Introduction
Lateral pelvic lymph nodes (LPLNs) are a common site
of metastasis in patients with middle-low rectal cancer. Previous
studies have indicated that, worldwide, LPLN metastasis (LPLNM)
ranges from 8.6 to 21.0% for rectal cancer (1-3).
Moreover, a retrospective study in Japan showed that in patients
with T3/T4 rectal cancer below the peritoneal reflection, lateral
lymph node dissection (LLND) could theoretically reduce local
recurrence by 50.3% and improve 5-year survival by 8% (4). However, LLND surgery is associated
with a long operation time, sizable blood loss and frequent
postoperative complications (5-9).
Furthermore, in the past 30 years, since preoperative neoadjuvant
chemoradiotherapy (CRT) + total mesorectal excision (TME) surgery
has become the standard treatment strategy for locally advanced
rectal cancer, local recurrence has been well controlled.
Therefore, the use of preventive LLND is controversial (10).
Despite the lack of data to support this, recent
treatment patterns have converged toward performing LLND in cases
with clinical suspected LPLNM in both Western and Eastern countries
(10,11). However, a clear consensus is
required on the indications for selective LLND. It is important to
determine the clinical characteristics of rectal cancer with
pathological LPLNM, which can guide precise treatment with
selective LLND and identify patients who may benefit from this
surgical procedure.
The present study retrospectively analyzed 64
patients who underwent TME + LLND surgery, and aimed to identify
the clinical characteristics of pathological LPLNM and to determine
predictive factors guiding pre-treatment decisions.
Materials and methods
Patients and treatment strategies
Between February 2019 and April 2024, 64 patients
received TME + LLND surgery at the Department of Colorectal
Surgery, Zhangzhou Municipal Hospital Affiliated of Fujian Medical
University (Zhangzhou, China). The patients were pathologically
confirmed as having rectal cancer and had suspected clinical LPLNM
based on preoperative MRI. The criteria for clinically suspicious
LPLNM were as follows: i) Short-axis diameter of LPLN ≥5 mm; and
ii) high-risk factors detected by MRI evaluation, such as irregular
shape and rough edges, heterogeneous or intense enhancement of
LPLN.
In the present study, patients with cT3-4 stage, cN2
stage, a short-axis diameter of LPLN ≥1.0 cm or mesorectal fascia
involvement were subjected to neoadjuvant therapy (chemotherapy or
CRT). The choice of neoadjuvant treatment, such as chemotherapy or
CRT, was determined by the wishes of the patient and through
multidisciplinary team meetings, including radiologists, and
medical and surgical oncologists. Surgery was performed 6-8 weeks
after the completion of CRT. Patients with pathological stage
III/IV, or with high-risk stage II (pT4, tumor perforation,
lymphatic invasion, perineural invasion) underwent adjuvant
chemotherapy after surgery (CAPE-OX or mFOLFOX6, 6 months).
In the present study, the LPLN was defined as the
lymph node located in the lateral pelvic area, including three
regions: The obturator nodes (283N), internal iliac nodes (263N)
and external iliac nodes (293N). Briefly, the scope of LLND surgery
was ureterohypogastric nerve fascia as the inner boundary,
vesicohypogastric fascia as the caudal boundary, pelvic wall fascia
as the lateral boundary, and iliac vessel bifurcation as the
cephalic boundary. All patients underwent a laparoscopic
procedure.
The Ethics Committee of Zhangzhou Hospital
Affiliated of Fujian Medical University (Zhangzhou, China) approved
this retrospective study (approval no. 2023LWB289), and it
conformed to the ethical standards of the World Medical Association
Declaration of Helsinki.
Data collection
Information regarding patient demographics, tumor
characteristics and clinical outcomes was obtained. In addition,
body mass index (BMI), LPLN size, tumor distance from the anal
verge and neoadjuvant therapy data were collected. All data were
obtained from the medical records of the patients.
The present study used MRI to detect and evaluate
LPLNM. The short-axis diameter of the largest LPLN assessed by MRI
was measured and set as a representative value. Tumor staging was
performed using the American Joint Committee on Cancer staging
system (8th edition) (12), based
on the available information after surgery (pTNM).
Statistical analysis
Categorical and continuous variables were compared
using the χ2 test and unpaired Student's t-test
respectively. Univariate and multivariate logistic regression
analyses were used to analyze risk factors of pathological LPLNM.
