1. Introduction
The cochlea is a highly differentiated and
anatomically isolated sensory organ located within the temporal
bones of mammals, including humans. The hearing function of the
cochlea is mainly dependent on the performance of outer and inner
hair cells in the organ of Corti and spiral ganglion neurons (SGNs)
in the Rosenthal's canal. These hair cells convert sound signals
into electrical signals, which are delivered to the auditory
pathway of the brain by SGNs along the auditory nerve.
However, the hair cells of the organ of Corti as
well as SGNs of the Rosenthal's canal are terminally differentiated
cells, and their failure to recover or regenerate following
exposure to ototoxic insults, including chemicals and noise, as
well as aging, results in irreversible hearing loss in mammals
(1–4). Despite advances in the development of
neuroprotective drugs, no clinically effective means of repairing
or preventing acoustic dysfunction are available as yet (5–7).
The cochlea is a relatively isolated structure
anatomically. Although it is difficult for systemically delivered
drugs to cross the blood-labyrinth barrier and enter the inner ear,
locally injected drugs can be concentrated in the cochlear lymph
fluid (8,9). There has been marked progress in
target gene identification as well as vector preparation
technologies, thus raising the potential for local gene
transfection into the cochlea.
More than 90 genes affecting inner ear development
have been identified to date, of which 19 are involved in
non-syndromic deafness. Viral and non-viral vectors have been
experimentally applied to the cochlea using different approaches
(10–13). Careful characterization of the
mechanisms underlying dysfunctions, such as presbycusis and genetic
deafness, is likely to expand the horizons of this novel
therapeutic modality. Thus, the number and types of cases to which
gene transfection can be applied for the treatment of chronic
degenerative disorders and congenital diseases of the ear using
gene modification approaches is likely to increase.
2. Principles of gene transfection in the
cochlea
Local gene transfection involves the insertion,
alteration or removal of genes in target tissues using a viral or
non-viral vector to drive or inhibit the expression of a functional
protein (14–16).
The most evident advantage of local gene
transfection into the cochlea is the steady and long-term
expression and effect on the target area, which shows marked
contrast to the effects of cytokine treatment (17). Exogenous expression of the X-linked
inhibitor of apoptosis protein (XIAP) gene delivered by an
adeno-associated virus (AAV) vector and of the neurotrophin gene by
an adenoviral (Adv) vector was able to maintain long-term effects
in cochlear cells and protect against deafness (14,18).
However, target gene introduction by a Herpes simplex virus (HSV)
vector showed poor transfection efficacy in the inner ear (19,20).
The safety of gene transfection should also be taken
into consideration. AAV is an ideal vector due to its low
antigenicity. Hydroxyapatite (HAT) nanoparticles also represent a
vector free from the risk of biological disaster (21). By contrast, the application of HSV
and Adv vectors has been restricted due to their potential
immunogenicity and cytotoxicity (22,23).
In addition, the injection procedure was shown to negatively affect
the cochlea. Injection by cochleostomy into the scala media or
scala tympani causes irreversible electrophysiological and
morphological damage to the cochlea (24,25).
The methods used to examine cochlear characteristics pre- and
post-gene transfection are presented in Table I.
 | Table IMethods used to examine the
characteristics of the cochlea pre- and post-gene transfection. |
Table I
Methods used to examine the
characteristics of the cochlea pre- and post-gene transfection.
| Examination | Methods | Ref. |
|---|
|
Electrophysiology |
| Outer hair cell
(OHC) | Cochlear
microphonic (CM), otoacoustic emission (OAE) | (21,22) |
| Inner hair cell
(IHC) | Compound action
potential (CAP) | (23,24) |
| Spiral ganglion
neurons (SGNs) | Compound action
potential (CAP) | (23) |
| Auditory
pathways | Auditory brainstem
responses (ABR), electrical auditory brainstem responses
(EABR) | (25,26) |
| Single neuron | Compound action
potential (CAP) | (27) |
| Staining |
| Hair cells | Silver nitrate,
hematoxylin-eosin (HE), phalloidin-TRITC and DAPI staining | (25,28) |
| SGNs | Prussian blue and
DAPI staining | (25) |
| Nerve fibers | Anti-neurofilament
200 (NF200) antibodies | (29) |
| Observation
(microscopy) |
| Light | Surface
preparation, paraffin section, semithin section | (30) |
| Fluorescence | Surface
preparation, frozen section | (31) |
| Laser scanning
confocal (LSCM) | Surface
preparation, frozen section | (32,33) |
| Scanning
electron | Sample coated by
conducting material | (28) |
| Transmission
electron | Ultrathin
section | (32) |
Approximately 45 deafness-related genes have been
identified for non-syndromic hereditary hearing loss, and another
30 genes associated with syndromic hearing loss have been
identified (26,27). The expression of exogenous genes
may be used to rescue, replace or silence mutant loci (28,29).
The introduction of genes such as glial cell line-derived
neurotrophic factor (GDNF), Bcl-2 and XIAP was shown to have
protective effects against ototoxic insults, including chemical-
and noise-mediated injury (30–32).
Moreover, mouse atonal homolog 1 (Math 1) and human atonal homolog
1 (Hath 1) were shown to induce the regeneration of hair cells in
the organ of Corti and utricular maculae (16,33).
In addition, the efficacy of transfection for
various vectors in subjects should be considered. Previous studies
showed that most transfected cells were located in the basal turn
of the cochlea or in the basilar membrane (34,35).
3. Viral vectors
Adv vectors
The first studies of cochlear gene transfection
utilized an Adv vector composed of a linear DNA molecule ~35 kb in
length. Adv vectors may be generated at high titers and are able to
accommodate large (8-kb) DNA inserts. They have the advantage of
not requiring cell division for transfection and may be used
successfully for the transfection of the terminally differentiated
cells in the mammalian inner ear. Adv vectors have been shown to
effectively transfect hair cells, and a transfection efficacy of
~90% in the inner hair cells (IHCs) and 50% in the outer hair cells
(OHCs) and SGNs has been observed in vivo and in
vitro(36–39).
