Introduction
In 1998, Hsu et al (1) found that the leucine-rich
repeat-containing G-protein coupled receptor 5 (Lgr5) was
homologous to the gonadotropin and thyrotropin receptors in
Drosophila (1). Lgr5 is expressed in
the eye, brain, mammary glands, hair follicles, stomach, intestines
and other tissues (2,3). Lgr5 serves an important role in the Wnt
signaling pathway, which is intimately involved in tumor
development (4–6). Lgr5 is a marker of stem cells in the
intestine, mammary glands and hair follicles. Lgr5 is also
expressed in intestinal tumors (7,8), which are
composed of crypt base columnar cells and stem cells. In addition,
Lgr5 is expressed in hepatocellular carcinoma, breast cancer and
gastric carcinoma. However, its role in normal lung and in lung
cancer remains unknown.
Lung cancer is the leading cause of
cancer-assocaited mortality among males in developed countries
(9). Few studies reporting on Lgr5 in
lung cancer have been published in recent years (10,11). To
investigate whether Lgr5 serves a role in lung cancer similar to
that in colorectal cancer and to determine whether Lgr5 functions
in the maintenance of lung structure, the present study assessed
the structure of the lungs in Lgr5 heterozygous knockout mice
(Lgr5+/−). Notbaly, it was demonstrated that the lungs
of Lgr5+/− mice were abnormal compared with those of
wild-type (WT) mice, which suggested that Lgr5 functions in the
maintenance of normal lung structure. Furthermore, Lgr5 expression
was analyzed in tumor tissues of patients with lung cancer. The
present study indicated that Lgr5 serves an important role in lung
adenocarcinoma and provided some meaningful insight into the
presence of Lgr5-positive cells in the lungs and Lgr5 expression in
lung cancer.
Materials and methods
Mice
Genetically engineered mice [B6.129P2-Lgr5tm1
(cre/ERT2) Cle/J] were donated to our laboratory by Professor Geng
(Department of Biologic and Materials Sciences, University of
Michigan School of Dentistry, Ann Arbor, MI, USA). This mouse model
was produced by knock-in of the Lgr5-EGFP-IRES-creERT2 allele,
which results in the loss of function of one Lgr5 allele and the
expression of the CreERT2-EGFP fusion protein. Lgr5 mice do not
exhibit any obvious abnormalities in terms of birth rate or any
abnormal physiological properties. By contrast, the homozygous
deletion of Lrg5 in mice resulted in neonatal lethality (12). To investigate the role of Lgr5 in the
lungs, Lgr5+/− mice were used in the present study. The
mice were housed in an individual ventilated cage (IVC) environment
at 24±2°C and 60±5% humidity with 12 h light/dark cycles. The mice
were fed a bacteria-free diet. All mice were sacrificed under
diethyl ether anesthesia. Guangdong College of Pharmacy Approved
Animal Ethics Committee approved all murine experiments. To
identify the genotype of the mice, DNA was extracted from a 0.5–1
cm portion of the tail. The DNA was amplified in polymerase chain
reaction (PCR) amplification buffer (2X PCR mix; Promega, Beijing,
China), and the PCR products were subsequently run on a 2% agarose
gel. The PCR product of the homozygous mice was 174 bp, the
heterozygous mice PCR product was 174 bp and the WT mice product
was 298 bp. The primers used were as follows: Common (sequence no.
8060), 5′-CTGCTCTCTGCTCCCAGTCT-3′; WT reverse (sequence no. 8061),
5′-ATACCCCATCCCTTTTGAGC-3′; mutant reverse (oIMR9402)
5′-GAACTTCAGGGTCAGCTTGC-3′. The PCR system and PCR reaction
conditions were in accordance with the protocols of the Jackson
Laboratory (Bar Harbor, ME, USA).
Collection of tissue specimens
Lgr5 expression was evaluated in a total of 42
primary non-small cell lung cancers (NSCLCs) and 28 matched normal
adjacent lung tissue samples by immunohistochemistry (IHC). The 42
samples included 22 lung adenocarcinoma samples and 20 squamous
cell carcinoma samples. In addition, a tissue microarray (TMA) that
contained 80 cases of lung adenocarcinoma was also included.
Therefore, the total number of lung adenocarcinoma cases was 102,
whereas the total number of squamous cell carcinoma samples was 20.