Multivariate analysis was performed on factors with a significant
effect (P<0.1) in the univariate analysis, and the effect of
each variable was assessed using the hazard ratio (HR) and 95%
confidence interval (95% CI). Receiver operating characteristic
(ROC) curve analysis was performed for the initial LPLN size. SPSS
(version 20.0; IBM Corp.) was used to perform all analyses and
GraphPad Prism (version 10.0; Dotmatics) was used to draw diagrams.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics of
patients
Patient details are shown in Table I. A total of 64 patients (average
age, 58.9±11.1 years; age range, 35-83 years; 37 men and 27 women)
were included. The distance of the tumor from the anal verge was
5.5±1.9 cm (range, 1-11 cm). Of the 64 patients, 28 (43.8%)
received neoadjuvant therapy, of which 16 received chemotherapy and
11 received CRT. The average initial LPLN size was 9.7±4.9 mm
(range, 4.5-27.0 mm), and the average number of LPLNs harvested was
7.9±5.2 (range, 0-30). Of the 64 patients with suspected LPLNM
under clinical criteria, 50 (78.1%) had a short-axis LPLN diameter
of ≥5 mm); 38 (59.4%) had high-risk factors, such as irregular
shape and rough edges, and heterogeneous or intense enhancement.
Finally, 24 cases of LPLNM were confirmed by pathology (37.5%).
 | Table IBaseline characteristics of
patients. |
Table I
Baseline characteristics of
patients.
| Characteristic | Values |
|---|
| Sex, n (%) | |
|
Male | 37 (57.8%) |
|
Female | 27 (42.2%) |
| Mean ± SD age, years
(range) | 58.9±11.1
(35-83) |
| Mean ± SD BMI,
kg/m2 (range) | 23.0±2.3
(18.2-28.1) |
| Mean ± SD distance of
tumor from the AV, cm (range) | 5.5±1.9 (1-11) |
| Rectal tumor
location, n (%) | |
|
>5 cm
from AV | 25 (39.1%) |
|
≤5 cm from
AV | 39 (60.9%) |
| Mean ± SD initial
LPLN sizea, mm
(range) | 9.7±4.9
(4.5-27.0) |
| Diagnostic criteria
for clinical | |
| LPLNM, n (%) | |
|
≥5 mm | 50 (78.1%) |