First-generation E1−, E3−
replication-deficient Adv vectors showed cytotoxicity and the
induction of an immune response to cochlear hair cells within 8–10
days of transfection (23,39,40).
The hair cell lesions induced by E1−, E3−
replication-deficient Adv vectors are inhibited by
immunosuppression using glucocorticoids, while morphological
evidence indicates that hair cells remained intact following the
injection of glucocorticoids prior to Adv vector treatment
(41). Second-generation Adv
vectors with deletions in the E1, E2b and E3 regions were
introduced into trials of cochlear injection and functional lesions
in the cochlea were delayed until 28 days following transfection of
the virus. This was suggested to be due to a delayed immune
response, which was eliminated using gutted adenoviral vectors with
deletions in E1, E2b, E3 and/or pol or another locus (39). The current focus of Adv vector
development is on the elimination of excess viral genes to minimize
host immune responses and cytotoxicity.
AAV vectors
AAVs consist of a single-stranded DNA parvovirus
capable of transfecting pre- and post-mitotic cells with no
requirement for actively dividing cells. AAVs accommodate DNA
inserts 3.5–4.0 kb in length (42). AAVs integrate into the host cell's
DNA, usually on chromosome 19, following induction by the viral rep
gene. It was reported previously that transfected AAVs may be
retained for 6 months and induce stable transgene expression in
cochlear cells (43). Moreover,
compared with Adv vectors, AAV vectors are based on a
non-pathogenic human virus that has not been associated with
disease and shows less ototoxicity (38).
There are at least 10 AAV serotypes based on amino
acid sequence differences in their respective capsid proteins. Of
these, AAV serotypes 1–5 are useful for gene therapeutic
applications due to their typical tropism and profile in
vivo(44). AAVs of serotype 2
have commonly been used to drive the expression of genes in several
cochlear cell types, including hair cells, especially OHCs, and
supporting cells in the organ of Corti, SGNs in the Rosenthal's
canal, cells of the spiral limbus and spiral ligament and sensory
and supporting cells of the crista ampullaris (45–47).
AAVs of serotype 5 exhibited high transfection efficacy in SGNs,
but failed to transfect hair cells, while AAV1 and AAV7 showed good
transfection efficacy in cells of the spiral ligament, and
expression driven by AAV5 and AAV8 was especially apparent in
Claudius cells (48). These
phenomena may be closely correlated with the expression of
co-receptors to various virus serotypes on the target cell surface
(49,50). However, it is difficult to produce
AAV vectors in high titers.
Additionally, the application of mutant AAVs needs
to be examined. A recombinant AAV vector with mutations in capsid
surface-exposed tyrosine residues showed a 10-fold increase in
transfection efficacy in HeLa cells, and a 30-fold increase in
murine hepatocytes in vitro compared with
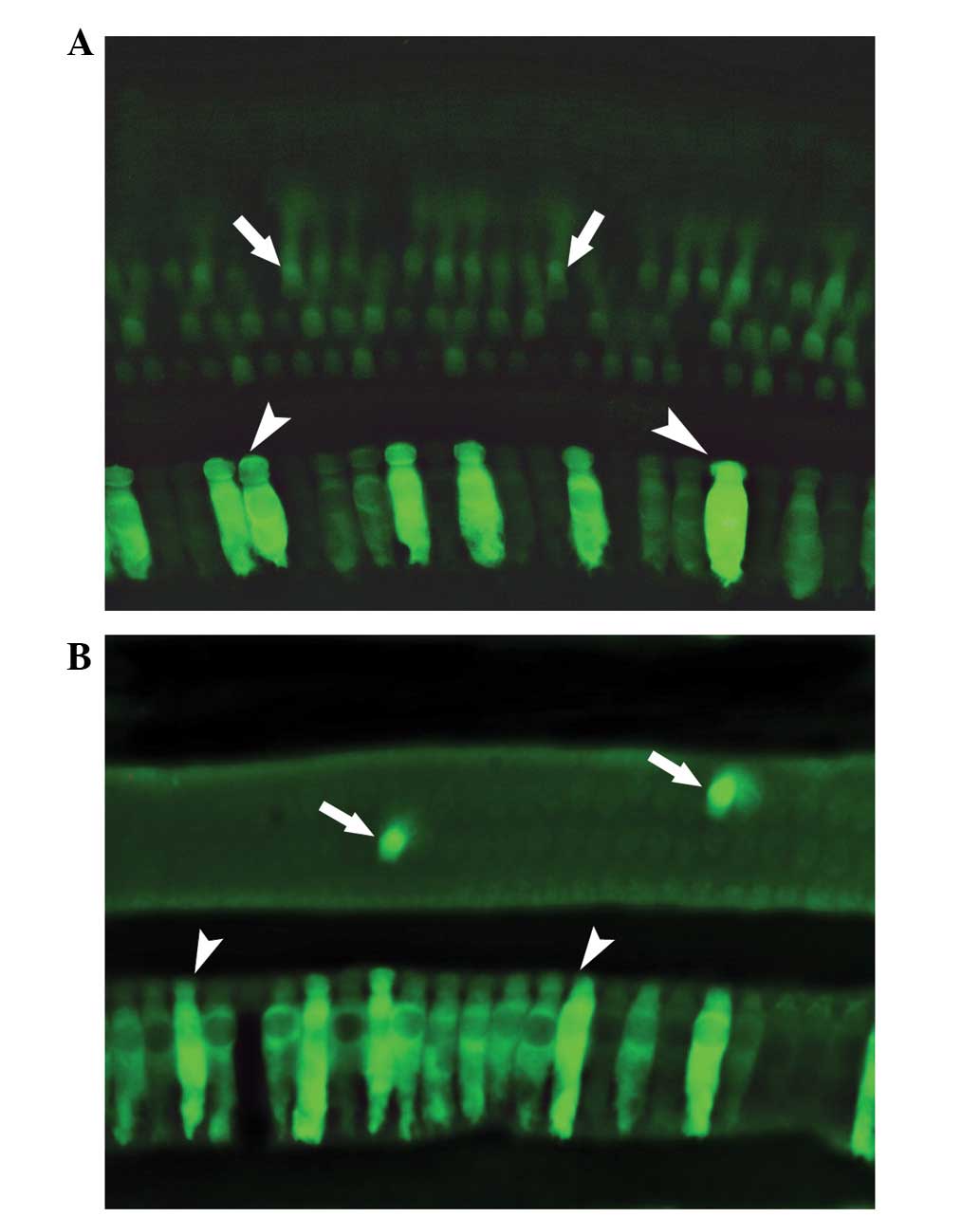
tyrosine-phosphorylated AAV vectors (51). This study indicates potential for
the development of a high-efficacy transfection system at a low
virus dose that is also an ideal candidate for use in human gene
therapy (Fig. 1).
