple steps are involved in the invasion of PTC
cells, including cancer cell attachment to ECM, degradation of ECM
components and subsequent infiltration into adjacent normal tissue
(42). The accomplishment of this
process, as shown by several lines of research, is largely
attributable to the activation of MMPs. Among the MMPs, a subset
called gelatinases, consisting of MMP-2 and MMP-9, has gained the
most attention in studies on the acquisition of invasive and
metastatic tumor properties, as they degrade collagen IV, the major
component of the basement membrane (18,43).
Furthermore, MMP-9 is of special interest since its
basal expression is normally low, whereas it is highly expressed in
most human cancers in response to various growth factors and
cytokines (43,44). It has been shown that
MMP-9-deficient exhibit impaired metastasis formation and tumor
growth. In this respect, upregulation of MMP-9 expression in
various types of human cancers contributes to tumor progression,
invasion and metastasis (18,45,46).
In addition, there are more evidence demonstrating that MMP-9 was
overexpressed in various types of tumors when compared to normal
tissue, including in papillary thyroid cancer (47,48).
On the other hand, emerging evidence indicate that the upregulation
of ROCK1 protein enhanced invasion of cells of various types of
tumors. To the best of our knowledge, however, the connection of
ROCK1 and MMP-9 and their impact on the outcome of PTC patients
have not been studied. This study first demonstrates the
pathological role of ROCK1 in enhancing MMP-9 expression and
mediating the invasive phenotype of thyroid cancer cells. Moreover,
levels of MMP-9 positively correlated with the ROCK1 levels in PTC
tissues.
In conclusion, to the best of our knowledge, this is
the first report on the relationship between ROCK1 and prognosis in
patients with PTC. This study indicates that ROCK1 is an
independent prognostic marker for human PTC and that its strong
expression contributes to papillary thyroid carcinoma progression
by enhancing MMP-9 expression and tumor invasion. These results,
together with the correlation between ROCK1/MMP-9 pathway and
metastasis in papillary thyroid carcinoma patients, point towards
the importance of targeting ROCK1 as a novel approach for the
treatment of papillary thyroid carcinoma.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (81702654), the
Natural Science Foundation of Guangdong Province (2017A030313642),
the grant (2013) 163 from the Key Laboratory of Malignant Tumor
Molecular Mechanism and Translational Medicine of Guangzhou Bureau
of Science and Information Technology and the grant K1809001 from
the Key Laboratory of Malignant Tumor Gene Regulation and Target
Therapy of Guangdong Higher Education Institutes.
Glossary
Abbreviations
Abbreviations:
|
ROCK1
|
Rho-associated protein kinase 1
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
IHC
|
immunohistochemistry
|
|
PTC
|
papillary thyroid carcinoma
|
|
ECM
|
extracellular matrix
|
|
FBS
|
fetal bovine serum
|
|
SI
|
staining index
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
LNRFS
|
lymph node recurrence-free
survival
|
|
DRFS
|
distant recurrence-free survival
|
References
|
1
|
Zeiger MA and Schneider EB: BRAF V600E
mutation independently predicts central compartment lymph node
metastasis in patients with papillary thyroid cancer. Ann Surg
Oncol. 20:3–4. 2013. View Article : Google Scholar
|
|
2
|
Zivaljevic V, Slijepcevic N, Sipetic S,
Paunovic I, Diklic A, Zoric G and Kalezic N: Risk factors for
well-differentiated thyroid cancer in men. Tumori. 99:458–462.
2013.PubMed/NCBI
|
|
3
|
Sipos JA and Mazzaferri EL: Thyroid cancer
epidemiology and prognostic variables. Clin Oncol (R Coll Radiol).
22:395–404. 2010. View Article : Google Scholar
|
|
4
|
Li Z, Huang X, Xu J, Su Q, Zhao J and Ma
J: miR-449 over-expression inhibits papillary thyroid carcinoma
cell growth by targeting RET kinase-β-catenin signaling pathway.
Int J Oncol. 49:1629–1637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng S, Serra S, Mercado M, Ezzat S and
Asa SL: A high-throughput proteomic approach provides distinct
signatures for thyroid cancer behavior. Clin Cancer Res.
17:2385–2394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agrawal N, Akbani R, Aksoy BA, Ally A,
Arachchi H, Asa SL, Auman JT, Balasundaram M, Balu S, Baylin SB, et
al Cancer Genome Atlas Research Network: Integrated genomic
characterization of papillary thyroid carcinoma. Cell. 159:676–690.