|
Imaging risk
factors | 38 (59.4%) |
| Mean ± SD CEA, ng/ml
(range) | 7.4±8.3
(1.3-56.0) |
| cT stage, n (%) | |
|
cT1-2 | 15 (23.4%) |
|
cT3-4 | 49 (76.6%) |
| cN stage, n (%) | |
|
cN0 | 36 (56.3%) |
|
cN1-2 | 28 (43.7%) |
| Neoadjuvant therapy,
n (%) | |
|
None | 37 (57.8%) |
|
Chemotherapy | 16 (25.0%) |
|
Chemoradiotherapy | 11 (17.2%) |
| Mean ± SD LPLN
harvested (range) | 7.9±5.2 (0-30) |
| Positive LPLN, n
(%) | 24 (37.5%) |
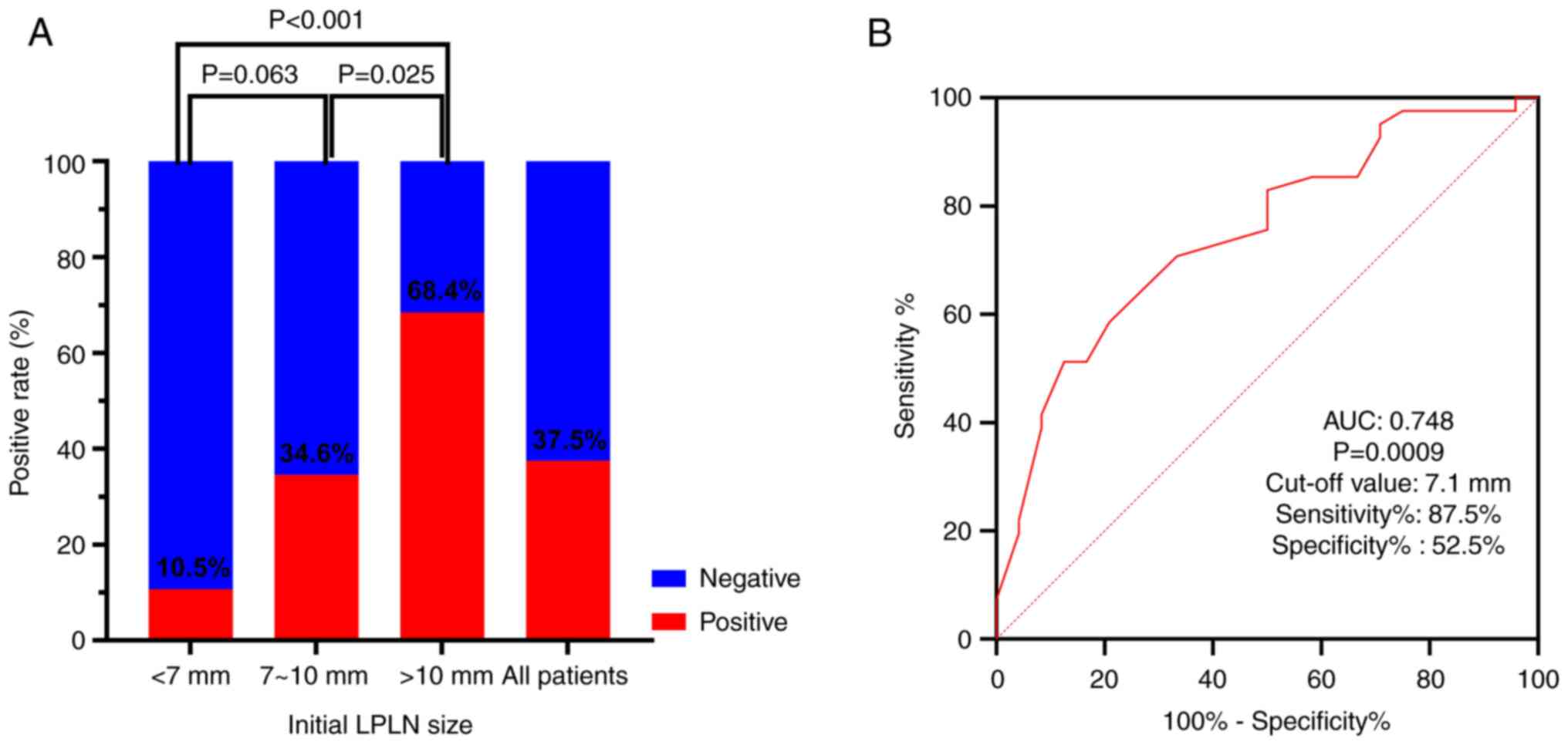
Pathological LPLNM is related to
initial lymph node size
Pathological LPLNM was revealed to be related to
initial lymph node size (Fig. 1A).
When the initial LPLN size was <7 mm, the pathological LPLNM
rate was 10.5%, whereas when the LPLN was between 7 and 10 mm, the
rate was 34.6%, and when the LPLN size was >10 mm, the rate was
68.4%. Furthermore, ROC curve analysis was performed for the
initial LPLN size. The area under the curve was 0.748 (P=0.0009;
Fig. 1B). When the cut-off initial
LPLN size was 7.1 mm, the sensitivity and specificity were 87.5 and
52.5%, respectively.
Clinical characteristics of
pathological LPLNM
The clinical characteristics of the patients in the
positive and negative LPLN groups are shown in Table II. After analyzing sex, age, BMI,
tumor distance from the anal verge, neoadjuvant therapy, average
initial LPLN size, cT/N stage and numbers of LPLNs, it was revealed
that initial LPLN size and cN stage were statistically different
between the positive and negative LPLN groups. The average initial
LPLN size in the positive LPLN group was bigger than that in the
negative LPLN group (12.1±5.6 mm vs. 8.3±4.0 mm; P<0.05). In
addition, the rates of cN1-2 in the positive LPN group were higher
than those in the negative LPLN group (P<0.05).
 | Table IIClinical characteristics of positive
and negative LPLN groups. |
Table II
Clinical characteristics of positive
and negative LPLN groups.