HSV vectors
HSV is a DNA virus with a 152-kb double-stranded DNA
genome, which may be used to transfect cells of neuronal origin.
This type of vector is easy to produce and capable of carrying
large DNA inserts. HSV type I has generally been used to transfect
cochlear cells. HSV-1-linked NT-3 has been used successfully for
the stable transfection of SGNs and for protection against the
ototoxicity of cisplatin (52).
However, HSV vectors are not the preferred approach for cochlear
transfection due to their low transfection efficacy in cochlear
cells (20). In addition, there
are concerns about the apparent immune response and inflammation
induced by viral infection of the inner ear (53,54).
Investigation of HSV for cochlear applications remains in the
developmental stage, yet these vectors show potential for promoting
the survival of neural and neural-derived cells.
Lentivirus vectors
Lentiviruses, such as human immunodeficiency virus,
may be used as retroviral vectors to transfect dividing as well as
non-dividing cells (55). Although
stable protein expression mediated by a lentivirus vector was
reported in rat brain without observable toxicity for 6 months
(56), the efficacy of
transfection into cochlear cells was rather poor in vivo and
in vitro. Lentivirus vectors show a narrower distribution
throughout the cochlea compared with AAV and Adv vectors. The
results of previous in vivo and in vitro studies
indicated an intact cellular and tissue cytoarchitecture within
lentivirus-infused cochlea and an absence of inflammation or
pathological changes (57).
Lentiviruses may be suitable as vectors for the transfection of
neurotrophins and other protective factors into the cochlea.
4. Non-viral vectors
Liposomes
Positively charged (cationic) liposomes coupled with
a negatively charged (anionic) integrated target gene are able to
bind the plasma membrane of target cells and release the gene into
the cytoplasm (58). The genes
delivered by liposomes have been shown to be incorporated into the
genome of the host, with the encoded protein expressed for only 14
days in the neurosensory epithelia and surrounding tissues of the
cochlea in guinea pigs (59).
Studies of cationic liposomes have demonstrated a wide distribution
of the reporter gene in hair cells and supporting cells in guinea
pigs, and in the spiral ligament, Reissner's membrane and SGNs in
mice (59,60). However, liposomes may affect the
physiological activity of cells, such as inhibition of the
mitochondrial inner membrane, protein kinase C and ATPase activity,
resulting in cytotoxicity (61–63).
Thus, liposome vectors may be suitable for gene transfection when
expression is required only for a short time.
HAT
Polylactic/glycolic acid was the first nanoparticle
vector used to deliver materials to the cochlea (64). However, HAT was the first
nanoparticle vector used successfully to transfect cochlear cells
(15). The infusion of HAT
particles 40–50 nm in length into the cochlea resulted in no
significant damage. In addition, the HAT-mediated gene transfection
of NT-3 has been shown to have a protective effect against the
excitotoxicity of kainic acid in SGNs. Thus, HAT may be a useful
candidate for cochlear transfection if the low transfection
efficacy (16% in HeLa cells) associated with these nanoparticles
can be improved (21).
Hemagglutinating virus of Japan envelope
(HVJ-E)
HVJ-E vector is a non-viral second generation HVJ
vector, which was first used for gene transfection into the central
nervous system (65). It is
relatively easy to produce HVJ-E and these vectors show higher
fusion activity compared with first-generation HVJ-liposome
vectors. An HVJ-E vector combining hepatocyte growth factor (HGF)
injected through the cerebrospinal fluid was shown to approach the
cochlea and the expression of HGF prevented kanamycin-induced
hearing loss (66). These results
indicate that HVJ-E vectors are more efficient compared with other
non-viral vectors and safer compared with viral vectors. Thus, they
represent another potentially useful therapeutic approach to
sensorineural hearing impairment. Table II briefly compares the advantages
and disadvantages of the various vector types described above.
 | Table IIOverview of the vectors for gene
transfection. |
Table II
Overview of the vectors for gene
transfection.