The clinical features of the patients with NSCLC are outlined in
Table I. All specimens were collected
from patients who were admitted to the Department of Thoracic
Surgery, Sun Yat-Sen University Cancer Center, (Guangzhou, China)
between October 9, 2009 and July 12, 2014 with patient consent and
institutional review board approval. All patients signed informed
consent for the use of their clinical specimens for medical
research. The samples in this study were approved by the Committee
for Ethical Review of Research at Sun Yat-Sen University.
 | Table I.Correlation between Lgr5 expression
and the clinical and pathological characteristics of 122 cases of
primary non-small cell lung cancer. |
Table I.
Correlation between Lgr5 expression
and the clinical and pathological characteristics of 122 cases of
primary non-small cell lung cancer.
|
|
| Lgr5 expression
(%) |
|
|---|
|
|
|
|
|
|---|
| Clinical feature | No. patients | Negative (%) | Positive (%) | P-value |
|---|
| Gender |
|
|
| 0.269 |
| Male | 75 | 49 (65.3) | 26 (34.7) |
|
|
Female | 47 | 26 (55.3) | 21 (44.7) |
|
| Age |
|
|
| 0.421 |
|
≤60-years-old | 68 | 46 (67.6) | 22
(32.4) |
|
|
>60-years-old | 64 | 39 (60.9) | 25 (39.1) |
|
| Tumor
sizea,b |
|
|
| 0.075 |
| ≤5
cm3 | 59 | 31 (52.5) | 27 (47.5) |
|
| >5
cm3 | 63 | 43 (84.10) | 20 (15.9) |
|
| Tumor
invasionc |
|
|
| 0.351 |
| T1 | 12 | 6 (50.0) | 6 (50.0) |
|
| T2 | 65 | 28 (43.1) | 37 (56.9) |
|
| T3 | 45 | 27 (60.0) | 18 (40.0) |
|
| Lymph node
metastasis |
|
|
| 0.569 |
| N0 | 82 | 52 (63.4) | 30 (36.6) |
|
| N1 | 40 | 23 (57.5) | 17 (42.5) |
|
| AD and SS |
|
|
| <0.001 |
|
Adenocarcinoma | 102 | 55 (53.9) | 47 (46.1) |
|
|
Squamous | 20 | 20 (100) | 0 (0.0) |
|
| TNM
staged |
|
|
| 0.026 |
| I–II | 81 | 56 (69.1) | 25 (30.9) |
|
|
III–IV | 41 | 19 (46.3) | 22 (53.7) |
|
Histology
The paraffin embedded tissues were sectioned at a
thickness of 3–5 µm, and hematoxylin and eosin (H&E) staining
was performed, as previously described (13). Briefly, IHC was performed as follows:
Antigen retrieval with ethylenediaminetetraacetic acid solution (pH
9.0), incubation of the slides in 3% H2O2 at
room temperature for 12 min, followed by washes and the blocking of
non-specific proteins. The antibody was applied to the slides,
which were subsequently incubated at 4°C overnight. The tissue
sections were washed again and the secondary antibody was applied
to the slides. Finally, diaminobenzidine was used to visualize the
positive staining on the slides. The primary Lgr5 antibody was used
at a dilution of 1:100 (cat. no. sc-135238; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The anti-rabbit
secondary antibody was conjugated to horseradish peroxidase and was
used at a dilution of 1:500 (cat. no. GK500705). The
diaminobenzidine chromogen system was purchased from Gene Tech Co.,
Ltd. (Shanghai, China). The slides were viewed on a BX51 Olympus
microscope (Olympus Corporation, Shibuya, Japan).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism software version 5 (Graphpad Software, CA, USA). The
χ2 test or Fisher's exact test was used to analyze the
association of Lgr5 expression with the clinicopathological
parameters of the patients. The survival curves were plotted
according to the Kaplan-Meier method to evaluate the Lgr5
expression levels with respect to the survival rate. The
multivariate survival analyses were performed by a Cox proportional
hazard model using the Wald test. P<0.05 was considered to
indicate a statistically significant difference..
Results
Lgr5-positive cells are present in
mouse lung tissue
Lgr5 is well-characterized as a marker of normal
stem cells in the intestine and as a marker of cancer stem cells.
The Lgr5 homozygous knockout in mice resulted in neonatal lethality
(12). The present study suggested
that Lgr5 serves an important role in development. However, the
role of Lgr5-positive cells in the lung remains unknown. To
investigate whether cells in normal mouse lung express Lgr5, normal
lung tissue was obtained from C57/BL WT mice and the expression of
Lgr5 was determined by IHC using a specific antibody. To confirm
that the antibody was specific, normal mouse intestine was stained.