| Characteristic | Positive LPLN
(n=24) | Negative LPLN
(n=40) | P-value |
|---|
| Sex, n (%) | | | 0.069 |
|
Male | 10 (41.7%) | 26 (65.0%) | |
|
Female | 14 (58.3%) | 14 (35.0%) | |
| Mean ± SD age, years
(range) | 58.3±12.0
(38-83) | 59.2±10.7
(35-78) | 0.759 |
| Mean ± SD BMI,
kg/m2 (range) | 22.9±2.1
(18.2-26.2) | 23.1±2.4
(18.9-28.1) | 0.857 |
| Mean ± SD distance of
tumor from the AV, cm (range) | 5.4±1.8 (1-10) | 5.6±1.0 (1-11) | 0.731 |
| Rectal tumor
location, n (%) | | | 0.074 |
|
>5 cm
from AV | 6 (25.0%) | 19 (47.5%) | |
|
≤5 cm from
AV | 18 (75.0%) | 21 (52.5%) | |
| Mean ± SD initial
LPLN sizea, mm
(range) | 12.1±5.6
(5.0-27.0) | 8.3±4.0
(4.5-25.0) | 0.003 |
| Imaging risk factors,
n (%) | | | 0.693 |
|
No | 9 (37.5%) | 17 (42.5%) | |
|
Yes | 15 (62.5%) | 23 (57.5%) | |
| Mean ± SD CEA,
ng/ml (range) | 8.5±12.0
(1.3-56.0) | 6.7±4.9
(1.7-24.6) | 0.411 |
| cT stage, n
(%) | | | 0.819 |
|
cT1-2 | 6 (25.0%) | 9 (22.5%) | |
|
cT3-4 | 18 (75.0%) | 31 (77.5%) | |
| cN stage, n
(%) | | | 0.004 |
|
cN0 | 8 (33.3%) | 28 (70.0%) | |
|
cN1-2 | 16 (66.7%) | 12 (30.0%) | |
| Neoadjuvant
therapy, n (%) | | | 0.835 |
|
No | 13 (54.2%) | 24 (60.0%) | |
|
Chemotherapy | 7 (29.2%) | 9 (22.5%) | |
|
Chemoradiotherapy | 4 (16.7%) | 7 (17.5%) | |
| Mean ± SD LPLN
harvested (range) | 8.6±6.4 (1-30) | 7.6±4.4 (0-20) | 0.517 |
Univariate and multivariate logistic
regression analyses of preoperative factors associated with
pathological LPLNM
In the univariate analysis of risk factors, initial
LPLN size (≥7.1 mm; HR=7.737, 95%CI 1.987-30.132, P=0.003) and cN
stage (N1-2; HR=4.667, 95% CI 1.577-13.813, P=0.005) were
significantly associated with pathological LPLNM (Table III). Notably, male sex may also
represent a potential risk factor for pathological LPLNM; however,
this was not significant (P=0.072). In the multivariate analysis of
risk factors, initial LPLN size (≥7.1 mm; HR=4.856, 95% CI
1.158-20.359, P=0.031) was the only independent risk factor for
pathological LPLNM.
 | Table IIIUnivariate and multivariate analyses
of preoperative factors associated with pathological LPLN
metastasis. |
Table III
Univariate and multivariate analyses
of preoperative factors associated with pathological LPLN
metastasis.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex
(male/female) | 2.600
(0.919-7.353) | 0.072 | 2.288
(0.707-7.412) | 0.167 |
| Age, (<60/≥60
years) | 0.944
(0.415-2.834) | 0.919 | | |
| Rectal tumor
distance from AV (>5/≤5 cm) | 2.714
(0.892-8.261) | 0.079 | | |
| BMI (<23/≥23
kg/m2) | 1.353
(0.490-3.739) | 0.560 | | |
| Imaging risk
factors (No/Yes) | 1.232
(0.437-3.476) | 0.694 | | |
| Initial LPLN
sizea,
(<7.1/≥7.1 mm) | 7.737
(1.987-30.132) | 0.003 | 4.856
(1.158-20.359) | 0.031 |
| CEA (≤5/>5
ng/ml) | 0.443
(0.157-1.251) | 0.124 | | |
| cT stage
(T1-2/T3-4) | 0.871
(0.266-2.849) | 0.819 | | |
| cN stage
(N0/N1-2) | 4.667
(1.577-13.813) | 0.005 | 3.185
(0.960-10.569) | 0.058 |
| Neoadjuvant therapy
(No/Yes) | 1.269
(0.457-3.528) | 0.648 | | |
| MRF (-/+) | 1.491
(0.401-5.543) | 0.551 | | |
| EMVI (-/+) | 2.000
(0.634-6.311) | 0.237 | | |
Association of pathological LPLNM rate
with LPLN size and cN
Table IV shows the
sensitivity, specificity, and positive and negative predictive
values for diagnosing LPLNM based on different diagnostic criteria.
