Glioblastoma (GBM) is a common primary malignant
brain tumor in the cranial cavity accounting for 45.2% of malignant
primary brain and central nervous system tumors (1). Tumors formed by brain glial cells,
including astrocytes, oligodendrocytes and ependymal cells, can be
referred to as GBM (2). In 2018,
the International and European Society for Pediatric Oncology found
that GBM grows fast, and 70-80% of patients have a course of 3-6
months, and only 10% of patients have a course of >1 year based
on clinical patient data from 10 countries (3,4).
Those with a longer course may evolve from low-malignant
astrocytomas (5). Due to the
rapid growth of GBM, cerebral edema and intracranial pressure are
markedly increased, and all patients have symptoms, including
headache and vomiting (6).
Although excision, radiation and chemotherapy are standard
treatments, the prognosis remains poor for patients with GBM
(7,8).
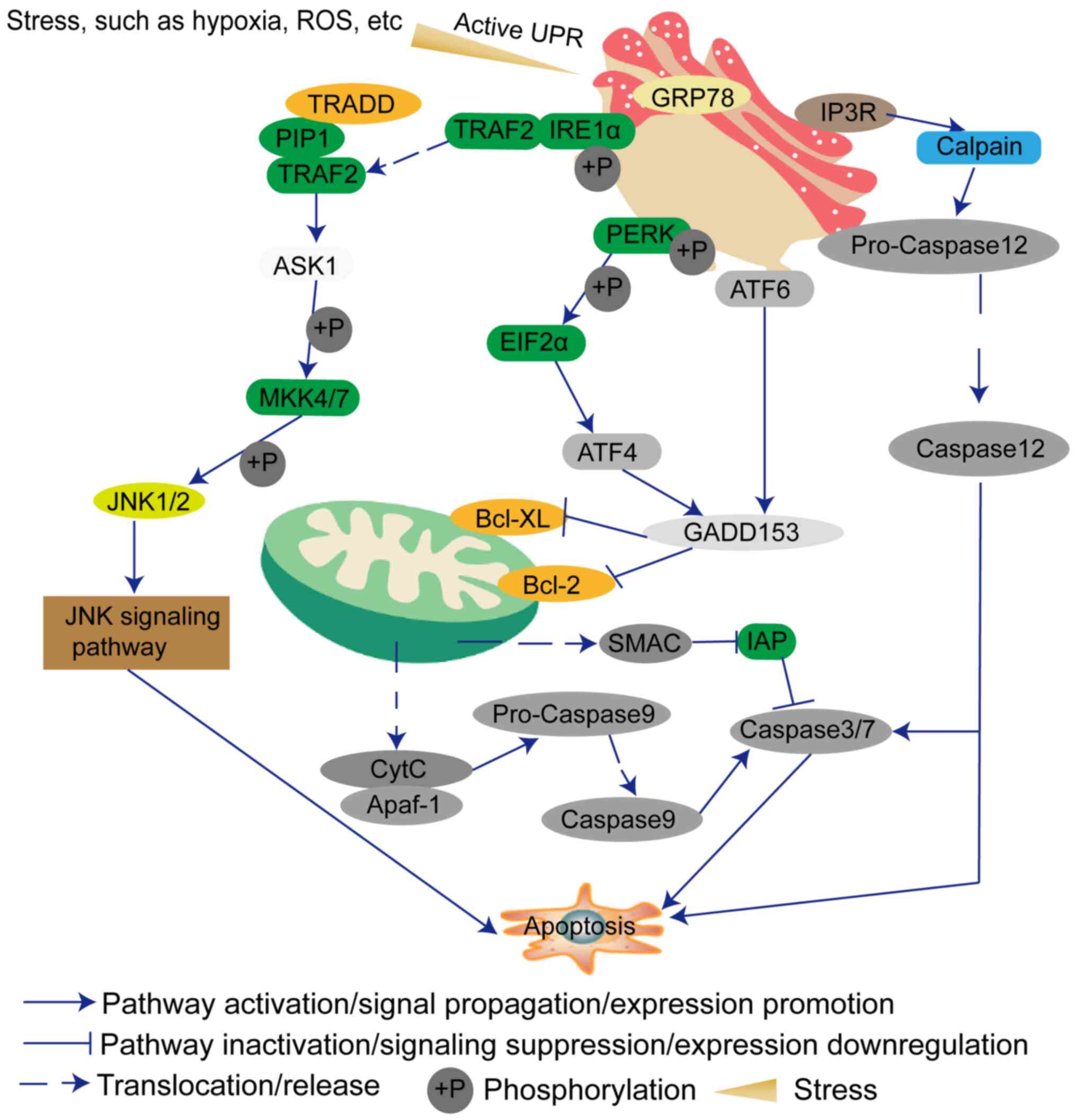
The main function of the endoplasmic reticulum (ER)
is to synthesize proteins and lipids. In addition to meeting its
own needs, the lipids are also provided to the Golgi apparatus,
lysosomes, plasma membranes, mitochondria and other membranous cell
structures (9). The accumulation
of unfolded or misfolded proteins in the ER will cause ER stress,
and in turn triggers the unfolded protein response (UPR) to ensure
that the protein is folded correctly (10). ER stress can induce the expression
of glucose regulatory proteins [78-kDa glucose-regulated protein
(GRP78) and GRP94] and other ER molecular chaperones to protect
cell proliferation (10).
However, ER stress can also independently induce endogenous cell
apoptosis and ultimately affect cell fate, such as adaptation,
injury or apoptosis (11).
Generally, if the ER stress is continuous, the protein kinase
R-like endoplasmic reticulum kinase (PERK), inositol-requiring
enzyme 1 (IRE1) and activating transcription factor (ATF)6
signaling pathways will be used to transduce the downstream
apoptotic pathways (12,13). Therefore, researchers are
gradually turning their attention to how to induce GBM cell
apoptosis by ER stress. This review discusses the factors that
influence the occurrence of ER stress, and the possibility of ER
stress as a treatment for GBM.
The perception and response to exogenous stress is
an important component of cell physiology. Certain studies have
demonstrated that the ER can initiate the cell response to
exogenous stress (14,15). ER synthetic proteins should be
properly folded, glycosylated and disulfide-bonded to form
functional proteins (16).
Therefore, a quality control mechanism is important for detecting
misfolded or unfolded proteins and performing cell functions, such
as cell division and cell-to-cell interactions (16,17). Due to the proliferation of cancer
cells, the probability of misfolded or unfolded proteins is higher
than that of normal cells (18).
Therefore, the UPR can prevent the continuous synthesis of
misfolded or unfolded proteins to a certain extent, and has a
protective effect on GBM cells (19). The UPR has three classic
transmembrane ER-resident UPR sensors: IRE1α (20), PERK (21) and ATF6 (22). These sensors can detect misfolded
and unfolded proteins, and accelerate the recovery and maintenance
of ER homeostasis (17).
Furthermore, the signaling network within the cells
is complex. When a signal changes, it must trigger a series of
signal changes, suppression or assistance (23). Additionally, sustained ER stress
can induce other signaling pathways, such as DNA damage signaling
and death receptor-mediated signaling, to promote cell apoptosis
(24). The PERK-elF2α-ATF4-DNA
damage inducible transcript 3 (GADD153) signaling pathway is one of
the classic signaling pathways of ER stress (25). Therefore, the expression levels of
members of this pathway will also cause changes in other genes,
which will synergistically promote cell apoptosis (25). p53 is a key player in the DNA
damage response (DDR), and its expression is specifically induced
by the PERK kinase during the UPR following ER stress (25). p53 activation during the DDR has
been well studied (26,27). Once activated, p53 will stimulate
and suppress different gene products, which aim to either prevent
abnormal proliferation by a reversible arrest of the cell cycle to
facilitate the repair processes, or to induce irreversible
outcomes, including apoptosis or senescence (25). In addition, PERK expression
decreases RNA component of mitochondrial RNA processing
endoribonuclease expression, and increases microRNA-206 to inhibit
Bcl-2, and consequently induces cleaved caspase3 (28). Cannabidiol (CBD) has the ability
to inhibit the proliferation of GBM cells, and CBD can promote the
expression of GADD153 to trigger ER stress, and induces
mitochondrial dysfunction and lethal mitophagy arrest via the
GADD153-tribbles pseudokinase 3-AKT-mTOR axis (29). Salinomycin and its ester
derivatives 5-7 increase the levels of phosphorylated
(p-)eukaryotic initiation factor (eIF)2α (Ser51) and IRE1α
proteins, and also increase the levels of DNA damage indicators,
such as γ-H2A histone family member X (γH2AX) protein and modified
guanine (8-oxoG), by upregulating the expression levels of GADD153
(30). eIF5B has been
demonstrated to serve a critical role in canonical translation, and
eIF5B depletion results in the upregulation of DNA damage-inducible
protein 34 (GADD34) transcription, and leads to activation of JNK
to promote cell death (31).
X-box binding protein 1 (XBP1) expression increases DNA damage,
protein ATM phosphorylation, and the expression levels of
MRE11-RAD50-NBS1 complex and γH2AX (32). In addition, IRE1 can interact with
adaptor protein TNF receptor-associated factor 2 (TRAF2) and then
initiates JNK, which has been demonstrated to be involved in cell
death (33). IRE1 may contribute
to apoptosis by activating the RIDD signaling pathway (34).
Although a few studies have demonstrated that the
existence of IRE1α is beneficial to the neovascularization of GBM,
IRE1α also induces cell death and serves a unique role in ER stress
(48-50). GRP78 can bind the luminal domain
of IRE1α (49). Under ER stress,
IRE1α dissociates from GRP78 and promotes the separation of TRAF2,
and also induces cleavage of XBP1 into a splicing variant of XBP1
and transcription of GADD153 (51,52). TRAF2 forms a complex with TNF
receptor superfamily member 1A-associated death domain protein,
transforming growth factor β-activated kinase 1 and
receptor-interacting protein 1 (53). This complex can activate apoptosis
signal-regulating kinase 1 (ASK1), and then activate
mitogen-activated protein kinase 4/7 (54-56) and the JNK apoptosis signaling
pathway (57).
ATF6 is a type II transmembrane protein. ATF6 has a
cytosolic bZIP transcription factor domain (58). During ER stress, ATF6 translocates
to the Golgi where it is cleaved by proteases (59). The cleaved ATF6 cytosolic fragment
can then act as a transcription factor (59). Prior to that, ATF6 needs to be
modified, such as by reduction and glycosylation (60). ATF6 also induces GADD153
expression (61,62).
Normal tissue cells need to undergo a series of
changes before they can become cancer cells, such as genetic
changes, or activation or inactivation of certain signaling
pathways (8). For example,
stearoyl CoA desaturase (SCD1) and proto-oncogene SEC61 translocon
subunit γ can promote ER homeostasis, avoid the production of
misfolded proteins and inhibit the occurrence of tumors (63). Paraoxonase 2 (PON2), a paraoxonase
protein, consists of lactone hydrolases with different substrate
specificities (64). When PON2 is
located on the nuclear membrane and ER, it can increase the
stability of the ER and protect cancer cells from adverse
environmental conditions and chemotherapy (64). Hypoxia-stimulated galectin-1
(Gal1) is an effective regulator of GBM cell migration and an
angiogenic molecule (65).
Additionally, the reduction of Gal1 weakens the expression levels
of seven genes related to chemical resistance: ORP150, BNIP3L,
HERP, TRA1, GADD45B, GRP78 and CYR61 (65). The absence of cytochrome P450 17A1
can induce the occurrence of ER stress and reactive oxygen species
(ROS) generation by regulating secretion-associated Ras-related
GTPase 1 (66). Furthermore,
heat-shock protein 27 and 90 can decrease the levels of cytochrome
C, caspase3, caspase9 and caspase12 in GBM cells (67,68). Additionally, cyclophilin B is a
prolyl isomerase residing in the ER, and its absence can damage the
ER structure (69).