| Vectors | Advantages | Disadvantages |
|---|
| Adv | Ability to
transfect post-mitotic cells
Easy to produce
Large insert size | Limited duration of
transgene expression
Immunogenic |
| AAV | Ability to
transfect post-mitotic cells
Long-term and stable expression
Nonpathogenic virus | Variable
transfection efficiencies
Low gene capacity
Unable to pass freely through the round window membrane
Hard to produce in high titers |
| HSV |
Neurotrophic
Easy to produce
Large insert size
Stable expression |
Immunogenic
Variable transfection efficiencies |
| Lentivirus | Stable
expression | Risk of insertional
mutagenesis
Low transfection efficiencies
Cell division required |
| Liposomes | Easy to
produce
Large insert size
Nonpathogenic | Low transfection
efficiencies |
| HAT | Easy to
produce
Large insert size
Nonpathogenic | Low transfection
efficiencies |
| HVJ-E | Easy to
produce
Large insert size
Nonpathogenic | Variable
transfection efficiencies |
5. Injection approaches
As the cochlea is surrounded by a bony wall and is
isolated due to the blood-labyrinth barrier, direct infusion into
the cochlea is usually necessary to achieve transgene expression in
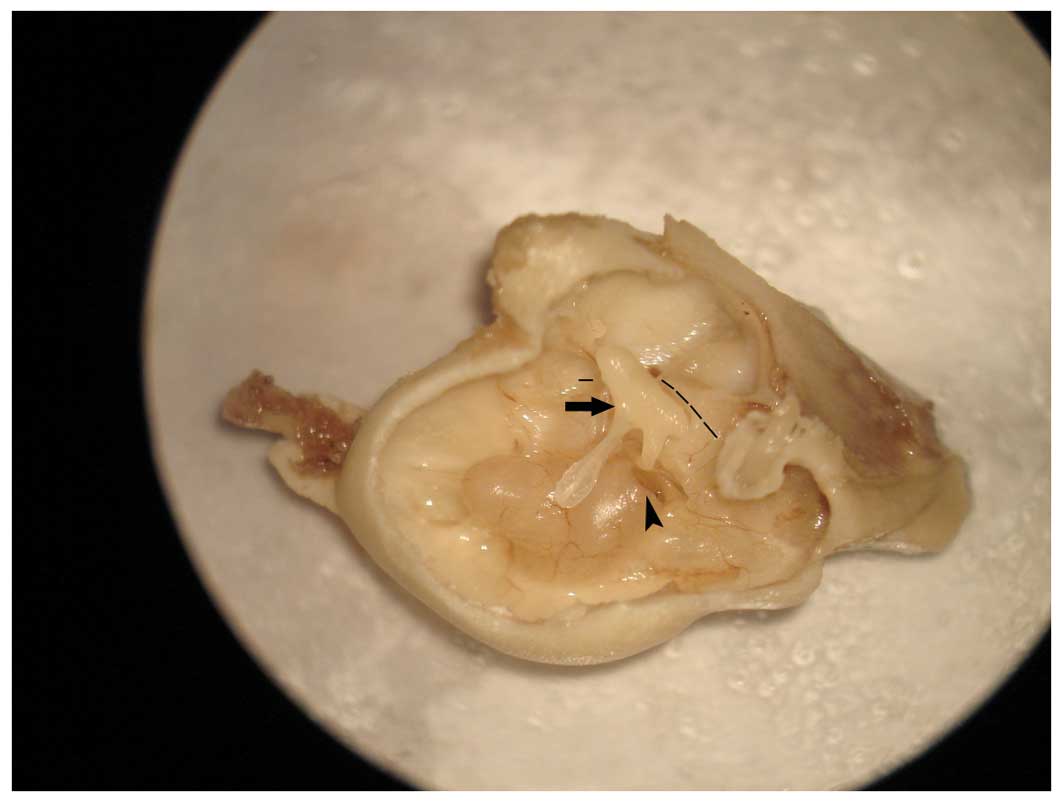
cells within this structure (Fig.
2). This is an ideal approach into the cochlea and does not
cause damage or at least functional impairment, and which allows
easy and convenient manipulation. There are three main approaches
to injection into the cochlea: the scala media, the semicircular
canals and the scala tympani.
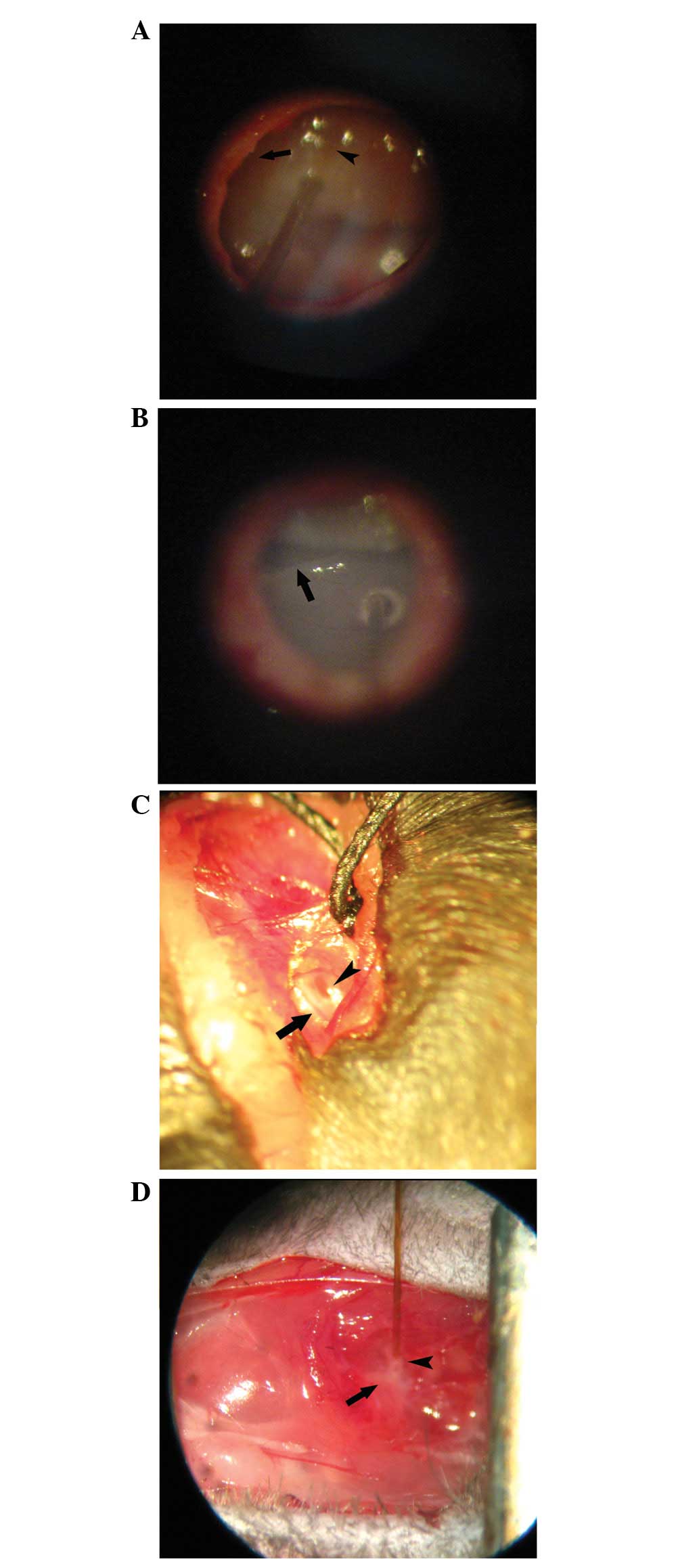
The scala media approach involves injection into the
endolymphatic system using a vector able to transfect sensory cells
in the organ of Corti (Fig. 3A).