Regarding the initial LPLN size, with a cut-off value of ≥7.1 mm,
the sensitivity for LPLNM was 87.5%, but the specificity was only
52.5%. When both LPLN size ≥7.1 mm and cN1-2 criteria were met, the
sensitivity was 66.7%, the specificity increased to 77.5%, and the
positive and negative predictive values were 64.0 and 79.5%,
respectively.
 | Table IVDiagnosis of LPLN metastasis
according to the initial LPLN size and cN stage in all patients
(n=64). |
Table IV
Diagnosis of LPLN metastasis
according to the initial LPLN size and cN stage in all patients
(n=64).
| Value |
Sensitivitya |
Specificityb | Positive predictive
valuec | Negative predictive
valued | Pathological
positivity ratee |
|---|
| LPLN size ≥7.1
mm | 87.5% (21/24) | 52.5% (21/40) | 52.5% (21/40) | 87.5% (21/24) | 52.5% (21/40) |
| cN1-2 | 66.7% (16/24) | 70.0% (28/40) | 57.1% (16/28) | 77.8% (28/36) | 57.1% (16/28) |
| LPLN size ≥7.1 mm
and cN1-2 | 66.7% (16/24) | 77.5% (31/40) | 64.0% (16/25) | 79.5% (31/39) | 69.6% (16/23) |
Furthermore, the pathological positivity rate of
different conditions was analyzed in 64 patients. Those with LPLN
≥7.1 mm and cN1-2 had a pathological positivity rate of 69.6%
(16/23). Those with LPLN <7.1 mm and cN1-2 had a pathological
positivity rate of 0% (0/5), while those with LPLN ≥7.1 mm and cN0
had a pathological positivity rate of 29.4% (5/17).
Discussion
In recent years, with the increase in evidence, and
the results of multi-center studies in Asia, Europe and the United
States (13-16),
TME + selective LLND surgery has been a mainstream strategy for the
treatment of rectal cancer with suspected LPLNM based on MRI or CT.
Although selective LLND can effectively enhance the detection rate
of LPLNM, the consideration of surgical trauma and associated risks
has necessitated a discussion on accurately selecting the
appropriate patient population for this treatment. The present
study conducted a retrospective analysis of 64 patients who
underwent selective LLND, aiming to characterize the clinical
features of pathological LPLNM for guiding pre-treatment
decisions.
The strongest predictor of LPLNM known to date is
LPLN size; however the size criteria vary between 5 and 10 mm, and
the pathological positivity rate between 7.3 and 34.3% (17-20).
Notably, the results vary widely and are controversial. In the
present study, patients with a LPLN size of ≥5 mm or with imaging
risk factors identified by preoperative MRI evaluation were
suspected of having clinical metastasis. Overall, the pathological
positivity rate of LPLNM by surgery was 37.5%. Moreover,
pathological LPLNM was related to initial LPLN size. When the
initial LPLN size was <7 mm, the pathological LPLNM rate was
10.5%, whereas when the LPLN size was 7-10 mm, the rate was 34.6%,
and when the LPLN size was >10 mm, the rate was 68.4%. The
results of the ROC curve analysis of initial LPLN sizes revealed
that the sensitivity and specificity were 87.5 and 52.5%,
respectively, when the cut-off initial LPLN size was 7.1 mm.
Furthermore, the multivariate analysis identified initial LPLN size
(≥7.1 mm) as the only independent risk factor for pathological
LPLNM (HR=4.856, 95% CI 1.158-20.359, P=0.031). The current study
revealed that an initial LPLN size of ≥7.1 mm may be a better
indicator for selective LLND than 5 mm. A study from Korea found
that an initial LPLN of 8 mm had the best sensitivity and
specificity (21). The sensitivity
for LPN metastasis was 100% with a cut-off value of 6 mm for the
initial LPN size, but the specificity was only 24.6%, whereas the
sensitivity and specificity were 94.4 and 47.8%, respectively, with
a cut-off value of 8 mm for the initial LPN size. Numerous studies
have revealed similar results, indicating that an initial LPLN of 5
mm may not be a good indicator for selective LLND (13,22-25).
It remains unclear as to whether initial LPLN size
or size post-treatment (chemotherapy or CRT) should be used as the
criterion. A previous report (17)
showed that LPLN size after CRT was a significant predictive factor
of LPLNM, but initial LPLN size was not. Another study (20) demonstrated that a LPLN size of ≥5
mm after CRT could be a cut-off value for selective LLND surgery.
The purpose of the present study was to accurately identify
pathological LPLNM when suspicious LPLNs were detected by MRI, and
then to arrange treatment strategies (surgery or CRT). The findings
revealed that the pathological positivity rate of initial LPLN size
(≥7 mm) was >30%, regardless of whether CRT was administered.
Considering such a high pathological positivity rate, it may be
acceptable to perform LLND surgery based on initial LPLN size.