In addition, knockdown of cAMP-responsive
element-binding protein 3 induces cell apoptosis by increasing
p-PERK, p-eIF2α, ATF4, Bax and caspase3 (70). Reversion inducing cysteine rich
protein with kazal motifs (RECK) is a key suppressor gene in
regulating cancer cell invasion and metastasis (71). Highly expressive RECK can modulate
ER stress by binding to or sequestering GRP78 to activate p-eIF2α
(71). Tumor necrosis factor
receptor-associated protein 1 can markedly induce the occurrence of
ER stress by activating ATF4 (72). In addition, neural precursor cells
can migrate to advanced astrocytoma via the release of the vanillin
receptor [transient receptor potential vanillin subfamily member 1
(TRPV1)] (73). TRPV1 induces GBM
cell death via ER stress (73).
Therefore, drugs that target these proteins located on the ER
membrane or stabilizing the structure of the ER can be
developed.
The redox environment of the ER determines the fate
of proteins entering the ER, and the level of redox signaling
mediators also regulates the level of ROS (74). ROS can induce the occurrence of ER
stress through redox signaling mediators, such as NADPH-P450
reductase, protein disulfide isomerase-endoplasmic reticulum
oxidoreductase 1, NADPH oxidase 4, glutathione/glutathione
disulfide and calcium (74,75).
In addition, ROS-mediated hypoxia-inducible factor
1α serves an important role in promoting tumor microenvironment,
anti-apoptosis and drug resistance in GBM (76). Mitochondrial PTEN-induced kinase 1
(PINK1), a regulator of the Warburg effect, is a negative regulator
of proliferation in GBM cells (77). PINK1 can inhibit ROS generation
and cell proliferation through FOXO3a (77). This finding highlights the
importance of the balance between PINK1 and ROS in normal cells and
cancer cells (77). Additionally,
luteolin, a common dietary flavonoid, can induce a lethal ER stress
pathway and mitochondrial dysfunction by increasing the
intracellular ROS levels (78).
Dihydroartemisinin (DHA) induces cell apoptosis through
mitochondrial membrane depolarization, cytochrome'C and caspase 9
(79). Furthermore, the
cytotoxicity of DHA can increase the expression levels of GRP78,
GADD153, eIF2α and caspase12 (79).
One of the main obstacles for tumor progression is
that cancer cells grow in a low-oxygen environment (80). Hypoxia can stimulate the adaptive
response to promote cell proliferation and survival, as well as
angiogenesis (81). However, some
researchers have illustrated that hypoxia can also cause cell
apoptosis or necrosis (81,82). Consequently, under hypoxic
conditions, understanding the decision-making process that
regulates cell death, adaptation and resistance for treatment is
crucial. Hypoxia has been demonstrated to induce ER stress
(83). Additionally, hypoxia can
induce the cell surface exposure of calreticulin, a hallmark of
immunogenic cell death (84,85).
Studies have demonstrated that after knock out of
the gene encoding endothelin-1, the expression levels of endothelin
receptor type B, endothelin 1, endothelin converting enzyme 1 and
endothelin receptor type A are upregulated, which will increase the
sensitivity of GBM cells to hypoxia-induced ER stress (86,87). Furthermore, under hypoxic
conditions, the expression levels of snail family transcriptional
repressor 2 and mesoderm specific transcript are upregulated, which
will amplify the inhibitory effect of IRE1 on various genes
(88). Hypoxia also leads to
upregulation of IGFBP6, IGFBP7, IGFBP10/CYR61, WISP1 and WISP2, and
downregulation of IGFBP9/NOV at the mRNA level (89). IRE1 markedly downregulates IGFBP7,
IGFBP10/CYR61, WISP1 and WISP2, and upregulates IGFBP9/NOV, which
shows that ER stress is an essential part of malignant GBM cell
proliferation (90).
The glucose metabolism allows energy to be oxidized
by its carbon bonds and then used in the form of ATP. The final
product of glucose can be lactate or carbon dioxide (91). In the 1920s, Otto Warburg and his
colleagues found that tumors were absorbing a lot of glucose
compared with surrounding tissues (92). Furthermore, in the presence of
oxygen, glucose can also be fermented to produce lactic acid
through aerobic glycolysis (92).
Subsequently, in 1929, the British biochemist Herbert Crabtree
confirmed Warburg's findings and further revealed that the
respiratory intensity of tumors was variable, and numerous tumors
exhibited this phenomenon (93).
Additionally, Racker developed his theory of the origin of the
Warburg effect in terms of intracellular pH imbalance and ATPase
activity defects (94). Research
on genetics and pharmacology demonstrated that the Warburg effect
is necessary for cancer cell proliferation (95). Studies have demonstrated that the
direct and indirect result of cancer-causing mutations is the
reprogramming of cancer cell metabolism (96,97). Obtaining the necessary nutrients
from the nutrient-deficient environment is a common feature of
tumor metabolism (98). Cancer
cells can use these nutrients to maintain viability and build new
biomass (98). Therefore, tumors
are considered to be a metabolic disease (99).
Low glucose means that cancer cells collect less
energy. GRP78 can be upregulated in a low-glycemic state, and
enables GBM cells to survive by inducing autophagy (100). Additionally, in the low-glycemic
stage, p-PERK and cleavage of ATF6 are upregulated, which indicates
that low glucose can lead to ER stress, activation of caspase, cell
dysfunction, cell arrest and cell death (101-103). Metformin is the first-line drug
for type 2 diabetes, and it can induce cancer cell death via the
ASK1/phorbol-12-myristate-13-acetate-induced protein 1 and
ROS/ASK1/JNK signaling pathways (104). The aforementioned can also
indicate that the ketogenic diet can partially inhibit cancer cell
proliferation.
In tumor tissues, due to increased glycolytic
activity, slow blood circulation and insufficient blood supply of
cancer cells, the pH of certain tumors (astrocytoma and squamous
cell carcinoma) is <6.0, while the normal physiological pH is
7.3 (105). The low outflow of
potentially toxic metabolic waste and a low influx of metabolites
further enhance acidic conditions where cancer cells are located
(106). The metastasis of solid
tumors to acidic sites can promote tumor growth, invasion,
angiogenesis, immunosuppression and chemotherapy resistance
(107). However, there are
conflicting reports on the effects of the acidic environment on
cancer cell physiology. For example, low pH can promote tumor
development; however, certain studies have demonstrated that low pH
can also induce cancer cell apoptosis (108,109). For example, acidic stress may
lead to upregulation of Src activity, VEGF and MMPs, which is
conducive to cancer cell survival and metastasis (110,111). Acid-mediated apoptosis is
considered to be the result of caspase activation (109). The acidic environment can
regulate GBM cell proliferation and radiosensitivity (112). Additionally, acidity induces the
expression of eIF2α, IRE1α, ATF6, GADD153 and caspase12 (113,114). This indicates that, in the brain
tissue, the acidic environment may kill cancer cells via the ER
stress pathway.
ER stress can give rise to changes in lipid
metabolism; however, certain evidence suggests that dysfunctional
lipid metabolism may activate UPR, regardless of whether there is a
misfolded protein in the ER lumen (124). Subsequently, after UPR, genes
involved in lipid metabolism will be upregulated (125). The brain is generally considered
to be one of the most fat-rich organs, and the lipid content itself
can affect various clinically relevant behavioral indicators
(126). These bioactive lipids
include steroids, diacyl glycerol, sphingolipids,
phosphatidylinositol phosphate, phosphatidylcholine and
polyunsaturated fatty acids (126). Furthermore, saturated fatty
acids have the effect of promoting ER stress, while unsaturated
fatty acids counteract this effect (126). Saturated fatty acids, such as
palmitic acid and stearic acid, are known inducers of ER stress in
various cell types, such as liver and breast cancer cells, and can
regulate cell survival and apoptosis signals (126). SCD1, a downstream gene of sterol
regulatory element-binding protein (SREBP), mediates lipid
desaturation, which has also been found to be a critical
determinant of cancer cell survival (127). SREBPs have important roles in
regulating lipid metabolism and mediate lipid synthesis in GBM
cells (127). Loss of SREBP and
lipid synthesis can block GBM cell proliferation in xenograft
models (128). In addition,
SREBP ablation is also accompanied by the activation of IRE1α and
PERK (129). These findings
indicate that proliferating cells need to establish a balance
between their proliferation rate and unsaturated lipid supply to
prevent ER stress. Normal cells can regulate their proliferation
rate in response to nutrient availability and retain a pool of
unsaturated lipid, which allows cells to maintain homeostasis and
avoid ER stress (129). However,
with the rapid proliferation of cancer cells, if the exogenous
unsaturated lipids are limited, the cancer cells will experience ER
stress, eventually leading to cell death (129).
Due to the rapid proliferation of cancer cells,
cancer cells need to synthesize a large amount of protein to
support their own needs, resulting in misfolded proteins occurring
in cancer cells. According to the ClinicalTrials.gov database (https://www.clinicaltrials.gov/), some studies have
been carried out on the effect of ER stress on tumor treatment.
Among them, a project investigating TN-TC11G
(9-tetrahydrocannabinol + CBD) in combination with temozolomide
(TMZ) and radiotherapy in patients with newly-diagnosed GBM is
recruiting patients.
TMZ is the first-line drug for clinical glioma
chemotherapy. It is an oral alkylated chemotherapy drug and
effectively crosses the blood-brain barrier. However, over time,
some GBM can gradually resist TMZ-induced damage. This resistance
may be associated with the DNA repair pathway (O6-methylguanine DNA
methyltransferase, DNA mismatch repair, base excision repair
system), EGFR, MDM2 proto-oncogene, p53 mutation and PTEN (130). Therefore, researchers pay
increasing attention to natural compounds, small molecules,
viruses, bacteria, and calcium activators or inhibitors, and
conduct basic research, aiming to one day treat glioma in clinical
settings.
GRP78 predominantly resides in the ER lumen within
normal cells, and most of the research on GRP78 has focused on
cytosolic or total GRP78 (131,132). However, in tumor
microenvironments where GRP78 expression is upregulated, GRP78 also
localizes to the surface of GBM cell membranes (133). GRP78 may influence not only GBM
cells, but also the surrounding microenvironmental vasculature
(133). Therefore, it has been
gradually revealed that some compounds, such as epigallocatechin
3-gallate (EGCG), honokiol, celecoxib and bortemozib, can inhibit
the growth of glioma by inhibiting GRP78 (133). IRE1 is the main mediator of the
UPR (134). When cancer cells
trigger ER stress in an unfavorable environment, the IRE1 signal
can be an adaptive mechanism (135). However, the Food and Drug
Administration-approved compounds methotrexate, cefoperazone,
folinic acid and fludarabine phosphate, as inhibitors of IRE1,
hinder the adaptation mechanism (135). In addition, flavokawain B, a
natural kava chalcone, exhibits potent anti-tumor activity in
various cancer types, such as lung cancer cells (136) and gastric cancer cells (134). It induces protective autophagy
by targeting the ATF4-GADD153-AKT-mTOR signaling pathway (137). Piperlongumine preferentially
kills high-grade glioma (HGG) cells but has little effect on normal
brain cells (138). It induces
ROS generation and disrupts protein folding in the ER by increasing
the oxidative deactivation of peroxide reduction 4 to activate the
ER stress pathway in HGG cells (138). Therefore, piperlongumine can be
regarded as an effective drug for the treatment of GBM.