The results are shown in Fig. 1A. In
the lung tissue, the results demonstrated that some Lgr5-positive
cells were present in the bronchi and alveoli (Fig. 1B and C). The present study next
determined whether the knockout of Lgr5 affected lung structure and
found that the structure of the lung tissue of Lgr5 heterozygous
knockout mice (Lgr5+/− mice) differed from that of the
WT mice. This finding indicated that the lung septa of
Lgr5+/− mice were thicker and that the lung structure
was irregular compared with the WT mice (Fig. 1E).
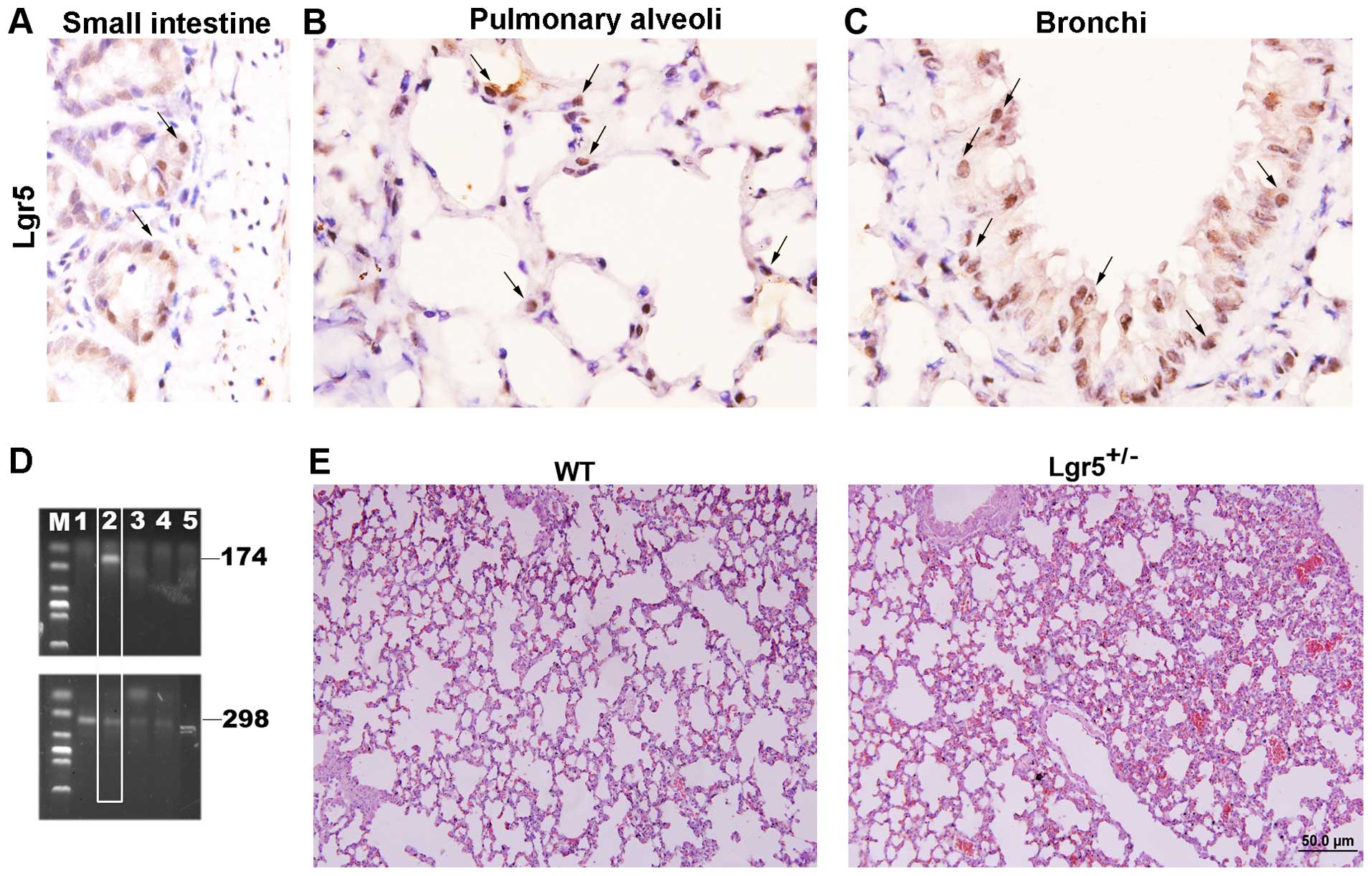 | Figure 1.Lgr5 expression in the mouse lung. (A)
The sensitivity of the Lgr5 antibody was confirmed by staining
intestinal tissue of WT mice. Lgr5-positive cells are indicated by
arrows. (B) A few Lgr5-positive cells are present in the alveoli of
mouse lung, as indicated by arrows (magnification, ×1,000). (C) A
few Lgr5-positive cells were present in the lung bronchi
(magnification, ×1,000), as indicated by arrows. (D) Identification
of Lgr5+/− mice was performed by genotyping. Bands of
298 bp and 174 bp were obtained by polymerase chain reaction
amplification and are indicative of the Lgr5+/−
genotype. Lanes 1 and 3–5 are WT mice and lane 2 is the
Lgr5+/− mouse. (E) Representative hematoxylin and eosin
staining of Lgr5+/− mouse lung and WT mouse lung tissue.