2014. View Article : Google Scholar :
|
|
7
|
Beninato T, Scognamiglio T, Kleiman DA,
Uccelli A, Vaca D, Fahey TJ III and Zarnegar R: Ten percent tall
cells confer the aggressive features of the tall cell variant of
papillary thyroid carcinoma. Surgery. 154:1331–1336; discussion
1336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee CW, Roh JL, Gong G, Cho KJ, Choi SH,
Nam SY and Kim SY: Risk factors for recurrence of papillary thyroid
carcinoma with clinically node-positive lateral neck. Ann Surg
Oncol. 22:117–124. 2015. View Article : Google Scholar
|
|
9
|
Zhu J, Wang X, Zhang X, Li P and Hou H:
Clinicopathological features of recurrent papillary thyroid cancer.
Diagn Pathol. 10:962015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saito K, Ozawa Y, Hibino K and Ohta Y:
FilGAP, a Rho/Rho-associated protein kinase-regulated
GTPase-activating protein for Rac, controls tumor cell migration.
Mol Biol Cell. 23:4739–4750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banyard J, Anand-Apte B, Symons M and
Zetter BR: Motility and invasion are differentially modulated by
Rho family GTPases. Oncogene. 19:580–591. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morgan-Fisher M, Wewer UM and Yoneda A:
Regulation of ROCK activity in cancer. J Histochem Cytochem.
61:185–198. 2013. View Article : Google Scholar :
|
|
13
|
Vigil D, Kim TY, Plachco A, Garton AJ,
Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL, et
al: ROCK1 and ROCK2 are required for non-small cell lung cancer
anchorage-independent growth and invasion. Cancer Res.
72:5338–5347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan R, Xu X, Chen M, Hu H, Ge H, Wen S,
Zhou S and Pi R: Advances in the studies of roles of Rho/Rho-kinase
in diseases and the development of its inhibitors. Eur J Med Chem.
70:613–622. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azab AK, Kleinstern J, Doviner V, Orkin B,
Srebnik M, Nissan A and Rubinstein A: Prevention of tumor
recurrence and distant metastasis formation in a breast cancer
mouse model by biodegradable implant of 131I-norcholesterol. J
Control Release. 123:116–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu FH, Kuo SF, Hsueh C, Chao TC and Lin
JD: Postoperative recurrence of papillary thyroid carcinoma with
lymph node metastasis. J Surg Oncol. 112:149–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Werb Z: ECM and cell surface proteolysis:
Regulating cellular ecology. Cell. 91:439–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bottino J, Gelaleti GB, Maschio LB,
Jardim-Perassi BV and de Campos Zuccari DA: Immunoexpression of
ROCK-1 and MMP-9 as prognostic markers in breast cancer. Acta
Histochem. 116:1367–1373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JR, Gan WJ, Li XM, Zhao YY, Li Y, Lu
XX, Li JM and Wu H: Orphan nuclear receptor Nur77 promotes
colorectal cancer invasion and metastasis by regulating MMP-9 and
E-cadherin. Carcinogenesis. 35:2474–2484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao J, Liu X, Yang F, Liu T, Yan Q and
Yang X: By inhibiting Ras/Raf/ERK and MMP-9, knockdown of EpCAM
inhibits breast cancer cell growth and metastasis. Oncotarget.
6:27187–27198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American Thyroid Association Management
Guidelines for Adult Patients with Thyroid Nodules and
Differentiated Thyroid Cancer: The American Thyroid Association
Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid
Cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar :
|
|
24
|
Li J, Guan HY, Gong LY, Song LB, Zhang N,
Wu J, Yuan J, Zheng YJ, Huang ZS and Li M: Clinical significance of
sphingo-sine kinase-1 expression in human astrocytomas progression
and overall patient survival. Clin Cancer Res. 14:6996–7003. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong C, Qu S, Liu B, Pan S, Jiao Y, Nie Y,
Su F, Liu Q and Song E: MiR-106b expression determines the
proliferation paradox of TGF-β in breast cancer cells. Oncogene.
34:84–93. 2015. View Article : Google Scholar
|
|
26
|
Chen J, Yao Y, Gong C, Yu F, Su S, Chen J,
Liu B, Deng H, Wang F, Lin L, et al: CCL18 from tumor-associated
macrophages promotes breast cancer metastasis via PITPNM3. Cancer
Cell. 19:541–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akaishi J, Sugino K, Kameyama K, Masaki C,
Matsuzu K, Suzuki A, Uruno T, Ohkuwa K, Shibuya H, Kitagawa W, et
al: Clinicopathologic features and outcomes in patients with
diffuse sclerosing variant of papillary thyroid carcinoma. World J
Surg. 39:1728–1735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lochhead PA, Wickman G, Mezna M and Olson
MF: Activating ROCK1 somatic mutations in human cancer. Oncogene.
29:2591–2598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pinner S and Sahai E: PDK1 regulates
cancer cell motility by antagonising inhibition of ROCK1 by RhoE.