There are still numerous compounds that can induce
cell apoptosis via the ER stress pathway in GBM cells. For example,
Isochaihulactone, a natural compound extracted from the Chinese
traditional herb Nan-Chai-Hu, can disrupt ER homeostasis in GBM
cells (139). The novel
resorcinol derivatives [2,4-bis (4-fluorophenylacetyl) resorcinol
(BFP)] can increase some characteristic ER stress markers, such as
GRP78, IRE1, eIF2α and GADD153, in human GBM cell lines (U251 and
U87), and a mouse GBM cell line (C6 cells) (140). In addition, treatment with BFP
can increase ROS generation and downstream caspase activation, such
as caspase12, caspase9 and caspase7 (140). Cannabinoids can inhibit the
epithelial-mesenchymal transition of several tumors in rats and
mice, and enhance tumor immune surveillance (141). Therefore, cannabinoids may be
considered as potential anticancer drugs. In 2003, cannabinoids
were used to explore the anticancer mechanism (142). The results demonstrated that the
main mediator of cannabinoid is the stress-regulating protein p8
(also designated as a candidate for metastasis 1) (142). Further research revealed that p8
has an apoptotic effect by upregulating ER stress-related genes
ATF4 and GADD153 (142).
Additionally, Shikonin (one of the main active ingredients of
Chinese herbal medicine Lithospermum erythro-rhizon)
(143), fatsioside A (a novel
baccharane-type triterpene glycoside) (144), garlic compounds (diallyl sulfide
and diallyl disulfide) (145),
apigenin, (-)-epigallocatechin, and genistein (146,147), desipramine (a tricyclic
antidepressant) (148), curcumin
(149,150), xanthatin (a natural
sesquiterpene lactone purified from Xanthium strumarium L.)
(151), honokiol (a cell-wall
component of M. grandiflora) (152), sinomenine hydrochloride (the
main biologically active alkaloid isolated from Leymus
chinensis) (152), radicol
(a novel trinorguaiane type sesquiterpene) (153), phenyl isothiocyanate (a member
of the isothiocyanate family) (154,155), redox organoruthenium compound 11
(RDC11; one of the most active compounds among the novel
ruthenium-derived compounds) (156) and obtusaquinone [OBT; a natural
compound from the heartwood of Dalbergia retusa (cocobolo)]
(157) have also been reported
to induce ER stress (Table I).
Among them, after injecting GBM cells into the mouse cranial
cavity, RDC11 and OBT can reduce tumor progression, and improve the
survival rate of the mouse (156,157). Therefore, whether other natural
compounds can pass through the blood-brain barrier at the
individual level requires in-depth research. In addition, there are
also numerous reports on other tumors, which suggest that natural
compounds can induce cell apoptosis through ER stress. For example,
aspirin can induce multiple myeloma (MM) cell apoptosis by
inhibiting Blimp1, activating the ATF4/CHOP apoptotic pathway
(158). Valosin containing
protein (p97/VCP) is an ER-associated protein, and novel p97/VCP
inhibitor induces ER stress and apoptosis in both
bortezomib-sensitive and -resistant MM cells (159). Furthermore, there are numerous
other compounds that can also affect cancer cell proliferation
through ER stress in other tumors, such as 18βH (a semisynthetic
derivative of -glycyrrhetinic acid) in breast cancer cells (MCF-7
and MDA-MBA-231) (160),
resveratrol (a natural polyphenol compound) (161) and 2-pyrazine-PPD (a novel
dammarane derivative) (162) in
gastric cancer, sothiocyanates (natural compounds abundant in
cruciferous vegetables) in non-small cell lung cancer cells
(163), and honokiol (a
hydroxylated biphenyl natural product) in prostate cancer,
melanoma, lung cancer, leukemia and colorectal cancer (164). Therefore, whether these natural
compounds can induce ER stress and be used for the treatment of GBM
in vivo still requires basic verification and clinical
trials.
GBM stem cells (GSCs) can be considered key drivers
of tumor growth, aggressiveness and therapy resistance in GBM
(8). PERK is a well-characterized
switch between survival and death during persistent ER stress and
mediates cell death through induction of GADD153 (165). A previous study has demonstrated
that ER stress aggravation targets GSCs, and PERK directly
regulates SOX2 downregulation at the protein level to induce GSC
differentiation, independent from eIF2α/ATF4 signaling (165). Furthermore, ionizing radiation
potentiates ER stress, which reduces proliferation in a
PERK-dependent manner (166).
Adding PERK inhibitor, ER stress inducer (2DG) and GADD34
phosphatase inhibitor (Sal003) to irradiated GBM cells can reduce
cell viability (166). In
addition, other highly selective PERK inhibitors may provide a
ground-breaking, anticancer treatment strategy in a PERK-dependent
manner. 7-Methyl-5-(1-2,3-dihydro-1H-indo
l-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414) is an oral,
effective and selective PERK inhibitor, which inhibited the growth
of a human tumor xenograft in mice by inducing ER stress (167). Therefore, GSK2606414 treatment
may be considered as an effective drug therapy for GBM.
Small-molecule inhibitor 42215 of PERK can markedly induce
apoptosis after treatment of cancer cells (168). ATF4 is the master regulator of
the cellular stress response and the core regulator of the
PERK-eIF2α signaling pathway (169). Kurarinone (Extract of
S. flavescens Roots) activates ATF4 to induce cancer
cell apoptosis (169). A mixture
of sixteen previously selected small molecules ('active mixture';
AM16: l-arginine, l-tyrosine, l-histidine, l-tryptophan,
l-methionine, l-phenylalanine, adenine, l-(-)-malic acid,
2-deoxy-d-ribose, orotic acid, d-(+)-mannose, hippuric acid,
pyridoxine, d-biotin, (-)-riboflavin and l-ascorbic acid) can
upregulate ATF4 and GADD153 to induce cell apoptosis (170). Furthermore, salicylaldehyde
analogs (MK0186893) and umbelliferones (4 µ8c) can be used
as IRE1 RNase inhibitors, and have shown promise as a potential
therapeutic strategy to counteract disease pathogenesis associated
with overactive IRE1 signaling (171,172). Palmitoylation inhibitors
(2-bromopalmitate, cerulenin and tunicamycin) induce cell death by
promoting the accumulation of XBP1 (173). In addition, >10,000 non-toxic
compounds that may activate IRE1-dependent XBP1 splicing through a
mechanism independent of binding the IRE1 kinase active site have
been identified by a high-throughput screening approach in July
2020 (174). This undoubtedly
provides more drugs for the treatment of tumors via ER stress and
requires in-depth exploration and screening.
In addition to specific small molecule modulators
of UPR pathways and their potential use in developing anticancer
therapies, numerous small molecule compounds have been demonstrated
to induce GBM cell apoptosis (Table
I). For example, NEO214 (rolipram-perillyl alcohol conjugate)
is produced by the covalent linkage of carbamate linkage and
cyclopropanol (175). It can
cross the blood-brain barrier, and induce cell death via ER stress
and the death receptor 5/TRAIL/TNF superfamily member 10 signaling
pathway (175). Therefore,
NEO214 may be considered as a potential clinical antitumor drug.
Asiatic acid (AsA) is a natural small molecule, and it is widely
used to cure various neurological disorders. AsA also induces ER
stress by increasing GRP78, IRE1α and calpain, to damage a cellular
organization in human GBM cells (LN18, U87MG and U118MG) (176). Notably, AsA can cross the
blood-brain barrier (12).
Therefore, AsA may be considered as a potential clinical antitumor
drug. In addition, 2-amino-N-acetamide (176), NEO212 (a combination of TMZ and
perillyl alcohol) (177), NEO100
(a high-purity and high-quality polychlorohydrin) (178), Compound-7g (179), platinum thiopyridine (II)
complex (180), endothelial
monocyte activating polypeptide II (181), berberine (an isoquinoline
quaternary alkaloid isolated from a variety of medicinal plants)
(182),
N-methyl-4-isoleucine-cyclosporine (a small molecule cyclophilin
binding inhibitor) (183),
celecoxib (a non-steroidal anti-inflammatory drug) (184), the silent mating type
information regulation 2 homolog activator
(R)-N-(2-(3-((3-Hydroxypyrrolidin-1-yl)methyl)imidazo[2,1-b]
thiazol-6-yl)phenyl)-2-naphthamide (185), neuro-steroid, 5-androstene
3β,17α diol (186), minocycline
(187) and ursolic acid
(188-190) can also induce GBM cell apoptosis
via the ER stress pathway. Whether these small molecules can be
used in clinical trials needs to be examined in animal models to
ensure that they can cross the blood-brain barrier and reduce
damage to normal cells.
In addition to compounds, some viruses, bacteria,
and calcium activators or inhibitors have been reported to induce
GBM cell apoptosis via ER stress (Table I). For instance, ovitriol A (a
fungal sesterterpene from Bipolaris oryzae) can induce
paralysis-like cell death in human glioma cell lines (T98G, U251MG,
U343, U373MG and A172), accompanied by the expansion of the ER
(191). Unconjugated bilirubin
may cause bilirubin neurotoxicity (192). It can also cause cell death t by
increasing GADD153 in U87MG cells (192). Rhabdovirus is an important
regulator of rhabdovirus-mediated cytotoxicity and mediates the ER
stress response pathway to induce cell apoptosis (193). Chikungunya virus (CHIKV), an
old-world alphavirus, can induce DNA fragmentation, loss of
mitochondrial membrane potential, poly(ADP-ribose) polymerase
(PARP) cleavage, nuclear enrichment and visible cytopathic effects
in a dose- and time-dependent manner (194).
The focus of tumor research has always been the
optimization of treatment strategies for malignant tumors. Silica
nanoparticles (SiNPs) are such a strategy, which is rapidly
developing into a promising tool for cancer diagnosis, imaging and
treatment (195). SiNPs lead to
impaired mitochondrial function, ROS generation and cell death by
elevating levels of ER stress genes, including GRP94, GRP78,
GADD153 and cyclooxygenase-2 (COX2) (195). Yessotoxin (YTX) is a polycyclic
ether compound produced by dinoflagellate and accumulates in
filter-fed shellfish (196). YTX
can upregulate p-PERK, p-eIF2α, s-XBP1 and GADD153 in human glioma
cell lines (SF539, SF295 and SNB75) (196). Additionally, YTX induces cell
cycle arrest and increases cholesterol and polar lipid content in
glioma cells (196). In
addition, amiodarone is a widely used antiarrhythmic drug (197). Amiodarone can inhibit a variety
of ion channels, including Na+/Ca2+
exchangers, L-type Ca2+ channels and Na+
channels, and increases the intracellular Ca(2+) level and GADD153
expression (197). Although
viruses, bacteria, and calcium activators or inhibitors can induce
cancer cell apoptosis through ER stress, their safety still needs
to be considered.
Various studies on GRP78 have also demonstrated
that GRP78 has an important role in recurrent GBM and tumor
progression after initial treatment (198,199). Of particular importance is TMZ,
the standard-of-care chemotherapeutic treatment for GBM. TMZ has
been demonstrated to result in activation of the UPR in GBM cells,
inducing increased levels of UPR markers, GRP78 and GADD153
(100). Therefore, certain drugs
can be used in combination with TMZ to enhance the sensitivity of
GBM to TMZ by increasing ER stress (Table II). For example, bufothionine is
extracted from the skins and parotid venom glands of the toad
Bufo bufo gargarizans Cantor (200). Bufothionine can synergize with
TMZ to exert an anti-growth effect by triggering ER stress
(200). PI3K/mTOR signaling is
ubiquitous in GBM (201). XL765
(Voxtalisib/SAR245409), an effective dual inhibitor of PI3Ks and
mTOR, inhibits the proliferation of GBM cells by inducing ER
stress-dependent apoptosis (201). The combination of XL765 and TMZ
can achieve improved therapeutic effects in A172, U87MG and T98G
cells (201). Fluoxetine (FLT),
as a drug widely used in cancer-related depression, has strong
anticancer effects in different types of cancer cells, such as
human ovarian granulosa tumor COV434 cells, and SKBR3 and MCF-7
breast cancer cells (202). The
combination of FLT and TMZ can also induce the activation of ATF6,
PERK, eIF2α, ATF4 and GADD153 (202). The upregulation of prolyl
4-hydroxylase-β polypeptide (P4HB) expression is associated with
the increase of the IC50 of TMZ, and is relatively
upregulated in resistant GBM cells (203). Targeting P4HB blocks its
protective function and makes GBM cells sensitive to TMZ (203). Chloroquine (CQ), a
quinoline-based antimalarial drug, can kill the plasmodium
falciparum parasite in the red blood cell stage by blocking the
acidic food vacuole heme to detoxify (198). Under physiological pH
conditions, CQ has unique chemical properties and is a weak base
that easily crosses the lipid bilayer of cells (198). The combination of TMZ and CQ can
trigger cell death by enhancing the formation of LC3B-II, the
accumulation of polyubiquitinated proteins, GADD153 and the
cleavage of PARP (198,204,205).