However, this pathway is difficult for clinical application due to
the complexity of manipulation and possible surgical side-effects,
such as disruption of the cochlear structure (the stria vascularis
and the spiral ligament) and hearing loss (67,68).
Animal studies indicated that surgical exposure from the level of
the mandible to the acoustic bulla is required, which may increase
the risk of functional impairment, and serious threshold shifts and
hair cell loss were observed following surgery in animal models
(67,69). Thus, this is not the preferred
option for the introduction of transgenes into the cochlea from the
viewpoint of functional recovery.
Conversely, the scala tympani approach, represented
by cochleostomy and the trans-round window membrane (RWM)
technique, is a comparatively convenient and simple method for
animal experiments as well as clinical application. Cochleostomy
(Fig. 3B) may be better than the
trans-RWM technique (Fig. 3C) for
the administration of accurate volumes and to prevent potential
fluid leakage. In addition, there is no evidence of threshold
shifts by ABR tests following surgery for cochleostomy infusion
(70,71), however, studies of
histopathological changes in cochlear cells following cochleostomy
should be conducted to ensure the protection of function (24).
Several vectors, including liposomes, Adv vectors
and HSV, have been shown to travel into the cochlea from the middle
ear space via the RWM, resulting in cochlear cell transfection
(34,72). The inflammatory response induced by
macrophages and precipitated in the inner ear to such vectors
probably enhances their ability to enter the inner ear space due to
an increase in permeability of the RWM (73). Conversely, AAVs are unable to
traverse the RWM freely without specific treatments to enhance the
permeability of the membrane (74). Methods to alter the permeability of
the RWM may also be used to enhance the rate of passage of other
vectors.
Based on the structure of the basilar membrane,
which lacks tight junctions, but consists of fibrils, a homogeneous
ground substance and mesothelial cells, vectors in the perilymph
space are able to cross the basilar membrane to approach hair cells
(75,76). Another pathway into the cochlea
involves passage through the habenula perforata to enter the organ
of Corti (77).
The semicircular canal approach, also termed
canalostomy (Fig. 3D), is used to
introduce vectors into the cochlea as well as the vestibular system
(78). The semicircular canal is
relatively simple to expose and the procedure carries little risk
of injuring the cochlea and surrounding blood vessels compared with
cochleostomy. However, the main disadvantage of the semicircular
canal approach is that it is impossible to determine whether the
tip of the needle opens into the endolymphatic compartment or into
the perilymph during surgery, and the seal between the bone and the
tube is usually ruptured, resulting in leakage of the inner ear
fluid and vector suspension. Furthermore, canalostomy was shown to
be associated with temporary vestibular function disorders in mice,
including adverse effects on circling behavior, head tilt and
swimming ability, but they recovered within 2 weeks following
surgery (79).
6. Future directions
The future of gene transfection is likely to include
improving the properties of vectors to achieve a higher
transfection efficacy and cell targeting, refining the methods of
gene delivery to minimize lesions to the cochlea, while confirming
widespread transfection throughout the cochlea or localized
transfection within specific areas.
In addition to treating chemical- and noise-induced
hearing loss, gene therapy may be used to improve cochlear implant
function. Neurotrophins promote the survival of and delay the
degeneration of SGNs. Neurotrophin gene transfection performed in
conjunction with cochlear implant surgery may enhance neurite
growth to the cochlear implant. The development of cochlear
implants with improved performance would improve the quality of
life for a number of deaf children and elderly people.
The genes and factors involved in cell fate
determination in the sensory epithelium of the inner ear have been
explored. Math 1 and Hath 1 have been shown to drive regeneration
of hair cells posterior to the lesions induced by ototoxic factors.
Further basic investigation of drug delivery in fetal and neonatal
animals is likely to facilitate the development of novel
methodologies for the effective treatment of genetic diseases.
Acknowledgements
This study was supported by the China National Funds
for Distinguished Young Scientists (grant no. 30925035).
References
|
1
|
Henry KR, Chole RA, McGinn MD and Frush
DP: Increased ototoxicity in both young and old mice. Arch
Otolaryngol. 107:92–95. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishiyama G, Ishiyama A, Kerber K and Baloh
RW: Gentamicin ototoxicity: clinical features and the effect on the
human vestibulo-ocular reflex. Acta Otolaryngol. 126:1057–1061.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eshraghi AA, Frachet B, Van De Water TR
and Eter E: Hearing loss in adults. Rev Prat. 59:645–652. 2009.(In
French).
|
|
4
|
Fetoni AR, Mancuso C, Eramo SL, et al: In
vivo protective effect of ferulic acid against noise-induced
hearing loss in the guinea pig. Neuroscience. 169:1575–1588. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell KC, Meech RP, Klemens JJ, et al:
Prevention of noise- and drug-induced hearing loss with
D-methionine. Hear Res. 226:92–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CK, Shin JI and Cho YS: Protective
effect of minocycline against cisplatin-induced ototoxicity. Clin
Exp Otorhinolaryngol. 4:77–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maniu A, Perde-Schrepler M and Cosgarea M:
Protective effect of L-N-acetylcysteine against gentamycin
ototoxicity in the organ cultures of the rat cochlea. Rom J Morphol
Embryol. 52:159–164. 2011.PubMed/NCBI
|
|
8
|
Agrup C, Gleeson M and Rudge P: The inner
ear and the neurologist. J Neurol Neurosurg Psychiatry. 78:114–122.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swan EE, Mescher MJ, Sewell WF, Tao SL and
Borenstein JT: Inner ear drug delivery for auditory applications.