Secondly, it was revealed that neoadjuvant therapy could not
completely kill tumor cells; 2/64 patients in the current study
were pathologically confirmed as having LPLNM, even though LPLN
size was 4 mm after neoadjuvant treatment. A similar finding was
also reported in a survey from Korea (26). Furthermore, there were a number of
cases in which neoadjuvant CRT was not performed for various
reasons, such as patient refusal, old age or comorbidity in
clinical practice. Therefore, it could be hypothesized that it is
more appropriate to utilize initial LPLN size as the criteria for
LPLND, rather than LPLN size after CRT.
Another preoperative risk factor for pathological
LPLNM was cN stage. In the univariate analysis of risk factors,
cN1-2 stage was significantly associated with pathological LPLNM
(HR=4.667, 95% CI 1.577-13.813, P=0.005); those with N1-2 had a
higher pathological positivity rate of LPLNM. However, there is a
clinical difficulty in estimating neoplasm staging accurately. Some
previous studies have reported that high-resolution MRI has a high
sensitivity (88%) and specificity (85%) in diagnosing LPLNM
(27,28). The combination of
diffusion-weighted imaging and thin-layer imaging has been
suggested to further improve the sensitivity and specificity of
diagnosis (29). The consensus of
Chinese experts recommend that high-resolution MRI is the preferred
diagnostic method for LPLNM (30).
Therefore, the current study balanced the complexity of patient
selection with the likelihood of benefit from treatment using MRI
evaluation as the selection criteria.
In the present study, initial LPLN size was the only
independent risk factor for pathological LPLNM, while cN was not.
However, the specificity was only 52.5% when a cut-off initial LPLN
size of 7.1 mm was set. Therefore, it is worth considering how to
judge positive LPLN more accurately in clinical decision-making.
When both LPLN size ≥7.1 mm and cN1-2 criteria were met, the
specificity increased to 77.5%. These findings indicated that
patients with an initial LPLN size of ≥7.1 mm and also with cN1-2
stage cancer, may benefit from TME + LLND surgery. Notably,
patients with LPLN size <7.1 mm and cN1-2 had a pathological
positivity rate of 0% (0/5), whereas those with LPLN size ≥7.1 mm
and cN0 had a pathological positivity rate of 29.6% (5/19). These
findings indicated that cN1-2 alone, without LPLN size or imaging
risk factors, is not enough to be the standard of diagnosis and to
decide on the treatment for LPLNM. These findings coincide with
those of the JCOG0212 trial, which showed that in patients with
advanced rectal cancer and a LPLN size of ≤10 mm, the rate of
pathological LPLNM was 7%; however, for those with a LPLN size
<5 mm, the pathological metastasis rate was only 5.2% (10).
The present study had several limitations. Firstly,
as the study was retrospective, inherent and unintentional
selection biases cannot be dismissed. Secondly, the study
population was too small to set the indications for LLND after
chemotherapy and CRT. In the future, individualized treatment based
on LPLN response after neoadjuvant therapy may be more precise.
Finally, pathological LPLNM is the most important factor in
formulating treatment strategies.
In conclusion, initial LPLN size and cN stage were
identified as significant clinical risk factors of pathological
LPLNM. Patients with an initial LPLN size of ≥7.1 mm and with cN1-2
stage cancer could benefit from TME + LLND surgery.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Startup Fund for
Scientific Research, Fujian Medical University (grant no.
2022QH1276).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XiajX, HS and MC contributed to the study conception
and design. YY and XiaozX acquired materials, and collected and
analyzed data. All authors substantially contributed to critically
reviewing the manuscript for important intellectual content. XiajX
and HS confirm the authenticity of all the raw data. YY prepared
the first draft of the manuscript and all authors commented on
previous versions of the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants adhered to the ethical standards of the institutional
and national research committee, and to The 1964 Declaration of
Helsinki and its later amendments or comparable ethical standards.
The study was approved by the Institutional Research Ethics
Committee of Zhangzhou Hospital Affiliated with Fujian Medical
University (approval no. 2023LWB289). As a retrospective study, the
requirement for informed consent was waived by the ethics
committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sato H, Maeda K and Maruta M: Prognostic
significance of lateral lymph node dissection in node positive low
rectal carcinoma. Int J Colorectal Dis. 26:881–889. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ishihara S, Kawai K, Tanaka T, Kiyomatsu
T, Hata K, Nozawa H, Morikawa T and Watanabe T: Oncological
outcomes of lateral pelvic lymph node metastasis in rectal cancer
treated with preoperative chemoradiotherapy. Dis Colon Rectum.