N,N-[(8-hydroxyquinoline)methyl]-substituted benzylamine (JLK1486)
is a novel type of ER stress inducer (206). The combined use of TMZ and
JLK1486 can cause long-term ER stress in human GBM cell lines
(U87MG, A172 and T98G) by increasing the levels of GRP78, ATF4 and
GADD153 (206). In addition,
celecoxib is a selective inhibitor of COX2. Increasing reports have
described that this drug has powerful anti-proliferation and
pro-apoptotic effects without the obvious involvement of COX2
(207,208). Celecoxib causes ER stress by
leaking calcium from the ER into the cytoplasm (207). The combination of bortezomib and
celecoxib can increase the expression levels of ER stress markers
GRP78 and GADD153, and cause the activation of c-jun (207). In addition, whether other
compounds enhance the sensitivity of GBM to TMZ by enhancing ER
stress needs to be verified.
The resistance of GBM to TMZ treatment is the
bottleneck of clinical treatment of this disease. Numerous cellular
processes, including inflammation, autophagy and apoptosis, are
regulated by the ER stress pathway. Furthermore, ER stress is a key
regulator of TMZ sensitivity and is more likely to function in a
cell-specific manner. Under low-dose and short-term TMZ treatment,
ER stress may have cytoprotective effects. However, persistent ER
stress can induce cell apoptosis. Therefore, numerous external
factors and internal changes can induce ER stress in GBM cells.
Although internal factors cannot be directly influenced, external
factors can be used to delay GBM cell proliferation. For example,
in a daily diet, patients with glioma can eat foods with less sugar
and saturated fatty acids to inhibit cancer cell proliferation.
In addition, this review also summarizes some
natural compounds and small molecule compounds, which are expected
to treat GBM via ER stress. These compounds are also expected to be
combined with TMZ. Additionally, there are numerous reports on
other tumors that certain specific small-molecule regulators and
natural compounds can specifically induce ER stress (165,209,210). Whether they can cross the
blood-brain barrier and induce GBM cell apoptosis still requires
verification. Additionally, the safety of the drug should also be
worthy of consideration. Overall, although limited research has
explored the pro-apoptosis function of ER stress for GBM cells, it
has demonstrated that induced ER stress appears to be a potential
treatment for GBM in the future.
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
PS and ZZ wrote this manuscript. JX prepared the
table and figure. LZ revised grammar and polished vocabulary after
the first review. HC drafted the manuscript. Data authentication is
not applicable. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The authors would like to thank Dr Zhen Dong (State
Key Laboratory of Silkworm Genome Biology, Southwest University,
Chongqing, China) for revising the manuscript and providing kind
suggestions.
This work was supported by the National Science Foundation of
China (grant nos. 81872071 and 81902664), the Natural Science
Foundation of Chongqing of China (grant no.
cstc2019jcyj-zdxmX0033), the Graduate Research and Innovation
Project of Chongqing of China (grant no. 2019CYB19117), the
Fundamental Research Funds for the Central Universities (grant no.
XYDS201912) and the State Key Laboratory of Silkworm Genome Biology
(grant no. SKLSGB-ORP202002).
|
1
|
Zhao Y, He J, Li Y, Lv S and Cui H: NUSAP1
potentiates chemoresistance in glioblastoma through its SAP domain
to stabilize ATR. Signal Transduct Target Ther. 5:442020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weller M, Wick W, Aldape K, Brada M,
Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R and
Reifenberger G: Glioma. Nat Rev Dis Primers. 1:150172015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reimunde P, Pensado-López A, Carreira
Crende M, Lombao Iglesias V, Sánchez L, Torrecilla-Parra M, Ramírez
CM, Anfray C and Torres Andón F: Cellular and molecular mechanisms
underlying glioblastoma and zebrafish models for the discovery of
new treatments. Cancers (Basel). 13:10872021. View Article : Google Scholar
|
|
4
|
Hoffman LM, Veldhuijzen van Zanten SEM,
Colditz N, Baugh J, Chaney B, Hoffmann M, Lane A, Fuller C, Miles
L, Hawkins C, et al: Clinical, radiologic, pathologic, and
molecular characteristics of long-term survivors of diffuse
intrinsic pontine glioma (DIPG): A collaborative report from the
International and european society for pediatric oncology DIPG
registries. J Clin Oncol. 36:1963–1972. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fareh M, Almairac F, Turchi L,
Burel-Vandenbos F, Paquis P, Fontaine D, Lacas-Gervais S, Junier
MP, Chneiweiss H and Virolle T: Cell-based therapy using
miR-302-367 expressing cells represses glioblastoma growth. Cell
Death Dis. 8:e27132017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brandes AA, Tosoni A, Franceschi E, Reni
M, Gatta G and Vecht C: Glioblastoma in adults. Crit Rev Oncol
Hematol. 67:139–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka S, Louis DN, Curry WT, Batchelor TT
and Dietrich J: Diagnostic and therapeutic avenues for
glioblastoma: No longer a dead end? Nat Rev Clin Oncol. 10:14–26.
2013. View Article : Google Scholar
|
|
8
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5:82020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aoyama-Ishiwatari S and Hirabayashi Y:
Endoplasmic reticulum-mitochondria contact sites-emerging
intracellular signaling hubs. Front Cell Dev Biol. 9:6538282021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzalez-Gronow M, Gopal U, Austin RC and
Pizzo SV: Glucose-regulated protein (GRP78) is an important cell
surface receptor for viral invasion, cancers, and neurological
disorders. IUBMB Life. 73:843–854. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yadav RK, Chae SW, Kim HR and Chae HJ:
Endoplasmic reticulum stress and cancer. J Cancer Prev. 19:75–88.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kavitha CV, Jain AK, Agarwal C, Pierce A,
Keating A, Huber KM, Serkova NJ, Wempe MF, Agarwal R and Deep G:
Asiatic acid induces endoplasmic reticulum stress and apoptotic
death in glioblastoma multiforme cells both in vitro and in vivo.
Mol Carcinog. 54:1417–1429. 2015. View Article : Google Scholar :
|
|
13
|
Zhang D, Wang F, Pang Y, Ke XX, Zhu S,
Zhao E, Zhang K, Chen L and Cui H: Down-regulation of CHERP
inhibits neuroblastoma cell proliferation and induces apoptosis
through ER stress induction. Oncotarget. 8:80956–80970. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McGrath EP, Centonze FG, Chevet E, Avril T
and Lafont E: Death sentence: The tale of a fallen endoplasmic
reticulum. Biochim Biophys Acta Mol Cell Res. 1868:1190012021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei J and Fang D: Endoplasmic reticulum
stress signaling and the pathogenesis of hepatocarcinoma. Int J Mol
Sci. 22:17992021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernales S, Papa FR and Walter P:
Intracellular signaling by the unfolded protein response. Annu Rev
Cell Dev Biology. 22:487–508. 2006. View Article : Google Scholar
|
|
17
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stöhr D, Jeltsch A and Rehm M: TRAIL
receptor signaling: From the basics of canonical signal
transduction toward its entanglement with ER stress and the
unfolded protein response. Int Rev Cell Mol Biol. 351:57–99. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim C and Kim B: Anti-cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10:10212018. View Article : Google Scholar :
|
|
20
|
Wang XZ, Harding HP, Zhang Y, Jolicoeur
EM, Kuroda M and Ron D: Cloning of mammalian Ire1 reveals diversity
in the ER stress responses. EMBO J. 17:5708–5717. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Walter P and Ron D: The unfolded protein
response: From stress pathway to homeostatic regulation. Science.
334:1081–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haze K, Yoshida H, Yanagi H, Yura T and
Mori K: Mammalian transcription factor ATF6 is synthesized as a
transmembrane protein and activated by proteolysis in response to
endoplasmic reticulum stress. Mol Biol Cell. 10:3787–3799. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elmore JM, Griffin BD and Walley JW:
Advances in functional proteomics to study plant-pathogen
interactions. Curr Opin Plant Biol. 63:1020612021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Chen DQ, Han JX, Zhao TT and Li SJ:
A review of traditional Chinese medicine in treating renal
interstitial fibrosis via endoplasmic reticulum stress-mediated
apoptosis. Biomed Res Int. 2021:66677912021.PubMed/NCBI
|
|
25
|
Fusée LTS, Marín M, Fåhraeus R and López
I: Alternative mechanisms of p53 action during the unfolded protein
response. Cancers(Basel). 12:4012020.
|
|
26
|
Storchova R, Burdova K, Palek M, Medema RH
and Macurek L: A novel assay for screening WIP1 phosphatase
substrates in nuclear extracts. FEBS J. May 13–2021.Epub ahead of
prin. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vodicka P, Andera L, Opattova A and
Vodickova L: The interactions of DNA repair, telomere homeostasis,
and p53 mutational status in solid cancers: Risk, prognosis, and
prediction. Cancers (Basel). 13:4792021. View Article : Google Scholar
|
|
28
|
Yukimoto A, Watanabe T, Sunago K, Nakamura
Y, Tanaka T, Koizumi Y, Yoshida O, Tokumoto Y, Hirooka M, Abe M and
Hiasa Y: The long noncoding RNA of RMRP is downregulated by PERK,
which induces apoptosis in hepatocellular carcinoma cells. Sci Rep.
11:79262021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang T, Xu T, Wang Y, Zhou Y, Yu D, Wang
Z, He L, Chen Z, Zhang Y, Davidson D, et al: Cannabidiol inhibits
human glioma by induction of lethal mitophagy through activating
TRPV4. Autophagy. Feb 25–2021.Epub ahead of prin. View Article : Google Scholar
|
|
30
|
Kuran D, Flis S, Antoszczak M, Piskorek M
and Huczyński A: Ester derivatives of salinomycin efficiently
eliminate breast cancer cells via ER-stress-induced apoptosis. Eur
J Pharmacol. 893:1738242021. View Article : Google Scholar
|
|
31
|
Bressler KR, Ross JA, Ilnytskyy S, Vanden
Dungen K, Taylor K, Patel K, Zovoilis A, Kovalchuk I and Thakor N:
Depletion of eukaryotic initiation factor 5B (eIF5B) reprograms the
cellular transcriptome and leads to activation of endoplasmic
reticulum (ER) stress and c-Jun N-terminal kinase (JNK). Cell
Stress Chaperones. 26:253–264. 2021. View Article : Google Scholar
|
|
32
|
González-Quiroz M, Blondel A, Sagredo A,
Hetz C, Chevet E and Pedeux R: When endoplasmic reticulum
proteostasis meets the DNA damage response. Trends Cell Biol.
30:881–891. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ventura JJ, Hübner A, Zhang C, Flavell RA,
Shokat KM and Davis RJ: Chemical genetic analysis of the time
course of signal transduction by JNK. Mol Cell. 21:701–710. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Farshbaf M, Khosroushahi AY,
Mojarad-Jabali S, Zarebkohan A, Valizadeh H and Walker PR: Cell
surface GRP78: An emerging imaging marker and therapeutic target
for cancer. J Control Release. 328:932–941. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Otero JH, Lizak B and Hendershot LM: Life
and death of a BiP substrate. Semin Cell Dev Biol. 21:472–478.