Adv Drug Deliv Rev. 60:1583–1599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staecker H, Gabaizadeh R, Federoff H and
Van De Water TR: Brain-derived neurotrophic factor gene therapy
prevents spiral ganglion degeneration after hair cell loss.
Otolaryngol Head Neck Surg. 119:7–13. 1998.PubMed/NCBI
|
|
11
|
Suzuki M, Yamasoba T, Suzukawa K and Kaga
K: Adenoviral vector gene delivery via the round window membrane in
guinea pigs. Neuroreport. 14:1951–1955. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan M, Venail F, Spencer N and Mezzina M:
Treatment of peripheral sensorineural hearing loss: gene therapy.
Gene Ther. 11(Suppl 1): S51–S56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Praetorius M, Pfannenstiel S, Klingmann C,
Baumann I, Plinkert PK and Staecker H: Expression patterns of
non-viral transfection with GFP in the organ of Corti in vitro and
in vivo. Gene therapy of the inner ear with non-viral vectors. HNO.
56:524–529. 2008.(In German).
|
|
14
|
Cooper LB, Chan DK, Roediger FC, et al:
AAV-mediated delivery of the caspase inhibitor XIAP protects
against cisplatin ototoxicity. Otol Neurotol. 27:484–490. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang M, Zhang YQ, He GX and Sun H:
Protective effect of NT-3 gene mediated by hydroxyapatite
nanoparticle on the cochlea of guinea pigs injured by
excitotoxicity. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 32:563–567.
2007.(In Chinese).
|
|
16
|
Kawamoto K, Ishimoto S, Minoda R, Brough
DE and Raphael Y: Math1 gene transfer generates new cochlear hair
cells in mature guinea pigs in vivo. J Neurosci. 23:4395–4400.
2003.PubMed/NCBI
|
|
17
|
Ylikoski J, Pirvola U, Virkkala J, et al:
Guinea pig auditory neurons are protected by glial cell
line-derived growth factor from degeneration after noise trauma.
Hear Res. 124:17–26. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghilardi JR, Freeman KT, Jimenez-Andrade
JM, et al: Sustained blockade of neurotrophin receptors TrkA, TrkB
and TrkC reduces non-malignant skeletal pain but not the
maintenance of sensory and sympathetic nerve fibers. Bone.
48:389–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michael AE, Collins TD, Norgate DP,
Gregory L, Wood PJ and Cooke BA: Relationship between ovarian
cortisol:cortisone ratios and the clinical outcome of in vitro
fertilization and embryo transfer (IVF-ET). Clin Endocrinol (Oxf).
51:535–540. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carnicero E, Garrido JJ, Alonso MT and
Schimmang T: Roles of fibroblast growth factor 2 during innervation
of the avian inner ear. J Neurochem. 77:786–795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frolenkov GI, Belyantseva IA, Kurc M,
Mastroianni MA and Kachar B: Cochlear outer hair cell
electromotility can provide force for both low and high intensity
distortion product otoacoustic emissions. Hear Res. 126:67–74.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liberman MC, Zuo J and Guinan JJ Jr:
Otoacoustic emissions without somatic motility: can stereocilia
mechanics drive the mammalian cochlea? J Acoust Soc Am.
116:1649–1655. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye HB, Shi HB, Wang J, et al: Bilirubin
induces auditory neuropathy in neonatal guinea pigs via auditory
nerve fiber damage. J Neurosci Res. 90:2201–2213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Badry MM and McFadden SL:
Electrophysiological correlates of progressive sensorineural
pathology in carboplatin-treated chinchillas. Brain Res.
1134:122–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia L, Yin S and Wang J: Inner ear gene
transfection in neonatal mice using adeno-associated viral vector:
a comparison of two approaches. PLoS One. 7:e432182012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landry TG, Wise AK, Fallon JB and Shepherd
RK: Spiral ganglion neuron survival and function in the deafened
cochlea following chronic neurotrophic treatment. Hear Res.
282:303–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walton JP, Barsz K and Wilson WW:
Sensorineural hearing loss and neural correlates of temporal acuity
in the inferior colliculus of the C57BL/6 mouse. J Assoc Res
Otolaryngol. 9:90–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Yin S, Yu Z, Huang Y and Wang J:
Dynamic changes in hair cell stereocilia and cochlear transduction
after noise exposure. Biochem Biophys Res Commun. 409:616–621.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang SM, Doi T, Asako M, Matsumoto A and
Yamashita T: Optical recording of membrane potential in dissociated
mouse vestibular ganglion cells using a voltage-sensitive dye.
Auris Nasus Larynx. 27:15–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poirrier AL, Van den Ackerveken P, Kim TS,
et al: Ototoxic drugs: difference in sensitivity between mice and
guinea pigs. Toxicol Lett. 193:41–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishimoto S, Kawamoto K, Stöver T, Kanzaki
S, Yamasoba T and Raphael Y: A glucocorticoid reduces adverse
effects of adenovirus vectors in the cochlea. Audiol Neurootol.