Interlobular septa of Lgr5+/− mouse lung are thickened
and abnormal hyperplasia in the bronchi and light pulmonary
congestion were also observed (magnification, ×200). WT, wild-type;
Lgr5, Leucine-rich repeat-containing G-protein coupled receptor 5;
bp, base pairs; M, marker. |
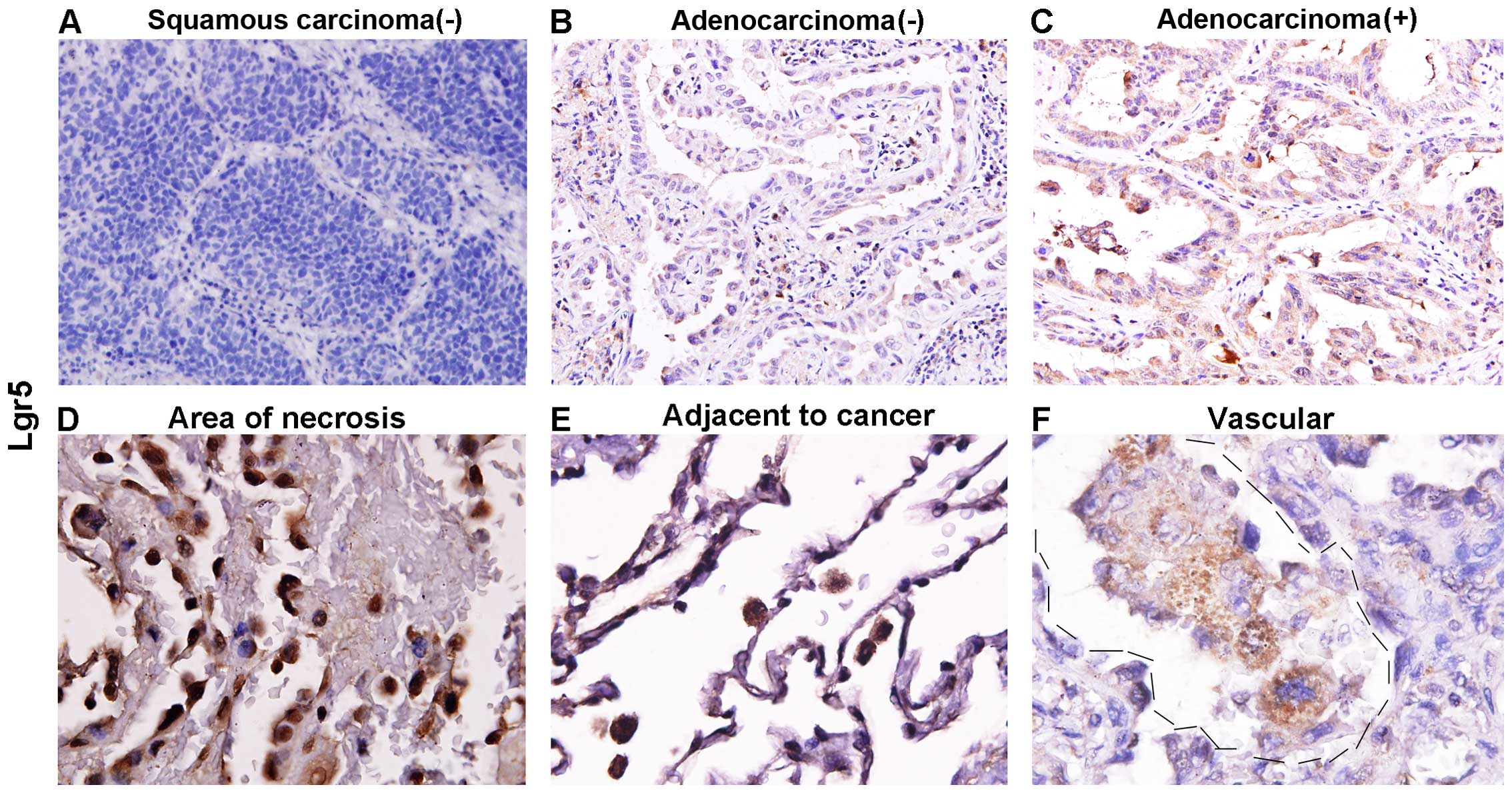
Lgr5 is expressed in lung
adenocarcinoma, but not in squamous carcinoma
Based on the above IHC and histology results, the
present study hypothesized that Lgr5 may serve a function in the
maintenance of the morphology of bronchi or alveoli in the normal
lung. It must be considered that lung tumor initiating cells are
partly derived from the bronchi and that Lgr5 is a well-known
marker of intestinal cancer stem cells. Therefore, in lung cancer,
the present study assumed that Lgr5 is associated with tumor
formation or to the tumor, node, metastasis (TNM) stage. Next, 122
lung cancer cases, including 102 cases of adenocarcinoma and 20
cases of squamous carcinoma, were subjected to IHC. The results
showed that Lgr5 is not expressed in squamous cell carcinoma (0/20;
P<0.001; Table I; Fig. 2A), however, is expressed in
adenocarcinoma (47/122). The representative image of the staining
is presented in Fig. 2B and C.
Notably, in certain cases, Lgr5-positive cells were present in
areas of tumor necrosis (Fig. 2D),
areas adjacent to the tumor (Fig.
2E), and the lymphatic spaces and vascular lumina (Fig. 2F).