2010. View Article : Google Scholar :
|
|
37
|
Bertolotti A, Zhang Y, Hendershot LM,
Harding HP and Ron D: Dynamic interaction of BiP and ER stress
transducers in the unfolded-protein response. Nat Cell Biol.
2:326–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen J, Snapp EL, Lippincott-Schwartz J
and Prywes R: Stable binding of ATF6 to BiP in the endoplasmic
reticulum stress response. Mol Cell Biol. 25:921–932. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pincus D, Chevalier MW, Aragon T, van
Anken E, Vidal SE, El-Samad H and Walter P: BiP binding to the
ER-stress sensor Ire1 tunes the homeostatic behavior of the
unfolded protein response. PLoS Biol. 8:e10004152010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Harding HP, Zhang Y, Bertolotti A, Zeng H
and Ron D: Perk is essential for translational regulation and cell
survival during the unfolded protein response. Mol Cell. 5:897–904.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Harding HP, Zhang Y and Ron D: Protein
translation and folding are coupled by an
endoplasmic-reticulum-resident kinase. Nature. 397:271–274. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kadowaki H and Nishitoh H: Signaling
pathways from the endoplasmic reticulum and their roles in disease.
Genes. 4:306–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hetz C and Mollereau B: Disturbance of
endoplasmic reticulum proteostasis in neurodegenerative diseases.
Nat Rev Neurosci. 15:233–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dai C, Li J, Tang S, Li J and Xiao X:
Colistin-induced nephrotoxicity in mice involves the mitochondrial,
death receptor, and endoplasmic reticulum pathways. Antimicrob
Agents Chemother. 58:4075–4085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lien JC, Huang CC, Lu TJ, Tseng CH, Sung
PJ, Lee HZ, Bao BY, Kuo YH and Lu TL: Naphthoquinone derivative
PPE8 induces endoplasmic reticulum stress in p53 null H1299 cells.
Oxid Med Cell Longev. 2015:4536792015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kimata Y, Kimata YI, Shimizu Y, Abe H,
Farcasanu IC, Takeuchi M, Rose MD and Kohno K: Genetic evidence for
a role of BiP/Kar2 that regulates Ire1 in response to accumulation
of unfolded proteins. Mol Biol Cell. 14:2559–2569. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jabouille A, Delugin M, Pineau R, Dubrac
A, Soulet F, Lhomond S, Pallares-Lupon N, Prats H, Bikfalvi A,
Chevet E, et al: Glioblastoma invasion and cooption depend on
IRE1alpha eSndoribonuclease activity. Oncotarget. 6:24922–24934.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Korennykh AV, Egea PF, Korostelev AA,
Finer-Moore J, Zhang C, Shokat KM, Stroud RM and Walter P: The
unfolded protein response signals through high-order assembly of
Ire1. Nature. 457:687–693. 2009. View Article : Google Scholar
|
|
52
|
Yoshida H, Matsui T, Yamamoto A, Okada T
and Mori K: XBP1 mRNA is induced by ATF6 and spliced by IRE1 in
response to ER stress to produce a highly active transcription
factor. Cell. 107:881–891. 2001. View Article : Google Scholar
|
|
53
|
Lim R, Barker G and Lappas M: TRADD,
TRAF2, RIP1 and TAK1 are required for TNF-alpha-induced pro-labour
mediators in human primary myometrial cells. Am J Reprod Immunol.
Mar 24–2017.Epub ahead of print. View Article : Google Scholar
|
|
54
|
Bluher M, Bashan N, Shai I, Harman-Boehm
I, Tarnovscki T, Avinaoch E, Stumvoll M, Dietrich A, Klöting N and
Rudich A: Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway
in human omental fat tissue may link macrophage infiltration to
whole-body Insulin sensitivity. J Clin Endocrinol Metab.
94:2507–2515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Syc-Mazurek SB, Rausch RL, Fernandes KA,
Wilson MP and Libby RT: MKK4 and MKK7 are important for retinal
development and axonal injury-induced retinal ganglion cell death.
Cell Death Dis. 9:10952018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sujitha S, Dinesh P and Rasool M:
Berberine modulates ASK1 signaling mediated through TLR4/TRAF2 via
upregulation of miR-23a. Toxicol Appl Pharmacol. 359:34–46. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Matsui Y, Kuwabara T, Eguchi T, Nakajima K
and Kondo M: Acetylation regulates the MKK4-JNK pathway in T cell
receptor signaling. Immunol Lett. 194:21–28. 2018. View Article : Google Scholar
|
|
58
|
Cao S, Tang J, Huang Y, Li G, Li Z, Cai W,
Yuan Y, Liu J, Huang X and Zhang H: The road of solid tumor
survival: From drug-induced endoplasmic reticulum stress to drug
resistance. Front Mol Biosci. 8:6205142021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bagchi AK, Malik A, Akolkar G, Zimmer A,
Belló-Klein A, De Angelis K, Jassal DS, Fini MA, Stenmark KR and
Singal PK: Study of ER stress and apoptotic proteins in the heart
and tumor exposed to doxorubicin. Biochim Biophys Acta Mol Cell
Res. 119039:20211868.
|
|
60
|
Lynch JM, Maillet M, Vanhoutte D,
Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ,
Osinska H, et al: A thrombospondin-dependent pathway for a
protective ER stress response. Cell. 149:1257–1268. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Adachi Y, Yamamoto K, Okada T, Yoshida H,
Harada A and Mori K: ATF6 is a transcription factor specializing in
the regulation of quality control proteins in the endoplasmic
reticulum. Cell Struct Funct. 33:75–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bommiasamy H, Back SH, Fagone P, Lee K,
Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ
and Brewer JW: ATF6alpha induces XBP1-independent expansion of the
endoplasmic reticulum. J Cell Sci. 122:1626–1636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pinkham K, Park DJ, Hashemiaghdam A, Kirov
AB, Adam I, Rosiak K, da Hora CC, Teng J, Cheah PS, Carvalho L, et
al: Stearoyl CoA desaturase is essential for regulation of
endoplasmic reticulum homeostasis and tumor growth in glioblastoma
cancer stem cells. Stem Cell Reports. 12:712–727. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shakhparonov MI, Antipova NV, Shender VO,
Shnaider PV, Arapidi GP, Pestov NB and Pavlyukov MS: Expression and
intracellular localization of paraoxonase 2 in different types of
malignancies. Acta Naturae. 10:92–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Le Mercier M, Lefranc F, Mijatovic T,
Debeir O, Haibe-Kains B, Bontempi G, Decaestecker C, Kiss R and
Mathieu V: Evidence of galectin-1 involvement in glioma
chemoresistance. Toxicol Appl Pharmacol. 229:172–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin HY, Ko CY, Kao TJ, Yang WB, Tsai YT,
Chuang JY, Hu SL, Yang PY, Lo WL and Hsu TI: CYP17A1 Maintains the
survival of glioblastomas by regulating SAR1-mediated endoplasmic
reticulum health and redox homeostasis. Cancers (Basel).
11:13782019. View Article : Google Scholar
|
|
67
|
Jakubowicz-Gil J, Langner E, Badziul D,
Wertel I and Rzeski W: Silencing of Hsp27 and Hsp72 in glioma cells
as a tool for programmed cell death induction upon temozolomide and
quercetin treatment. Toxicol Appl Pharmacol. 273:580–589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Horibe T, Torisawa A, Kohno M and Kawakami
K: Molecular mechanism of cytotoxicity induced by Hsp90-targeted
Antp-TPR hybrid peptide in glioblastoma cells. Mol Cancer.
11:592012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Choi JW, Schroeder MA, Sarkaria JN and
Bram RJ: Cyclophilin B supports Myc and mutant p53-dependent
survival of glioblastoma multiforme cells. Cancer Res. 74:484–496.
2014. View Article : Google Scholar
|
|
70
|
Hu Y, Chu L, Liu J, Yu L, Song SB, Yang H
and Han F: Knockdown of CREB3 activates endoplasmic reticulum
stress and induces apoptosis in glioblastoma. Aging (Albany NY).
11:8156–8168. 2019. View Article : Google Scholar
|
|
71
|
Chen Y, Tsai YH and Tseng SH: HDAC
inhibitors and RECK modulate endoplasmic reticulum stress in tumor
cells. Int J Mol Sci. 18:2582017. View Article : Google Scholar :
|
|
72
|
Nguyen TTT, Ishida CT, Shang E, Shu C,
Bianchetti E, Karpel-Massler G and Siegelin MD: Activation of LXR
receptors and inhibition of TRAP1 causes synthetic lethality in
solid tumors. Cancers (Basel). 11:7882019. View Article : Google Scholar
|
|
73
|
Stock K, Kumar J, Synowitz M, Petrosino S,
Imperatore R, Smith ES, Wend P, Purfürst B, Nuber UA, Gurok U, et
al: Neural precursor cells induce cell death of high-grade
astrocytomas through stimulation of TRPV1. Nat Med. 18:1232–1238.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zeeshan HM, Lee GH, Kim HR and Chae HJ:
Endoplasmic reticulum stress and associated ROS. Int J Mol Sci.
17:3272016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kyani A, Tamura S, Yang S, Shergalis A,
Samanta S, Kuang Y, Ljungman M and Neamati N: Discovery and
mechanistic elucidation of a class of protein disulfide isomerase
inhibitors for the treatment of glioblastoma. ChemMedChem.
13:164–177. 2018. View Article : Google Scholar :
|
|
76
|
Chen WL, Wang CC, Lin YJ, Wu CP and Hsieh
CH: Cycling hypoxia induces chemoresistance through the activation
of reactive oxygen species-mediated B-cell lymphoma extra-long
pathway in glioblastoma multiforme. J Transl Med. 13:3892015.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Agnihotri S, Golbourn B, Huang X, Remke M,
Younger S, Cairns RA, Chalil A, Smith CA, Krumholtz SL, Mackenzie
D, et al: PINK1 is a negative regulator of growth and the warburg
effect in glioblastoma. Cancer Res. 76:4708–4719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang Q, Wang H, Jia Y, Pan H and Ding H:
Luteolin induces apoptosis by ROS/ER stress and mitochondrial
dysfunction in gliomablastoma. Cancer Chemother Pharmacol.
79:1031–1041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Qu C, Ma J, Liu X, Xue Y, Zheng J, Liu L,
Liu J, Li Z, Zhang L and Liu Y: Dihydroartemisinin exerts
anti-tumor activity by inducing mitochondrion and endoplasmic
reticulum apoptosis and autophagic cell death in human glioblastoma
cells. Front Cell Neurosci. 11:3102017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Schito L and Semenza GL: Hypoxia-inducible
factors: Master regulators of cancer progression. Trends Cancer.
2:758–770. 2016. View Article : Google Scholar
|
|
81
|
Zhou J, Schmid T, Schnitzer S and Brune B:
Tumor hypoxia and cancer progression. Cancer Lett. 237:10–21. 2006.
View Article : Google Scholar
|
|
82
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bischoff FC, Werner A, John D, Boeckel JN,
Melissari MT, Grote P, Glaser SF, Demolli S, Uchida S, Michalik KM,
et al: Identification and functional characterization of
hypoxia-induced endoplasmic reticulum stress regulating lncRNA
(HypERlnc) in pericytes. Circ Res. 121:368–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Han YK, Park GY, Bae MJ, Kim JS, Jo WS and
Lee CG: Hypoxia induces immunogenic cell death of cancer cells by
enhancing the exposure of cell surface calreticulin in an
endoplasmic reticulum stress-dependent manner. Oncol Lett.