8:70–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Murphy R, Taaffe D, et al:
Efficient cochlear gene transfection in guinea-pigs with
adeno-associated viral vectors by partial digestion of round window
membrane. Gene Ther. 19:255–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maeda Y, Fukushima K, Kawasaki A,
Nishizaki K and Smith RJ: Cochlear expression of a
dominant-negative GJB2R75W construct delivered through the round
window membrane in mice. Neurosci Res. 58:250–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun H, Jiang M and Zhu SH: In vitro and in
vivo studies on hydroxyapatite nanoparticles as a novel vector for
inner ear gene therapy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za
Zhi. 43:51–57. 2008.(In Chinese).
|
|
35
|
Lalwani AK and Mhatre AN: Cochlear gene
therapy. Ear Hear. 24:342–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thomas CE, Ehrhardt A and Kay MA: Progress
and problems with the use of viral vectors for gene therapy. Nat
Rev Genet. 4:346–358. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dimitrov EA and Duckert LG: Morphologic
changes in the guinea pig cochlea following cochleostomy - a
preliminary scanning electron microscope study. Otolaryngol Head
Neck Surg. 93:408–413. 1985.
|
|
38
|
Iizuka T, Kanzaki S, Mochizuki H, et al:
Noninvasive in vivo delivery of transgene via adeno-associated
virus into supporting cells of the neonatal mouse cochlea. Hum Gene
Ther. 19:384–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kesser BW and Lalwani AK: Gene therapy and
stem cell transplantation: strategies for hearing restoration. Adv
Otorhinolaryngol. 66:64–86. 2009.PubMed/NCBI
|
|
40
|
Newton VE: Aetiology of bilateral
sensori-neural hearing loss in young children. J Laryngol Otol
Suppl. 10:1–57. 1985.PubMed/NCBI
|
|
41
|
Qu C, Gardner P and Schrijver I: The role
of the cytoskeleton in the formation of gap junctions by Connexin
30. Exp Cell Res. 315:1683–1692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Holt JR: Viral-mediated gene transfer to
study the molecular physiology of the Mammalian inner ear. Audiol
Neurootol. 7:157–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yagi M, Magal E, Sheng Z, Ang KA and
Raphael Y: Hair cell protection from aminoglycoside ototoxicity by
adenovirus-mediated overexpression of glial cell line-derived
neurotrophic factor. Hum Gene Ther. 10:813–823. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng G, Liu L, Wang P, et al: An in vivo
transfection approach elucidates a role for Aedes aegypti
thioester-containing proteins in flaviviral infection. PLoS One.
6:e227862011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pfannenstiel SC, Praetorius M, Plinkert
PK, Brough DE and Staecker H: Bcl-2 gene therapy prevents
aminoglycoside-induced degeneration of auditory and vestibular hair
cells. Audiol Neurootol. 14:254–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shou J, Zheng JL and Gao WQ: Robust
generation of new hair cells in the mature mammalian inner ear by
adenoviral expression of Hath1. Mol Cell Neurosci. 23:169–179.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jero J, Mhatre AN, Tseng CJ, et al:
Cochlear gene delivery through an intact round window membrane in
mouse. Hum Gene Ther. 12:539–548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Derby ML, Sena-Esteves M, Breakefield XO
and Corey DP: Gene transfer into the mammalian inner ear using
HSV-1 and vaccinia virus vectors. Hear Res. 134:1–8. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lei L and Han D: Efficient transduction of
spiral ganglion cells using adenovirus type 5 vector in the rat.
Acta Otolaryngol. 130:810–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duan ML, Ulfendahl M, Laurell G, et al:
Protection and treatment of sensorineural hearing disorders caused
by exogenous factors: experimental findings and potential clinical
application. Hear Res. 169:169–178. 2002. View Article : Google Scholar
|
|
51
|
Luebke AE, Foster PK, Muller CD and Peel
AL: Cochlear function and transgene expression in the guinea pig
cochlea, using adenovirus- and adeno-associated virus-directed gene
transfer. Hum Gene Ther. 12:773–781. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Luebke AE, Steiger JD, Hodges BL and
Amalfitano A: A modified adenovirus can transfect cochlear hair
cells in vivo without compromising cochlear function. Gene Ther.
8:789–794. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Holt JR, Johns DC, Wang S, et al:
Functional expression of exogenous proteins in mammalian sensory
hair cells infected with adenoviral vectors. J Neurophysiol.
81:1881–1888. 1999.PubMed/NCBI
|
|
54
|
Ishimoto S, Kawamoto K, Stover T, Kanzaki
S, Yamasoba T and Raphael Y: A glucocorticoid reduces adverse
effects of adenovirus vectors in the cochlea. Audiol Neurootol.
8:70–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Duan M, Bordet T, Mezzina M, Kahn A and
Ulfendahl M: Adenoviral and adeno-associated viral vector mediated
gene transfer in the guinea pig cochlea. Neuroreport. 13:1295–1299.
2002.PubMed/NCBI
|
|
56
|
Lalwani A, Walsh B, Reilly P, et al:
Long-term in vivo cochlear transgene expression mediated by
recombinant adeno-associated virus. Gene Ther. 5:277–281. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Stone IM, Lurie DI, Kelley MW and Poulsen
DJ: Adeno-associated virus-mediated gene transfer to hair cells and
support cells of the murine cochlea. Mol Ther. 11:843–848. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lalwani AK, Walsh BJ, Reilly PG, Muzyczka
N and Mhatre AN: Development of in vivo gene therapy for hearing
disorders: introduction of adeno-associated virus into the cochlea
of the guinea pig. Gene Ther. 3:588–592. 1996.
|
|
59
|
Lalwani AK, Walsh BJ, Carvalho GJ,
Muzyczka N and Mhatre AN: Expression of adeno-associated virus
integrated transgene within the mammalian vestibular organs. Am J
Otol. 19:390–395. 1998.PubMed/NCBI
|
|
60
|
Kilpatrick LA, Li Q, Yang J, Goddard JC,
Fekete DM and Lang H: Adeno-associated virus-mediated gene delivery
into the scala media of the normal and deafened adult mouse ear.
Gene Ther. 18:569–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu Y, Okada T, Sheykholeslami K, et al:
Specific and efficient transduction of Cochlear inner hair cells
with recombinant adeno-associated virus type 3 vector. Mol Ther.