60:469–476. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nagasaki T, Akiyoshi T, Fujimoto Y,
Konishi T, Nagayama S, Fukunaga Y and Ueno M: Preoperative
chemoradiotherapy might improve the prognosis of patients with
locally advanced low rectal cancer and lateral pelvic lymph node
metastases. World J Surg. 41:876–883. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sugihara K, Kobayashi H, Kato T, Mori T,
Mochizuki H, Kameoka S, Shirouzu K and Muto T: Indication and
benefit of pelvic sidewall dissection for rectal cancer. Dis Colon
Rectum. 49:1663–1672. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Georgiou P, Tan E, Gouvas N, Antoniou A,
Brown G, Nicholls RJ and Tekkis P: Extended lymphadenectomy versus
conventional surgery for rectal cancer: A meta-analysis. Lancet
Oncol. 10:1053–1062. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moriya Y: Treatment of lateral pelvic
nodes metastases from rectal cancer: The future prospective. G
Chir. 34:245–248. 2013.PubMed/NCBI
|
|
7
|
Yano H and Moran BJ: The incidence of
lateral pelvic side-wall nodal involvement in low rectal cancer may
be similar in Japan and the West. Br J Surg. 95:33–49.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Kobayashi H, Mochizuki H, Kato T, Mori T,
Kameoka S, Shirouzu K and Sugihara K: Outcomes of surgery alone for
lower rectal cancer with and without pelvic sidewall dissection.
Dis Colon Rectum. 52:567–576. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim HJ, Choi GS, Park JS, Park SY, Lee HJ,
Woo IT and Park IK: Selective lateral pelvic lymph node dissection:
A comparative study of the robotic versus laparoscopic approach.
Surg Endosc. 32:2466–2473. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fujita S, Mizusawa J, Kanemitsu Y, Ito M,
Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, et al:
Mesorectal excision with or without lateral lymph node dissection
for clinical stage II/III lower rectal cancer (JCOG0212): A
multicenter, randomized controlled, noninferiority trial. Ann Surg.
266:201–207. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim MJ, Chang GJ, Lim HK, Song MK, Park
SC, Sohn DK, Chang HJ, Kim DY, Park JW, Jeong SY and Oh JH:
Oncological impact of lateral lymph node dissection after
preoperative chemoradiotherapy in patients with rectal cancer. Ann
Surg Oncol. 27:3525–3533. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Amin MB, Edge SB, Greene FL and Brierley
JD: AJCC cancer staging manual, 8th edition. New York: Springer,
2017.
|
|
13
|
Ogura A, Konishi T, Cunningham C,
Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee
P, et al: Neoadjuvant (chemo)radiotherapy with total mesorectal
excision only is not sufficient to prevent lateral local recurrence
in enlarged nodes: Results of the multicenter lateral node study of
patients with low cT3/4 rectal cancer. J Clin Oncol. 37:33–43.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kroon HM, Malakorn S, Dudi-Venkata NN,
Bedrikovetski S, Liu J, Kenyon-Smith T, Bednarski BK, Ogura A, van
de Velde CJH, Rutten HJT, et al: Local recurrences in western low
rectal cancer patients treated with or without lateral lymph node
dissection after neoadjuvant (chemo)radiotherapy: An international
multi-centre comparative study. Eur J Surg Oncol. 47:2441–2449.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kroon HM, Hoogervorst LA, Hanna-Rivero N,
Traeger L, Dudi-Venkata NN, Bedrikovetski S, Kusters M, Chang GJ,
Thomas ML and Sammour T: Systematic review and meta-analysis of
long-term oncological outcomes of lateral lymph node dissection for
metastatic nodes after neoadjuvant chemoradiotherapy in rectal
cancer. Eur J Surg Oncol. 48:1475–1482. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yang X, Yang S, Hu T, Gu C, Wei M, Deng X,
Wang Z and Zhou Z: What is the role of lateral lymph node
dissection in rectal cancer patients with clinically suspected
lateral lymph node metastasis after preoperative chemoradiotherapy?
A meta-analysis and systematic review. Cancer Med. 9:4477–4489.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Oh HK, Kang SB, Lee SM, Lee SY, Ihn MH,
Kim DW, Park JH, Kim YH, Lee KH, Kim JS, et al: Neoadjuvant
chemoradiotherapy affects the indications for lateral pelvic node
dissection in mid/low rectal cancer with clinically suspected
lateral node involvement: A multicenter retrospective cohort study.