18:6269–6274. 2019.PubMed/NCBI
|
|
85
|
Minchenko DO, Riabovol OO, Ratushna OO and
Minchenko OH: Hypoxic regulation of the expression of genes encoded
estrogen related proteins in U87 glioma cells: Effect of IRE1
inhibition. Endocr Regul. 51:8–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Minchenko DO, Tsymbal DO, Riabovol OO,
Viletska YM, Lahanovska YO, Sliusar MY, Bezrodnyi BH and Minchenko
OH: Hypoxic regulation of EDN1, EDNRA, EDNRB, and ECE1 gene
expressions in ERN1 knockdown U87 glioma cells. Endocr Regul.
53:250–262. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Minchenko OH, Tsymbal DO, Minchenko DO,
Kovalevska OV, Karbovskyi LL and Bikfalvi A: Inhibition of ERN1
signaling enzyme affects hypoxic regulation of the expression of
E2F8, EPAS1, HOXC6, ATF3, TBX3 and FOXF1 genes in U87 glioma cells.
Ukr Biochem J. 87:76–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Minchenko OH, Tsymbal DO, Minchenko DO and
Kubaychuk OO: Hypoxic regulation of MYBL1, MEST, TCF3, TCF8, GTF2B,
GTF2F2 and SNAI2 genes expression in U87 glioma cells upon IRE1
inhibition. Ukr Biochem J. 88:52–62. 2016. View Article : Google Scholar
|
|
89
|
Minchenko DO, Kharkova AP, Tsymbal DO,
Karbovskyi LL and Minchenko OH: IRE1 inhibition affects the
expression of insulin-like growth factor binding protein genes and
modifies its sensitivity to glucose deprivation in U87 glioma
cells. Endocr Regul. 49:185–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Minchenko OH, Kharkova AP, Minchenko DO
and Karbovskyi LL: Effect of hypoxia on the expression of genes
that encode some IGFBP and ccn proteins in U87 glioma cells depends
on IRE1 signaling. Ukr Biochem J. 87:52–63. 2015. View Article : Google Scholar
|
|
91
|
Minami N, Tanaka K, Sasayama T, Kohmura E,
Saya H and Sampetrean O: Lactate reprograms energy and lipid
metabolism in glucose-deprived oxidative glioma stem cells.
Metabolites. 11:3252021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Liberti MV and Locasale JW: The warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Crabtree HG: Observations on the
carbohydrate metabolism of tumours. Biochem J. 23:536–545. 1929.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Racker E: Bioenergetics and the problem of
tumor growth. Am Sci. 60:56–63. 1972.PubMed/NCBI
|
|
95
|
Shim H, Chun YS, Lewis BC and Dang CV: A
unique glucose-dependent apoptotic pathway induced by c-Myc. Proc
Natl Acad Sci USA. 95:1511–1516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Gu M, Gao Y and Chang P: KRAS mutation
dictates the cancer immune environment in pancreatic ductal
adenocarcinoma and other adenocarcinomas. Cancers (Basel).
13:24292021. View Article : Google Scholar
|
|
97
|
Khrabrova DA, Yakubovskaya MG and Gromova
ES: AML-associated mutations in DNA methyltransferase DNMT3A.
Biochemistry (Mosc). 86:307–318. 2021. View Article : Google Scholar
|
|
98
|
Pavlova NN and Thompson CB: The emerging
hallmarks of cancer metabolism. Cell Metab. 23:27–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Mechelli R, Romano S, Romano C, Morena E,
Buscarinu MC, Bigi R, Bellucci G, Reniè R, Pellicciari G, Salvetti
M and Ristori G: MAIT cells and microbiota in multiple sclerosis
and other autoimmune diseases. Microorganisms. 9:11322021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Pyrko P, Schonthal AH, Hofman FM, Chen TC
and Lee AS: The unfolded protein response regulator GRP78/BiP as a
novel target for increasing chemosensitivity in malignant gliomas.
Cancer Res. 67:9809–9816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Soejima E, Ohki T, Kurita Y, Yuan X,
Tanaka K, Kakino S, Hara K, Nakayama H, Tajiri Y and Yamada K:
Protective effect of 3-hydroxybutyrate against endoplasmic
reticulum stress-associated vascular endothelial cell damage
induced by low glucose exposure. PLoS One. 13:e01911472018.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang Y, Ishida CT, Ishida W, Lo SL, Zhao
J, Shu C, Bianchetti E, Kleiner G, Sanchez-Quintero MJ, Quinzii CM,
et al: Combined HDAC and bromodomain protein inhibition reprograms
tumor cell metabolism and elicits synthetic lethality in
glioblastoma. Clin Cancer Res. 24:3941–3954. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Minchenko DO, Hubenya OV, Terletsky BM,
Moenner M and Minchenko OH: Effect of glutamine or glucose
deprivation on the expression of cyclin and cyclin-dependent kinase
genes in glioma cell line U87 and its subline with suppressed
activity of signaling enzyme of endoplasmic reticulum-nuclei-1. Ukr
Biokhim Zh (1999). 83:18–29. 2011.
|
|
104
|
Ma L, Wei J, Wan J, Wang W, Wang L, Yuan
Y, Yang Z, Liu X and Ming L: Low glucose and metformin-induced
apoptosis of human ovarian cancer cells is connected to ASK1 via
mitochondrial and endoplasmic reticulum stress-associated pathways.
J Exp Clin Cancer Res. 38:772019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Schornack PA and Gillies RJ: Contributions
of cell metabolism and H+ diffusion to the acidic pH of tumors.
Neoplasia. 5:135–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tannock IF and Rotin D: Acid pH in tumors
and its potential for therapeutic exploitation. Cancer Res.
49:4373–4384. 1989.PubMed/NCBI
|
|
107
|
Rofstad EK, Mathiesen B, Kindem K and
Galappathi K: Acidic extracellular pH promotes experimental
metastasis of human melanoma cells in athymic nude mice. Cancer
Res. 66:6699–6707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hjelmeland AB, Wu Q, Heddleston JM,
Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan
A and Rich JN: Acidic stress promotes a glioma stem cell phenotype.
Cell Death Differ. 18:829–840. 2011. View Article : Google Scholar :
|
|
109
|
Park HJ, Lyons JC, Ohtsubo T and Song CW:
Acidic environment causes apoptosis by increasing caspase activity.
Br J Cancer. 80:1892–1897. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kato Y, Lambert CA, Colige AC, Mineur P,
Noël A, Frankenne F, Foidart JM, Baba M, Hata R, Miyazaki K and
Tsukuda M: Acidic extracellular pH induces matrix
metalloproteinase-9 expression in mouse metastatic melanoma cells
through the phospholipase D-mitogen-activated protein kinase
signaling. J Biol Chem. 280:10938–10944. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xu L, Fukumura D and Jain RK: Acidic
extracellular pH induces vascular endothelial growth factor (VEGF)
in human glioblastoma cells via ERK1/2 MAPK signaling pathway:
Mechanism of low pH-induced VEGF. J Biol Chemistry.
277:11368–11374. 2002. View Article : Google Scholar
|
|
112
|
Reichert M, Steinbach JP, Supra P and
Weller M: Modulation of growth and radiochemosensitivity of human
malignant glioma cells by acidosis. Cancer. 95:1113–1119. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Xie ZY, Chen L, Wang F, Liu L, Zhang C,
Wang K, Cai F, Sinkemanni A, Hong X and Wu XT: Endoplasmic
reticulum stress is involved in nucleus pulposus degeneration and
attenuates low pH-Induced apoptosis of rat nucleus pulposus cells.
DNA Cell Biol. 36:627–637. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Dong L, Krewson EA and Yang LV: Acidosis
Activates endoplasmic reticulum stress pathways through GPR4 in
human vascular endothelial cells. Int J Mol Sci. 18:2782017.
View Article : Google Scholar :
|
|
115
|
Christensen SB, Andersen A, Kromann H,
Treiman M, Tombal B, Denmeade S and Isaacs JT: Thapsigargin
analogues for targeting programmed death of androgen-independent
prostate cancer cells. Bioorg Med Chem. 7:1273–1280. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Jansen S, Arning J and Beyersmann D:
Effects of the Ca ionophore a23187 on zinc-induced apoptosis in C6
glioma cells. Biol Trace Elem Res. 96:133–142. 2003. View Article : Google Scholar
|
|
117
|
Grant SK, Bansal A, Mitra A, Feighner SD,
Dai G, Kaczorowski GJ and Middleton RE: Delay of intracellular
calcium transients using a calcium chelator: Application to
high-throughput screening of the capsaicin receptor ion channel and
G-protein-coupled receptors. Anal Biochem. 294:27–35. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ciechomska IA, Gabrusiewicz K,
Szczepankiewicz AA and Kaminska B: Endoplasmic reticulum stress
triggers autophagy in malignant glioma cells undergoing
cyclosporine a-induced cell death. Oncogene. 32:1518–1529. 2013.
View Article : Google Scholar
|
|
119
|
Kaul A and Maltese WA: Killing of cancer
cells by the photoactivatable protein kinase C inhibitor,
calphostin C, involves induction of endoplasmic reticulum stress.
Neoplasia. 11:823–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Martinez NJ, Rai G, Yasgar A, Lea WA, Sun
H, Wang Y, Luci DK, Yang SM, Nishihara K, Takeda S, et al: A
high-throughput screen identifies 2,9-diazaspiro[5.5]Undecanes as
inducers of the endoplasmic reticulum stress response with
cytotoxic activity in 3D glioma cell models. PLoS One.
11:e01614862016. View Article : Google Scholar
|
|
121
|
Yu SN, Kim SH, Kim KY, Ji JH, Seo YK, Yu
HS and Ahn SC: Salinomycin induces endoplasmic reticulum
stressmediated autophagy and apoptosis through generation of
reactive oxygen species in human glioma U87MG cells. Oncol Rep.
37:3321–3328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
White MC, Johnson GG, Zhang W, Hobrath JV,
Piazza GA and Grimaldi M: Sulindac sulfide inhibits
sarcoendoplasmic reticulum Ca2+ ATPase, induces endoplasmic
reticulum stress response, and exerts toxicity in glioma cells:
Relevant similarities to and important differences from celecoxib.
J Neurosci Res. 91:393–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA,
Lim JH, Kwon TK and Choi KS: Monensin, a polyether ionophore
antibiotic, overcomes TRAIL resistance in glioma cells via
endoplasmic reticulum stress, DR5 upregulation and c-FLIP
downregulation. Carcinogenesis. 34:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Han J and Kaufman RJ: The role of ER
stress in lipid metabolism and lipotoxicity. J Lipid Res.
57:1329–1338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Thibault G, Shui G, Kim W, McAlister GC,
Ismail N, Gygi SP, Wenk MR and Ng DT: The membrane stress response
buffers lethal effects of lipid disequilibrium by reprogramming the
protein homeostasis network. Mol Cell. 48:16–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Guo W, Wong S, Xie W, Lei T and Luo Z:
Palmitate modulates intracellular signaling, induces endoplasmic
reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat
primary preadipocytes. Am J Physiol Endocrinol Metab.
293:E576–E586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Griffiths B, Lewis CA, Bensaad K, Ros S,
Zhang Q, Ferber EC, Konisti S, Peck B, Miess H, East P, et al:
Sterol regulatory element binding protein-dependent regulation of
lipid synthesis supports cell survival and tumor growth. Cancer
Metab. 1:32013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Williams KJ, Argus JP, Zhu Y, Wilks MQ,
Marbois BN, York AG, Kidani Y, Pourzia AL, Akhavan D, Lisiero DN,
et al: An essential requirement for the SCAP/SREBP signaling axis
to protect cancer cells from lipotoxicity. Cancer Res.