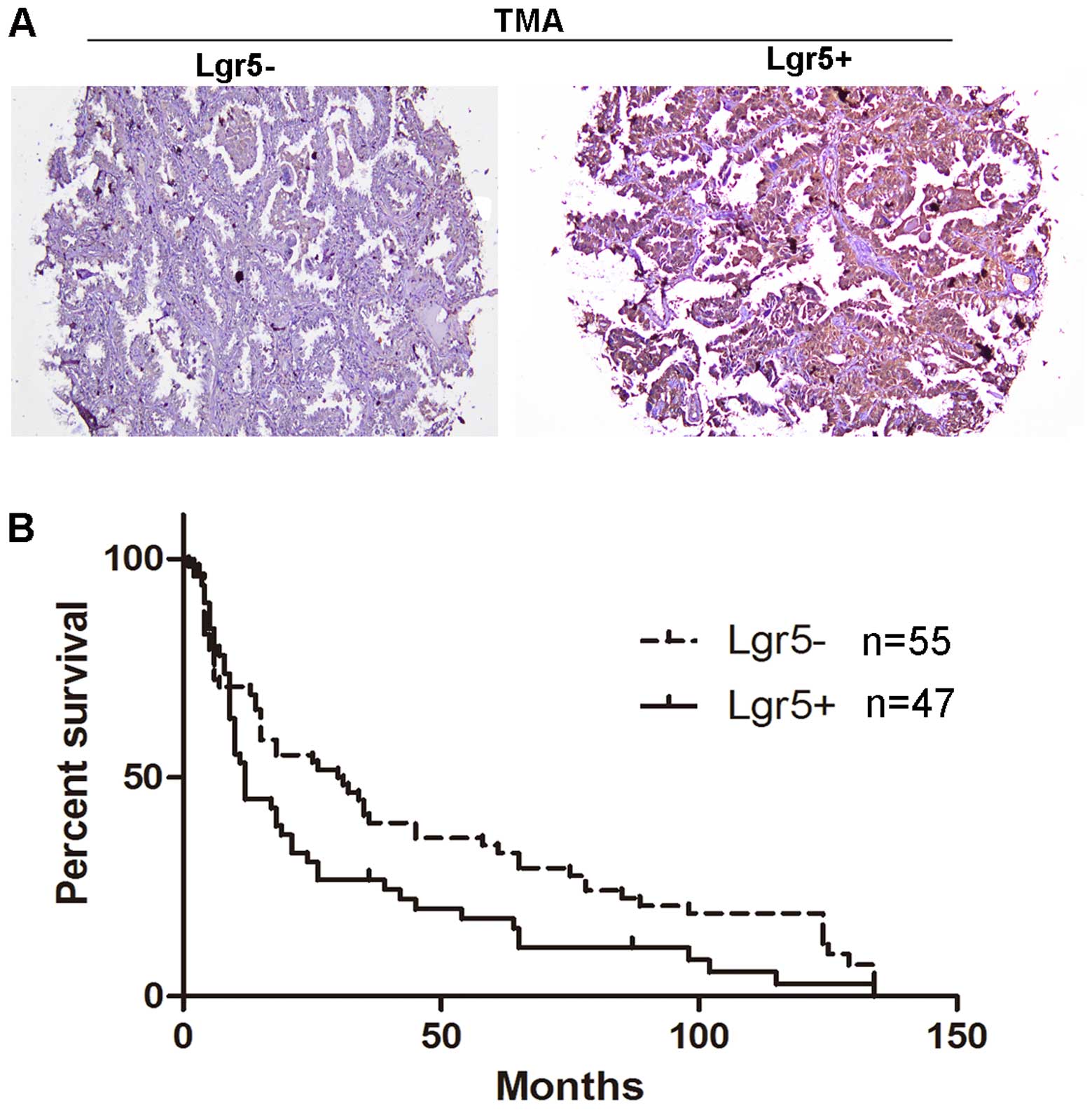
Survival analysis of patients with
lung adenocarcinoma as a function of Lgr5 expression
The above data showed that Lgr5 is expressed in lung
adenocarcinoma, but not in squamous carcinoma. Furthermore, the
present study stained one tissue microarray that included 60 cases
of lung adenocarcinoma to assess its expression by IHC using an
Lgr5 antibody. These cases were classified into two groups as
either ‘+’ or ‘-’, as shown in the representative image in Fig. 3A. The positive group was indicated by
‘+’ and the negative group was indicated by ‘-’.
A survival analysis was performed for all patients
with lung adenocarcinoma (paraffin blocks, 22 cases; TMA, 80 cases)
using the Kaplan-Meier method. The follow-up periods ranged between
4 and 157 months. To evaluate the association between Lgr5
expression in lung adenocarcinoma cells and the clinicopathological
parameters, the data from the patients was summarized in Table I. Lgr5 expression was not associated
with tumor size (P=0.075). In addition, no significant association
was observed between Lgr5 expression and age, gender, tumor
invasion or lymph node metastasis. However, Lgr5 expression was
associated with TNM stage (P=0.026).
Additionally, the median survival was 30.5 months
for patients in the Lgr5-negative group (n=55) and 12 months (n=47)
for patients in the Lgr5-positive group, which indicated a
significantly poorer survival rate of the Lgr5-positive group