Ann Surg Oncol. 21:2280–2287. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kawai K, Shiratori H, Hata K, Nozawa H,
Tanaka T, Nishikawa T, Murono K and Ishihara S: Optimal size
criteria for lateral lymph node dissection after neoadjuvant
chemoradiotherapy for rectal cancer. Dis Colon Rectum. 64:274–283.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Komori K, Fujita S, Mizusawa J, Kanemitsu
Y, Ito M, Shiomi A, Ohue M, Ota M, Akazai Y, Shiozawa M, et al:
Predictive factors of pathological lateral pelvic lymph node
metastasis in patients without clinical lateral pelvic lymph node
metastasis (clinical stage II/III): The analysis of data from the
clinical trial (JCOG0212). Eur J Surg Oncol. 45:336–340.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang P, Zhou S, Zhou H, Liang J and Zhou
Z: Evaluating predictive factors for determining the presence of
lateral pelvic node metastasis in rectal cancer patients following
neoadjuvant chemoradiotherapy. Colorectal Dis. 21:791–796.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bae JH, Song J, Kim JH, Kye BH, Lee IK,
Cho HM and Lee YS: Lateral lymph node size and tumor distance from
anal verge accurately predict positive lateral pelvic lymph nodes
in rectal cancer: A multi-institutional retrospective cohort study.
Dis Colon Rectum. 66:785–795. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ogura A, Konishi T, Beets GL, Cunningham
C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K,
et al: Lateral nodal features on restaging magnetic resonance
imaging associated with lateral local recurrence in low rectal
cancer after neoadjuvant chemoradiotherapy or radiotherapy. JAMA
Surg. 154(e192172)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schaap DP, Boogerd LSF, Konishi T,
Cunningham C, Ogura A, Garcia-Aguilar J, Beets GL, Suzuki C, Toda
S, Lee IK, et al: Rectal cancer lateral lymph nodes: Multicentre
study of the impact of obturator and internal iliac nodes on
oncological outcomes. Br J Surg. 108:205–213. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen JN, Liu Z, Wang ZJ, Mei SW, Shen HY,
Li J, Pei W, Wang Z, Wang XS, Yu J and Liu Q: Selective lateral
lymph node dissection after neoadjuvant chemoradiotherapy in rectal
cancer. World J Gastroenterol. 26:2877–2888. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou S, Zhang H, Liang J, Fu W, Lou Z,
Feng B, Yang Y, Xie Z and Liu Q: Chinese Lateral Node Collaborative
Group. Feasibility, indications, and prognostic significance of
selective lateral pelvic lymph node dissection after preoperative
chemoradiotherapy in middle/low rectal cancer: Results of a
multicenter lateral node study in China. Dis Colon Rectum.
67:228–239. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim MJ, Kim TH, Kim DY, Kim SY, Baek JY,
Chang HJ, Park SC, Park JW and Oh JH: Can chemoradiation allow for
omission of lateral pelvic node dissection for locally advanced
rectal cancer? J Surg Oncol. 111:459–464. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rooney S, Meyer J, Afzal Z, Ashcroft J,
Cheow H, De Paepe KN, Powar M, Simillis C, Wheeler J, Davies J and
Joshi H: The role of preoperative imaging in the detection of
lateral lymph node metastases in rectal cancer: A systematic review
and diagnostic test meta-analysis. Dis Colon Rectum. 65:1436–1446.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hoshino N, Murakami K, Hida K, Sakamoto T
and Sakai Y: Diagnostic accuracy of magnetic resonance imaging and
computed tomography for lateral lymph node metastasis in rectal
cancer: A systematic review and meta-analysis. Int J Clin Oncol.
24:46–52. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mizukami Y, Ueda S, Mizumoto A, Sasada T,
Okumura R, Kohno S and Takabayashi A: Diffusion-weighted magnetic
resonance imaging for detecting lymph node metastasis of rectal
cancer. World J Surg. 35:895–899. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Laparoscopic Surgery Committee of the
Endoscopist Branch in the Chinese Medical Doctor Association
(CMDA); Laparoscopic Surgery Committee of Colorectal Cancer
Committee of Chinese Medical Doctor Association (CMDA); Colorectal
Surgery Group of the Surgery Branch in the Chinese Medical
Association (CMA); Chinese Anti-Cancer Association Colorectal Tumor
Integrated Rehabilitation Committee; China International Exchange
and Promotive Association for Medical and Health Care Colorectal
Disease Branch. Chinese expert consensus on the diagnosis and
treatment for lateral lymph node metastasis of rectal cancer (2024
edition). Zhonghua Wei Chang Wai Ke Za Zhi. 27:1–14.
2024.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|