73:2850–2862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ackerman D and Simon MC: Hypoxia, lipids,
and cancer: Surviving the harsh tumor microenvironment. Trends Cell
Biol. 24:472–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
He Y, Su J, Lan B, Gao Y and Zhao J:
Targeting off-target effects: Endoplasmic reticulum stress and
autophagy as effective strategies to enhance temozolomide
treatment. Onco Targets Ther. 12:1857–1865. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zhang Y, Tseng CC, Tsai YL, Fu X, Schiff R
and Lee AS: Cancer cells resistant to therapy promote cell surface
relocalization of GRP78 which complexes with PI3K and enhances
PI(3,4,5)P3 production. PLoS One. 8:e800712013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Misra UK, Deedwania R and Pizzo SV:
Binding of activated alpha2-macroglobulin to its cell surface
receptor GRP78 in 1-LN prostate cancer cells regulates
PAK-2-dependent activation of LIMK. J Biol Chem. 280:26278–26286.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu K, Tsung K and Attenello FJ:
Characterizing cell stress and GRP78 in glioma to enhance tumor
treatment. Front Oncol. 10:6089112020. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hseu YC, Lin RW, Shen YC, Lin KY, Liao JW,
Thiyagarajan V and Yang HL: Flavokawain B and doxorubicin work
synergistically to impede the propagation of gastric cancer cells
via ROS-mediated apoptosis and autophagy Pathways. Cancers (Basel).
12:4752020. View Article : Google Scholar
|
|
135
|
Doultsinos D, Carlesso A, Chintha C, Paton
JC, Paton AW, Samali A, Chevet E and Eriksson LA:
Peptidomimetic-based identification of FDA-approved compounds
inhibiting IRE1 activity. FEBS J. 288:945–960. 2021. View Article : Google Scholar
|
|
136
|
Hua R, Pei Y, Gu H, Sun Y and He Y:
Antitumor effects of flavokawain-B flavonoid in
gemcitabine-resistant lung cancer cells are mediated via
mitochondrial-mediated apoptosis, ROS production, cell migration
and cell invasion inhibition and blocking of PI3K/AKT Signaling
pathway. J BUON. 25:262–267. 2020.PubMed/NCBI
|
|
137
|
Wang J, Qi Q, Zhou W, Feng Z, Huang B,
Chen A, Zhang D, Li W, Zhang Q, Jiang Z, et al: Inhibition of
glioma growth by flavokawain B is mediated through endoplasmic
reticulum stress induced autophagy. Autophagy. 14:2007–2022. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kim TH, Song J, Kim SH, Parikh AK, Mo X,
Palanichamy K, Kaur B, Yu J, Yoon SO, Nakano I and Kwon CH:
Piperlongumine treatment inactivates peroxiredoxin 4, exacerbates
endoplasmic reticulum stress, and preferentially kills high-grade
glioma cells. Neuro Oncol. 16:1354–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Tsai SF, Tao M, Ho LI, Chiou TW, Lin SZ,
Su HL and Harn HJ: Isochaihulactone-induced DDIT3 causes ER
stress-PERK independent apoptosis in glioblastoma multiforme cells.
Oncotarget. 8:4051–4061. 2017. View Article : Google Scholar :
|
|
140
|
Lu DY, Chang CS, Yeh WL, Tang CH, Cheung
CW, Leung YM, Liu JF and Wong KL: The novel phloroglucinol
derivative BFP induces apoptosis of glioma cancer through reactive
oxygen species and endoplasmic reticulum stress pathways.
Phytomedicine. 19:1093–1100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Guzman M: Cannabinoids: Potential
anticancer agents. Nat Rev Cancer. 3:745–755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Carracedo A, Lorente M, Egia A, Blázquez
C, García S, Giroux V, Malicet C, Villuendas R, Gironella M,
González-Feria L, et al: The stress-regulated protein p8 mediates
cannabinoid-induced apoptosis of tumor cells. Cancer Cell.
9:301–312. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ma X, Yu M, Hao C and Yang W: Shikonin
induces tumor apoptosis in glioma cells via endoplasmic reticulum
stress, and Bax/Bak mediated mitochondrial outer membrane
permeability. J Ethnopharmacol. 263:1130592020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Pan JM, Zhou L, Wang GB, Xia GW, Xue K,
Cui XG, Shi HZ, Liu JH and Hu J: Fatsioside A inhibits the growth
of glioma cells via the induction of endoplasmic reticulum
stress-mediated apoptosis. Mol Med Rep. 11:3493–3498. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Das A, Banik NL and Ray SK: Garlic
compounds generate reactive oxygen species leading to activation of
stress kinases and cysteine proteases for apoptosis in human
glioblastoma T98G and U87MG cells. Cancer. 110:1083–1095. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Das A, Banik NL and Ray SK: Flavonoids
activated caspases for apoptosis in human glioblastoma T98G and
U87MG cells but not in human normal astrocytes. Cancer.
116:164–176. 2010.
|
|
147
|
Djerir D, Iddir M, Bourgault S, Lamy S and
Annabi B: Biophysical evidence for differential gallated green tea
catechins binding to membrane type-1 matrix metalloproteinase and
its interactors. Biophys Chem. 234:34–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Ma J, Qiu Y, Yang L, Peng L, Xia Z, Hou
LN, Fang C, Qi H and Chen HZ: Desipramine induces apoptosis in rat
glioma cells via endoplasmic reticulum stress-dependent CHOP
pathway. J Neurooncol. 101:41–48. 2011. View Article : Google Scholar
|
|
149
|
Garrido-Armas M, Corona JC, Escobar ML,
Torres L, Ordóñez-Romero F, Hernández-Hernández A and
Arenas-Huertero F: Paraptosis in human glioblastoma cell line
induced by curcumin. Toxicol In Vitro. 51:63–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Sansalone L, Veliz EA, Myrthil NG,
Stathias V, Walters W, Torrens II, Schürer SC, Vanni S, Leblanc RM
and Graham RM: Novel Curcumin Inspired Bis-chalcone promotes
endoplasmic reticulum stress and glioblastoma neurosphere cell
death. Cancers (Basel). 11:3572019. View Article : Google Scholar
|
|
151
|
Ma YY, Di ZM, Cao Q, Xu WS, Bi SX, Yu JS,
Shen YJ, Yu YQ, Shen YX and Feng LJ: Xanthatin induces glioma cell
apoptosis and inhibits tumor growth via activating endoplasmic
reticulum stress-dependent CHOP pathway. Acta Pharmacol Sin.
41:404–414. 2020. View Article : Google Scholar :
|
|
152
|
Martin S, Lamb HK, Brady C, Lefkove B,
Bonner MY, Thompson P, Lovat PE, Arbiser JL, Hawkins AR and Redfern
CP: Inducing apoptosis of cancer cells using small-molecule plant
compounds that bind to GRP78. Br J Cancer. 109:433–443. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li ZY, Zhang C, Chen L, Chen BD, Li QZ,
Zhang XJ and Li WP: Radicol, a novel trinorguaiane-type
sesquiterpene, induces temozolomide-resistant glioma cell apoptosis
via ER stress and Akt/mTOR pathway blockade. Phytother Res.
31:729–739. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chou YC, Chang MY, Wang MJ, Harnod T, Hung
CH, Lee HT, Shen CC and Chung JG: PEITC induces apoptosis of human
brain glioblastoma GBM8401 cells through the extrinsic- and
intrinsic-signaling pathways. Neurochem Int. 81:32–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Chou YC, Chang MY, Wang MJ, Liu HC, Chang
SJ, Harnod T, Hung CH, Lee HT, Shen CC and Chung JG: Phenethyl
isothiocyanate alters the gene expression and the levels of protein
associated with cell cycle regulation in human glioblastoma GBM
8401 cells. Environ Toxicol. 32:176–187. 2017. View Article : Google Scholar
|
|
156
|
Meng X, Leyva ML, Jenny M, Gross I,
Benosman S, Fricker B, Harlepp S, Hébraud P, Boos A, Wlosik P, et
al: A ruthenium-containing organometallic compound reduces tumor
growth through induction of the endoplasmic reticulum stress gene
CHOP. Cancer Res. 69:5458–5466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Badr CE, Van Hoppe S, Dumbuya H,
Tjon-Kon-Fat LA and Tannous BA: Targeting cancer cells with the
natural compound obtusaquinone. J Natl Cancer Inst. 105:643–653.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Liu H, Xiong C, Liu J, Sun T, Ren Z, Li Y,
Geng J and Li X: Aspirin exerts anti-tumor effect through
inhibiting Blimp1 and activating ATF4/CHOP pathway in multiple
myeloma. Biomed Pharmacother. 125:1100052020. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Nishimura N, Radwan MO, Amano M, Endo S,
Fujii E, Hayashi H, Ueno S, Ueno N, Tatetsu H, Hata H, et al: Novel
p97/VCP inhibitor induces endoplasmic reticulum stress and
apoptosis in both bortezomib-sensitive and -resistant multiple
myeloma cells. Cancer Sci. 110:3275–3287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Alper P, Salomatina OV, Salakhutdinov NF,
Ulukaya E and Ari F: Soloxolone methyl, as a 18βH-glycyrrhetinic
acid derivate, may result in endoplasmic reticulum stress to induce
apoptosis in breast cancer cells. Bioorg Med Chem. 30:1159632021.
View Article : Google Scholar
|
|
161
|
Ren M, Zhou X, Gu M, Jiao W, Yu M, Wang Y,
Liu S, Yang J and Ji F: Resveratrol synergizes with cisplatin in
antineoplastic effects against AGS gastric cancer cells by inducing
endoplasmic reticulum stress-mediated apoptosis and G2/M phase
arrest. Oncol Rep. 44:1605–1615. 2020.PubMed/NCBI
|
|
162
|
De Wang X, Li T, Li Y, Yuan WH and Zhao
YQ: 2-Pyrazine-PPD, a novel dammarane derivative, showed anticancer
activity by reactive oxygen species-mediate apoptosis and
endoplasmic reticulum stress in gastric cancer cells. Eur J
Pharmacol. 881:1732112020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Zhang Q, Chen M, Cao L, Ren Y, Guo X, Wu X
and Xu K: Phenethyl isothiocyanate synergistically induces
apoptosis with Gefitinib in non-small cell lung cancer cells via
endoplasmic reticulum stress-mediated degradation of Mcl-1. Mol
Carcinog. 59:590–603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Zhu J, Xu S, Gao W, Feng J and Zhao G:
Honokiol induces endoplasmic reticulum stress-mediated apoptosis in
human lung cancer cells. Life Sci. 221:204–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Peñaranda-Fajardo NM, Meijer C, Liang Y,
Dijkstra BM, Aguirre-Gamboa R, den Dunnen WFA and Kruyt FAE: ER
stress and UPR activation in glioblastoma: Identification of a
noncanonical PERK mechanism regulating GBM stem cells through SOX2
modulation. Cell Death Dis. 10:6902019. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Dadey DYA, Kapoor V, Khudanyan A, Thotala
D and Hallahan DE: PERK regulates glioblastoma sensitivity to ER
stress although promoting radiation resistance. Mol Cancer Res.
16:1447–1453. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Axten JM, Medina JR, Feng Y, Shu A,
Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, et
al: Discovery of
7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine
(GSK2606414), a potent and selective first-in-class inhibitor of
protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J
Med Chem. 55:7193–7207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Rozpędek W, Pytel D, Wawrzynkiewicz A,
Siwecka N, Dziki A, Dziki Ł, Diehl JA and Majsterek I: Use of
Small-molecule inhibitory compound of PERK-dependent signaling
pathway as a promising target-based therapy for colorectal cancer.
Curr Cancer Drug Targets. 20:223–238. 2020. View Article : Google Scholar
|
|
169
|
Nishikawa S, Itoh Y, Tokugawa M, Inoue Y,
Nakashima KI, Hori Y, Miyajima C, Yoshida K, Morishita D, Ohoka N,
et al: Kurarinone from Sophora Flavescens roots triggers ATF4
activation and cytostatic effects through PERK phosphorylation.