compared with the Lgr5-negative group (P=0.033). Next, univariable
and multivariable analyses were performed to assess the effect of
Lgr5 expression on survival. A Cox proportional hazards regression
model was subsequently applied and the effect of Lgr5 on survival
was estimated. The crude hazard ratio (HR) of the Lgr5-positive
tumors compared with the Lgr5-negative tumors was 1.618 [95%
confidence interval (CI), 1.06–2.48; P=0.027]. To estimate the
independent pro-diagnostic effect of Lgr5 on survival, the present
study adjusted for confounding factors. The present study involved
59 lung adenocarcinoma patients with lung cancer-associated
mortalities and 5 variables that were included in the multivariate
regression model. To determine whether Lgr5 expression was a single
variable and not a potential confounding factor, a propensity score
was applied. The five variables were clinicopathological factors,
including age, tumor size, TNM stage, tumor invasion and
metastasis. The adjusted HR of the Lgr5-positive group was 0.396
(95% CI, −0.28–1.06; P=0.15) compared with the Lgr5-negative group,
which suggested that Lgr5 expression is not an independent risk
factor for poorer survival (Table
II).
 | Table II.Univariate and multivariate analyses
of the overall survival rate and the clinicopathological parameters
of 122 patients with lung cancer using the Cox proportional hazards
regression model. |
Table II.