12:725–733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Walters RW, Yi SM, Keshavjee S, et al:
Binding of adeno-associated virus type 5 to 2,3-linked sialic acid
is required for gene transfer. J Biol Chem. 276:20610–20616. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xiao W, Chirmule N, Berta SC, McCullough
B, Gao G and Wilson JM: Gene therapy vectors based on
adeno-associated virus type 1. J Virol. 73:3994–4003.
1999.PubMed/NCBI
|
|
64
|
Zhong L, Li B, Jayandharan G, et al:
Tyrosine-phosphorylation of AAV2 vectors and its consequences on
viral intracellular trafficking and transgene expression. Virology.
381:194–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen X, Frisina RD, Bowers WJ, Frisina DR
and Federoff HJ: HSV amplicon-mediated neurotrophin-3 expression
protects murine spiral ganglion neurons from cisplatin-induced
damage. Mol Ther. 3:958–963. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Keithley EM, Woolf NK and Harris JP:
Development of morphological and physiological changes in the
cochlea induced by cytomegalovirus. Laryngoscope. 99:409–414. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Stearns GS, Keithley EM and Harris JP:
Development of high endothelial venule-like characteristics in the
spiral modiolar vein induced by viral labyrinthitis. Laryngoscope.
103:890–898. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ailles LE and Naldini L: HIV-1-derived
lentiviral vectors. Curr Top Microbiol Immunol. 261:31–52.
2002.PubMed/NCBI
|
|
69
|
Blomer U, Naldini L, Kafri T, Trono D,
Verma IM and Gage FH: Highly efficient and sustained gene transfer
in adult neurons with a lentivirus vector. J Virol. 71:6641–6649.
1997.PubMed/NCBI
|
|
70
|
Han JJ, Mhatre AN, Wareing M, et al:
Transgene expression in the guinea pig cochlea mediated by a
lentivirus-derived gene transfer vector. Hum Gene Ther.
10:1867–1873. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Felgner PL, Gadek TR, Holm M, et al:
Lipofection: a highly efficient, lipid-mediated DNA-transfection
procedure. Proc Natl Acad Sci USA. 84:7413–7417. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wareing M, Mhatre AN, Pettis R, et al:
Cationic liposome mediated transgene expression in the guinea pig
cochlea. Hear Res. 128:61–69. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jero J, Tseng CJ, Mhatre AN and Lalwani
AK: A surgical approach appropriate for targeted cochlear gene
therapy in the mouse. Hear Res. 151:106–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Beavis AD: On the inhibition of the
mitochondrial inner membrane anion uniporter by cationic
amphiphiles and other drugs. J Biol Chem. 264:1508–1515.
1989.PubMed/NCBI
|
|
75
|
Bottega R and Epand RM: Inhibition of
protein kinase C by cationic amphiphiles. Biochemistry.
31:9025–9030. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Datiles MJ, Johnson EA and McCarty RE:
Inhibition of the ATPase activity of the catalytic portion of ATP
synthases by cationic amphiphiles. Biochim Biophys Acta.
1777:362–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tamura T, Kita T, Nakagawa T, et al: Drug
delivery to the cochlea using PLGA nanoparticles. Laryngoscope.
115:2000–2005. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shimamura M, Morishita R, Endoh M, et al:
HVJ-envelope vector for gene transfer into central nervous system.
Biochem Biophys Res Commun. 300:464–471. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Oshima K, Shimamura M, Mizuno S, et al:
Intrathecal injection of HVJ-E containing HGF gene to cerebrospinal
fluid can prevent and ameliorate hearing impairment in rats. FASEB
J. 18:212–214. 2004.PubMed/NCBI
|
|
80
|
Ishimoto S, Kawamoto K, Kanzaki S and
Raphael Y: Gene transfer into supporting cells of the organ of
Corti. Hear Res. 173:187–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yamasoba T, Yagi M, Roessler BJ, Miller JM
and Raphael Y: Inner ear transgene expression after adenoviral
vector inoculation in the endolymphatic sac. Hum Gene Ther.
10:769–774. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Shibata SB, Di Pasquale G, Cortez SR,
Chiorini JA and Raphael Y: Gene transfer using bovine
adeno-associated virus in the guinea pig cochlea. Gene Ther.
16:990–997. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Stover T, Yagi M and Raphael Y: Cochlear
gene transfer: round window versus cochleostomy inoculation. Hear
Res. 136:124–130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Konishi M, Kawamoto K, Izumikawa M,
Kuriyama H and Yamashita T: Gene transfer into guinea pig cochlea
using adeno-associated virus vectors. J Gene Med. 10:610–618. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nomura Y, Hara M and Kurata T:
Experimental herpes simplex virus and cytomegalovirus
labyrinthitis. Acta Otolaryngol Suppl. 457:57–66. 1989.PubMed/NCBI
|
|
86
|
Weiss MA, Frisancho JC, Roessler BJ and
Raphael Y: Viral-mediated gene transfer in the cochlea. Int J Dev
Neurosci. 15:577–583. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Nadol JB Jr: Intercellular junctions in
the organ of Corti. Ann Otol Rhinol Laryngol. 87:70–80. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kimura RS: The ultrastructure of the organ
of Corti. Int Rev Cytol. 42:173–222. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Fritzsch B, Farinas I and Reichardt LF:
Lack of neurotrophin 3 causes losses of both classes of spiral
ganglion neurons in the cochlea in a region-specific fashion. J
Neurosci. 17:6213–6225. 1997.PubMed/NCBI
|
|
90
|
Griffith AJ, Ji W, Prince ME, Altschuler
RA and Meisler MH: Optic, olfactory, and vestibular
dysmorphogenesis in the homozygous mouse insertional mutant Tg9257.
J Craniofac Genet Dev Biol. 19:157–163. 1999.PubMed/NCBI
|
|
91
|
Kawamoto K, Oh SH, Kanzaki S, Brown N and
Raphael Y: The functional and structural outcome of inner ear gene
transfer via the vestibular and cochlear fluids in mice. Mol Ther.
4:575–585. 2001. View Article : Google Scholar : PubMed/NCBI
|