Nat Cell Biol. 10:127–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Song Y, Wang Y, Luo J and Yu W:
MicroRNA-148a suppresses epithelial-to-mesenchymal transition by
targeting ROCK1 in non-small cell lung cancer cells. Mol Cell
Biochem. 380:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mertsch S, Oellers P, Wendling M, Stracke
W and Thanos S: Dissecting the inter-substrate navigation of
migrating glioblastoma cells with the stripe assay reveals a
causative role of ROCK. Mol Neurobiol. 48:169–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang W, Zhou X and Wei M: MicroRNA-144
suppresses osteosarcoma growth and metastasis by targeting ROCK1
and ROCK2. Oncotarget. 6:10297–10308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai H, Lin L, Cai H, Tang M and Wang Z:
Combined microRNA-340 and ROCK1 mRNA profiling predicts tumor
progression and prognosis in pediatric osteosarcoma. Int J Mol Sci.
15:560–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu B, Huang Y, Niu X, Tao T, Jiang L, Tong
N, Chen S, Liu N, Zhu W and Chen M: Hsa-miR-146a-5p modulates
androgen-independent prostate cancer cells apoptosis by targeting
ROCK1. Prostate. 75:1896–1903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kroiss A, Vincent S, Decaussin-Petrucci M,
Meugnier E, Viallet J, Ruffion A, Chalmel F, Samarut J and Allioli
N: Androgen-regulated microRNA-135a decreases prostate cancer cell
migration and invasion through downregulating ROCK1 and ROCK2.
Oncogene. 34:2846–2855. 2015. View Article : Google Scholar
|
|
37
|
Raviraj V, Fok S, Zhao J, Chien HY, Lyons
JG, Thompson EW and Soon L: Regulation of ROCK1 via Notch1 during
breast cancer cell migration into dense matrices. BMC Cell Biol.
13:122012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gilkes DM, Xiang L, Lee SJ, Chaturvedi P,
Hubbi ME, Wirtz D and Semenza GL: Hypoxia-inducible factors mediate
coordinated RhoA-ROCK1 expression and signaling in breast cancer
cells. Proc Natl Acad Sci USA. 111:E384–E393. 2014. View Article : Google Scholar :
|
|
39
|
Dai W, Teodoridis JM, Zeller C, Graham J,
Hersey J, Flanagan JM, Stronach E, Millan DW, Siddiqui N, Paul J,
et al: Systematic CpG islands methylation profiling of genes in the
wnt pathway in epithelial ovarian cancer identifies biomarkers of
progression-free survival. Clin Cancer Res. 17:4052–4062. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu H, Li W, Chen C, Pei Y and Long X:
MiR-335 acts as a potential tumor suppressor miRNA via
downregulating ROCK1 expression in hepatocellular carcinoma. Tumour
Biol. 36:6313–6319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Majid S, Dar AA, Saini S, Shahryari V,
Arora S, Zaman MS, Chang I, Yamamura S, Chiyomaru T, Fukuhara S, et
al: MicroRNA-1280 inhibits invasion and metastasis by targeting
ROCK1 in bladder cancer. PLoS One. 7:e467432012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kummer NT, Nowicki TS, Azzi JP, Reyes I,
Iacob C, Xie S, Swati I, Darzynkiewicz Z, Gotlinger KH, Suslina N,
et al: Arachidonate 5 lipoxygenase expression in papillary thyroid
carcinoma promotes invasion via MMP-9 induction. J Cell Biochem.
113:1998–2008. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brown-Clay JD, Shenoy DN, Timofeeva O,
Kallakury BV, Nandi AK and Banerjee PP: PBK/TOPK enhances
aggressive phenotype in prostate cancer via
β-catenin-TCF/LEF-mediated matrix metalloproteinases production and
invasion. Oncotarget. 6:15594–15609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wille C, Köhler C, Armacki M, Jamali A,
Gössele U, Pfizenmaier K, Seufferlein T and Eiseler T: Protein
kinase D2 induces invasion of pancreatic cancer cells by regulating
matrix metalloproteinases. Mol Biol Cell. 25:324–336. 2014.
View Article : Google Scholar :
|
|
46
|
Ai F, Zhang X, Li X, Qin Z, Ye Q, Tian L,
Tang A, Li N, Li G, Ma J, et al: Up-regulation of matrix
metalloproteinases in a mouse model of chemically induced
colitis-associated cancer: The role of microRNAs. Oncotarget.
6:5412–5425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Decock J, Thirkettle S, Wagstaff L and
Edwards DR: Matrix metalloproteinases: Protective roles in cancer.
J Cell Mol Med. 15:1254–1265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|