Univariate and multivariate analyses
of the overall survival rate and the clinicopathological parameters
of 122 patients with lung cancer using the Cox proportional hazards
regression model.
|
|
Univariatea |
Multivariateb |
|---|
|
|
|
|
|---|
| Factors | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age | 0.136 | 1.32
(0.944–2.435) | 0.094 |
|
≤60-years-old |
|
|
|
|
>60-years-old |
|
|
|
| Tumor size | 0.032 | 1.12
(0.832–1.482) | 0.696 |
| ≤10
cm3 |
|
|
|
| >10
cm3 |
|
|
|
| Tumor invasion | 0.033 | 1.58
(0.951–2.810) |
0.173 |
| T1 |
|
|
|
| T2 |
|
|
|
| T3 |
|
|
|
| Lymph node
metastasis | 0.001 | 1.812
(0.611–4.650) | 0.308 |
| N0 |
|
|
|
| N1 |
|
|
|
| TNM stage | 0.005 | 0.861
(0.470–2.667) | 0.897 |
|
I–II |
|
|
|
|
III–IV |
|
|
|
| Lgr5 | 0.076 | 3.36
(1.24–10.63) | 0.036 |
|
Negative |
|
|
|
|
Positive |
|
|
|
Discussion
Lgr5 is regarded as a marker of intestinal stem
cells, which has attracted a significant amount of attention to
this protein (8,14–19).
However, its role in the lungs and in lung cancer remains unclear
(10). The present study demonstrated
for the first time, to the best of our knowledge, that the lungs of
Lgr5 heterozygous mice exhibited structural abnormalities.
Specifically, the pulmonary interstitium was thickened according to
H&E staining. Additionally, Lgr5-positive cells were found in
the mouse lung, including the alveoli. In addition, a few cells
expressing Lgr5 were also found in the basal layer of the bronchial
tissue. The above results suggested that Lgr5 may serve a
significant role in the lung bronchi and alveoli. However, a
determination of the function of these positive cells requires
further clarification.
In both the human and mouse gut, Lgr5-positive cells
located in the intestinal crypts protect Paneth cells and are
regarded as tumor stem cells (18).
In the present study, it was revealed that certain cells in normal
lung tissue express Lgr5. This finding led us to question whether
Lgr5 serves a role in normal lung tissue or if it is associated
with lung cancer. Certain lung cancer cells are derived from the
lung bronchi; therefore, Lgr5 expression in lung tumor tissues from
patients with lung cancer was examined. Notably, Lgr5 is only
expressed in lung adenocarcinoma and its expression is associated
with TNM stage; however, Lgr5 expression not an independent factor
for survival. In another study, the Lgr5-positive rate in lung
adenocarcinoma was ~1/10 (11).
However, in the present study, the rate reached ~50%. However, as
the reasons that underlie this difference are unknown, additional
studies are required for clarification.
In previous studies, researchers have demonstrated
that Lgr5 expression is associated with colorectal tumorigenesis
and even colorectal cancer recurrence (20). Previous studies have also reported
that Lgr5 expression is correlated with tumor invasion and
metastasis (15,21,22).
However, in the present experiments, the data did not indicate this
phenomenon in lung adenocarcinoma. This difference may be because
Lgr5 serves different roles in lung tumors compared with intestinal
tumors. Notably, the presence of Lgr5-positive cells in lymphatic
vessels were observed. Therefore, more experiments are required to
investigate whether Lgr5 expression is associated with tumor
metastasis (Fig. 3C).
In conclusion, the present study provided novel
insights into the role of Lgr5 in normal lung tissue and lung
cancer. It will be conducive for us to learn the role and function
of Lgr5 in lung cancer.
Acknowledgements
The authors would like to thank the staff at the
Department of Pathology, Sun Yat-Sen University Cancer Center
(Guangzhou, China) for support in the collection of the clinical
samples. The present study was supported by the National Natural
Science Foundation of China (nos. 81472336 and 31471290), the
academic and professional development funds of the Guangdong
Provincial Department of Education (no. 2013KJCX0108) and the
non-profit foundation of Guangdong Province in China (nos.
2014A020212313 and 2015A030302086).
References
|
1
|
Hsu SY, Liang SG and Hsueh AJ:
Characterization of two LGR genes homologous to gonadotropin and
thyrotropin receptors with extracellular leucine-rich repeats and a
G protein-coupled, seven-transmembrane region. Mol Endocrinol.