Molecules. 24:31102019. View Article : Google Scholar :
|
|
170
|
Scheffer D, Kulcsár G, Nagyéri G,
Kiss-Merki M, Rékási Z, Maloy M and Czömpöly T: Active mixture of
serum-circulating small molecules selectively inhibits
proliferation and triggers apoptosis in cancer cells via induction
of ER stress. Cell Signal. 65:1094262020. View Article : Google Scholar
|
|
171
|
Guirao-Abad JP, Weichert M, Albee A, Deck
K and Askew DS: A Human IRE1 inhibitor blocks the unfolded protein
response in the pathogenic fungus aspergillus fumigatus and
suggests noncanonical functions within the pathway. mSphere.
5:e00879–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Cross BC, Bond PJ, Sadowski PG, Jha BK,
Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D
and Harding HP: The molecular basis for selective inhibition of
unconventional mRNA splicing by an IRE1-binding small molecule.
Proc Natl Acad Sci USA. 109:E869–E878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Chen X, Li H, Fan X, Zhao C, Ye K, Zhao Z,
Hu L, Ma H, Wang H and Fang Z: Protein palmitoylation regulates
cell survival by modulating XBP1 activity in glioblastoma
multiforme. Mol Ther Oncolytics. 17:518–530. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Grandjean JMD, Madhavan A, Cech L,
Seguinot BO, Paxman RJ, Smith E, Scampavia L, Powers ET, Cooley CB,
Plate L, et al: Pharmacologic IRE1/XBP1s activation confers
targeted ER proteostasis reprogramming. Nat Chem Biol.
16:1052–1061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Cho HY, Thein TZ, Wang W, Swenson SD,
Fayngor RA, Ou M, Marín-Ramos NI, Schönthal AH, Hofman FM and Chen
TC: The Rolipram-Perillyl alcohol conjugate (NEO214) is a mediator
of cell death through the death receptor pathway. Mol Cancer Ther.
18:517–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
McCubrey JA, Lahair MM and Franklin RA:
OSU-03012 in the treatment of glioblastoma. Mol Pharmacol.
70:437–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Cho HY, Wang W, Jhaveri N, Lee DJ, Sharma
N, Dubeau L, Schönthal AH, Hofman FM and Chen TC: NEO212,
temozolomide conjugated to perillyl alcohol, is a novel drug for
effective treatment of a broad range of temozolomide-resistant
gliomas. Mol Cancer Ther. 13:2004–2017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Marin-Ramos NI, Perez-Hernandez M, Tam A,
Swenson SD, Cho HY, Thein TZ, Hofman FM and Chen TC: Inhibition of
motility by NEO100 through the calpain-1/RhoA pathway. J
Neurosurgery. Aug 16–2019.Epub ahead of print. View Article : Google Scholar
|
|
179
|
Chen HY, He LJ, Li SQ, Zhang YJ, Huang JH,
Qin HX, Wang JL, Li QY and Yang DL: A derivate of
benzimidazole-isoquinolinone induces SKP2 transcriptional
inhibition to exert anti-tumor activity in glioblastoma cells.
Molecules. 24:27222019. View Article : Google Scholar :
|
|
180
|
Koncarevic S, Urig S, Steiner K, Rahlfs S,
Herold-Mende C, Sueltmann H and Becker K: Differential genomic and
proteomic profiling of glioblastoma cells exposed to
terpyridineplatinum(II) complexes. Free Radic Biol Med.
46:1096–1108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Li Z, Ma J, Liu L, Liu X, Wang P, Liu Y,
Li Z, Zheng J, Chen J, Tao W and Xue Y: Endothelial-monocyte
activating polypeptide II suppresses the in vitro
glioblastoma-induced angiogenesis by inducing autophagy. Front Mol
Neurosci. 10:2082017. View Article : Google Scholar :
|
|
182
|
Eom KS, Kim HJ, So HS, Park R and Kim TY:
Berberine-induced apoptosis in human glioblastoma T98G cells is
mediated by endoplasmic reticulum stress accompanying reactive
oxygen species and mitochondrial dysfunction. Biol Pharm Bull.
33:1644–1649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Wang L, Gundelach JH and Bram RJ:
Cycloheximide promotes paraptosis induced by inhibition of
cyclophilins in glioblastoma multiforme. Cell Death Dis.
8:e28072017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Suzuki K, Gerelchuluun A, Hong Z, Sun L,
Zenkoh J, Moritake T and Tsuboi K: Celecoxib enhances
radiosensitivity of hypoxic glioblastoma cells through endoplasmic
reticulum stress. Neuro Oncol. 15:1186–1199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Ye T, Wei L, Shi J, Jiang K, Xu H, Hu L,
Kong L, Zhang Y, Meng S and Piao H: Sirtuin1 activator SRT2183
suppresses glioma cell growth involving activation of endoplasmic
reticulum stress pathway. BMC Cancer. 19:7062019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Jia W, Loria RM, Park MA, Yacoub A, Dent P
and Graf MR: The neuro-steroid, 5-androstene 3β,17α diol; induces
endoplasmic reticulum stress and autophagy through PERK/eIF2α
signaling in malignant glioma cells and transformed fibroblasts.
Int J Biochem Cell Biol. 42:2019–2029. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Liu WT, Huang CY, Lu IC and Gean PW:
Inhibition of glioma growth by minocycline is mediated through
endoplasmic reticulum stress-induced apoptosis and autophagic cell
death. Neuro Oncol. 15:1127–1141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Shen S, Zhang Y, Zhang R, Tu X and Gong X:
Ursolic acid induces autophagy in U87MG cells via ROS-dependent
endoplasmic reticulum stress. Chem Biol Interact. 218:28–41. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Bown CD, Wang JF and Young LT: Increased
expression of endoplasmic reticulum stress proteins following
chronic valproate treatment of rat C6 glioma cells.
Neuropharmacology. 39:2162–2169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Park E, Gim J, Kim DK, Kim CS and Chun HS:
Protective effects of alpha-lipoic acid on glutamate-induced
cytotoxicity in C6 glioma cells. Biol Pharm Bull. 42:94–102. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Kim IY, Kwon M, Choi MK, Lee D, Lee DM,
Seo MJ and Choi KS: Ophiobolin A kills human glioblastoma cells by
inducing endoplasmic reticulum stress via disruption of thiol
proteostasis. Oncotarget. 8:106740–106752. 2017. View Article : Google Scholar :
|
|
192
|
Qaisiya M, Brischetto C, Jasprova J, Vitek
L, Tiribelli C and Bellarosa C: Bilirubin-induced ER stress
contributes to the inflammatory response and apoptosis in neuronal
cells. Arch Toxicol. 91:1847–1858. 2017. View Article : Google Scholar
|
|
193
|
Mahoney DJ, Lefebvre C, Allan K, Brun J,
Sanaei CA, Baird S, Pearce N, Grönberg S, Wilson B, Prakesh M, et
al: Virus-tumor interactome screen reveals ER stress response can
reprogram resistant cancers for oncolytic virus-triggered caspase-2
cell death. Cancer Cell. 20:443–456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Abraham R, Mudaliar P, Padmanabhan A and
Sreekumar E: Induction of cytopathogenicity in human glioblastoma
cells by chikungunya virus. PLoS One. 8:e758542013. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kusaczuk M, Kretowski R, Naumowicz M,
Stypulkowska A and Cechowska-Pasko M: Silica nanoparticle-induced
oxidative stress and mitochondrial damage is followed by activation
of intrinsic apoptosis pathway in glioblastoma cells. Int J
Nanomedicine. 13:2279–2294. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Rubiolo JA, Lopez-Alonso H, Martínez P,
Millán A, Cagide E, Vieytes MR, Vega FV and Botana LM: Yessotoxin
induces ER-stress followed by autophagic cell death in glioma cells
mediated by mTOR and BNIP3. Cell Signal. 26:419–432. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kim IY, Kang YJ, Yoon MJ, Kim EH, Kim SU,
Kwon TK, Kim IA and Choi KS: Amiodarone sensitizes human glioma
cells but not astrocytes to TRAIL-induced apoptosis via
CHOP-mediated DR5 upregulation. Neuro Oncol. 13:267–279. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Golden EB, Cho HY, Jahanian A, Hofman FM,
Louie SG, Schönthal AH and Chen TC: Chloroquine enhances
temozolomide cytotoxicity in malignant gliomas by blocking
autophagy. Neurosurg Focus. 37:E122014. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Przystal JM, Waramit S, Pranjol MZI, Yan
W, Chu G, Chongchai A, Samarth G, Olaciregui NG, Tabatabai G,
Carcaboso AM, et al: Efficacy of systemic temozolomide-activated
phage-targeted gene therapy in human glioblastoma. EMBO Mol Med.
11:e84922019. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Sun Y and Zhang X: Bufothionine promotes
apoptosis via triggering ER stress and synergizes with temozolomide
in glioblastoma multiforme cells. Anat Rec (Hoboken).
302:1950–1957. 2019. View Article : Google Scholar
|
|
201
|
Zhao H, Chen G and Liang H: Dual PI3K/mTOR
inhibitor, XL765, suppresses glioblastoma growth by inducing ER
stress-dependent apoptosis. Onco Targets Ther. 12:5415–5424. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Ma J, Yang YR, Chen W, Chen MH, Wang H,
Wang XD, Sun LL, Wang FZ and Wang DC: Fluoxetine synergizes with
temozolomide to induce the CHOP-dependent endoplasmic reticulum
stress-related apoptosis pathway in glioma cells. Oncol Rep.
36:676–684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Sun S, Lee D, Ho AS, Pu JK, Zhang XQ, Lee
NP, Day PJ, Lui WM, Fung CF and Leung GK: Inhibition of prolyl
4-hydroxylase, beta polypeptide (P4HB) attenuates temozolomide
resistance in malignant glioma via the endoplasmic reticulum stress
response (ERSR) pathways. Neuro Pncol. 15:562–577. 2013.
|
|
204
|
Golden EB, Cho HY, Hofman FM, Louie SG,
Schonthal AH and Chen TC: Quinoline-based antimalarial drugs: A
novel class of autophagy inhibitors. Neurosurg Focus. 38:E122015.
View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Shteingauz A, Porat Y, Voloshin T,
Schneiderman RS, Munster M, Zeevi E, Kaynan N, Gotlib K, Giladi M,
Kirson ED, et al: AMPK-dependent autophagy upregulation serves as a
survival mechanism in response to Tumor Treating Fields (TTFields).
Cell Death Dis. 9:10742018. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Weatherbee JL, Kraus JL and Ross AH: ER
stress in temozolomide-treated glioblastomas interferes with DNA
repair and induces apoptosis. Oncotarget. 7:43820–43834. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Kardosh A, Golden EB, Pyrko P, Uddin J,
Hofman FM, Chen TC, Louie SG, Petasis NA and Schönthal AH:
Aggravated endoplasmic reticulum stress as a basis for enhanced
glioblastoma cell killing by bortezomib in combination with
celecoxib or its non-coxib analogue, 2,5-dimethyl-celecoxib. Cancer
Res. 68:843–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Wang D, Fu L, Sun H, Guo L and DuBois RN:
Prostaglandin E2 promotes colorectal cancer stem cell expansion and
metastasis in mice. Gastroenterology. 149:1884–1895.e4. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Grandjean JM and Wiseman RL: Small
molecule strategies to harness the unfolded protein response: Where
do we go from here? J Biol Chemistry. 295:15692–15711. 2020.
View Article : Google Scholar
|
|
210
|
Bian T, Tagmount A, Vulpe C, Vijendra KC
and Xing C: CXL146, a novel 4H-chromene derivative, targets GRP78
to selectively eliminate multidrug-resistant cancer cells. Mol
Pharmacol. 97:402–408. 2020. View Article : Google Scholar : PubMed/NCBI
|