12:1830–1845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sukhdeo K, Koch CE, Miller TE, Zhou H,
Rivera M, Yan K, Cepko CL, Lathia JD and Rich JN: The Lgr5
transgene is expressed specifically in glycinergic amacrine cells
in the mouse retina. Exp Eye Res. 119:106–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vroegindeweij E, van Mourik I, Cupedo T
and Cornelissen JJ: Characterization of Lgr5-positive epithelial
cells in the murine thymus. Eur J Immunol. 43:1243–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Lau W, Barker N, Low TY, Koo BK, Li VS,
Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M,
et al: Lgr5 homologues associate with Wnt receptors and mediate
R-spondin signalling. Nature. 476:293–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huch M, Dorrell C, Boj SF, van Es JH, Li
VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, et
al: In vitro expansion of single Lgr5+ liver stem cells induced by
Wnt-driven regeneration. Nature. 494:247–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flanagan DJ, Phesse TJ, Barker N, Schwab
RH, Amin N, Malaterre J, Stange DE, Nowell CJ, Currie SA, Saw JT,
et al: Frizzled7 functions as a Wnt receptor in intestinal
epithelial Lgr5(+) stem cells. Stem Cell Reports. 4:759–767. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ, et al: Identification of stem cells in small intestine
and colon by marker gene Lgr5. Nature. 449:1003–1007. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Snippert HJ, van der Flier LG, Sato T, van
Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van
Rheenen J, Simons BD and Clevers H: Intestinal crypt homeostasis
results from neutral competition between symmetrically dividing
Lgr5 stem cells. Cell. 143:134–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao F, Zhou B, Xu JC, Gao X, Li SX, Zhu
GC, Zhang XG and Yang C: The role of LGR5 and ALDH1A1 in non-small
cell lung cancer: Cancer progression and prognosis. Biochem Biophys
Res Commun. 462:91–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryuge S, Sato Y, Jiang SX, Wang G,
Kobayashi M, Nagashio R, Katono K, Iyoda A, Satoh Y and Masuda N:
The clinicopathological significance of Lgr5 expression in lung
adenocarcinoma. Lung Cancer. 82:143–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morita H, Mazerbourg S, Bouley DM, Luo CW,
Kawamura K, Kuwabara Y, Baribault H, Tian H and Hsueh AJ: Neonatal
lethality of LGR5 null mice is associated with ankyloglossia and
gastrointestinal distension. Mol Cell Biol. 24:9736–9743. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takeda K, Kinoshita I, Shimizu Y, Matsuno
Y, Shichinohe T and Dosaka-Akita H: Expression of LGR5, an
intestinal stem cell marker, during each stage of colorectal
tumorigenesis. Anticancer Res. 31:263–270. 2011.PubMed/NCBI
|
|
15
|
He S, Zhou H, Zhu X, Hu S, Fei M, Wan D,
Gu W, Yang X, Shi D, Zhou J, et al: Expression of Lgr5, a marker of
intestinal stem cells, in colorectal cancer and its
clinicopathological significance. Biomed Pharmacother. 68:507–513.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Metcalfe C, Kljavin NM, Ybarra R and de
Sauvage FJ: Lgr5+ stem cells are indispensable for
radiation-induced intestinal regeneration. Cell Stem Cell.
14:149–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buczacki SJ, Zecchini HI, Nicholson AM,
Russell R, Vermeulen L, Kemp R and Winton DJ: Intestinal
label-retaining cells are secretory precursors expressing Lgr5.
Nature. 495:65–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato T, van Es JH, Snippert HJ, Stange DE,
Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M
and Clevers H: Paneth cells constitute the niche for Lgr5 stem
cells in intestinal crypts. Nature. 469:415–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Becker L, Huang Q and Mashimo H:
Immunostaining of Lgr5, an intestinal stem cell marker, in normal
and premalignant human gastrointestinal tissue.
ScientificWorldJournal. 8:1168–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuji S, Kawasaki Y, Furukawa S, Taniue K,
Hayashi T, Okuno M, Hiyoshi M, Kitayama J and Akiyama T: The
miR-363-GATA6-Lgr5 pathway is critical for colorectal
tumourigenesis. Nat Commun. 5:31502014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kleist B, Xu L, Li G and Kersten C:
Expression of the adult intestinal stem cell marker Lgr5 in the
metastatic cascade of colorectal cancer. Int J Clin Exp Pathol.
4:327–335. 2011.PubMed/NCBI
|
|
22
|
Wang Y, Jiang CQ and Fan LF: Correlation
of Musashi-1, Lgr5, and pEGFR expressions in human small intestinal
adenocarcinomas. Tumour Biol. 36:6075–6082. 2015. View Article : Google Scholar : PubMed/NCBI
|