1. Introduction
Acute vascular diseases, such as myocardial
infarction, stroke, pulmonary embolism (PE), deep vein thrombosis
(DVT), atrial fibrillation (AF), peripheral arterial occlusion, and
other thromboses of the blood system constitute major health risks.
As of 2008, there were ~2.3 million people in the United States
diagnosed with AF, a number estimated to increase to 5.6–10 million
by 2050. The incidence of hospital-acquired DVT is ~10–40% among
medical patients or those undergoing general surgery and 40–60% for
those following major orthopedic surgery without prophylaxis.
Moreover, ~10% of deaths in the hospital are caused by PE. Vascular
diseases are caused by either partial or total occlusion of a blood
vessel by a thrombus, which contains fibrin and platelets. Blood
coagulation is a crucial process involved in thrombosis (1).
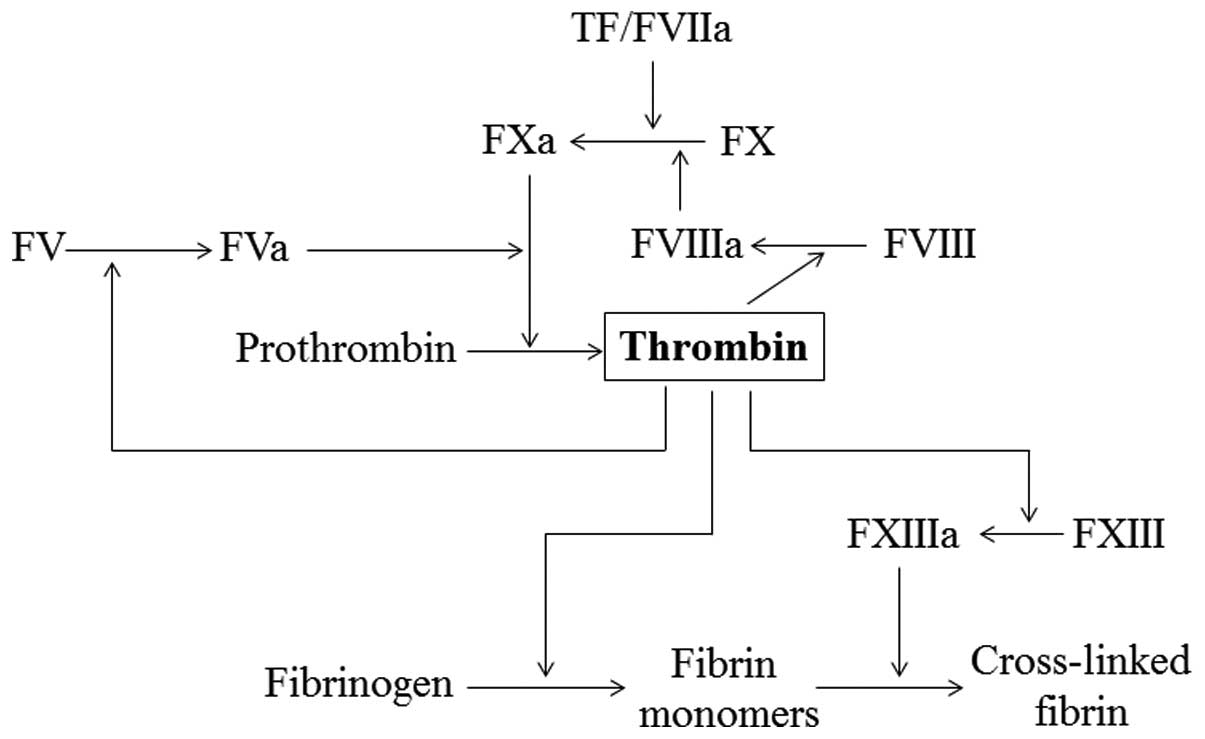
Thrombin plays a central role in blood coagulation.
It is a Na+-activated (2), trypsin-like serine protease,
activated from a larger precursor protein (pro-thrombin). The
active form of thrombin (α-thrombin) consists of a 36-amino acid
light chain (A chain) and a 259-amino acid peptidase domain (B
chain) covalently linked by a disulfide bridge (3). The active site of thrombin has three
pockets: S1, S2 and S3. Pocket S1 contains an aspartic acid residue
(Asp-189) at its bottom, which serves as the recognition site for
the basic side chain. Pocket S2 occludes a hydrophobic pocket in
the proximity of the Trp-60D residue; this pocket can accept larger
aliphatic residues, such as valine and proline. Pocket S3 is flat
and exposed to the solvent. Besides the active site, thrombin has
two important regulatory regions, exosites 1 and 2. Exosite 1 was
shown to be involved in the binding to fibrinogen, factor V, VIII,
thrombomodulin and platelet protease-activated receptors (PARs),
and exosite 2 was shown to be involved in the binding to factor V,
VIII, platelet glycoprotein Ibα (GPIbα) and heparin (4). Thrombin plays a central role in
maintaining the integrity of hemostasis. It interacts with most of
the zymogens and their cofactors, and plays both pro- and
anticoagulant roles in the blood (Fig.
1). Thrombin activates platelets, leading to platelet
aggregation. It converts fibrinogen into fibrin monomers, which
spontaneously polymerize into fibrin polymers and activate factor
XIII, a protein involved in fibrin cross-linking and clot
stabilization. Thrombin also activates factor V and VIII in a
positive feedback reaction (5).
Anticoagulant therapy plays an essential role in the
primary and secondary prevention of thromboembolic diseases
(6). Unfractionated heparins
(UFH), low molecular weight heparins (LMWH) and vitamin K
antagonists (VKAs) are classified as indirect thrombin inhibitors.
They have been widely and effectively used in certain
cardiovascular and thromboembolic diseases for a number of years
(7). However, their limitations
are important and well-recognized. Heparins have to be parenterally
administered and their activity requires cofactors such as
anti-thrombin III. Moreover, their anticoagulant effects are
variable due to non-specific protein binding, and therefore their
dosage must be monitored by laboratory tests. In addition,
treatment with heparins can cause a serious immune disorder known
as heparin-induced thrombocytopenia (HIT) and can lead to
osteoporosis in the long term. The main disadvantages of VKAs are
the requirement of regular dose adjustments by monitoring of their
anticoagulant effects; multiple food-drug and drug-drug
interactions; severe intracranial and extracranial bleeding
complications, and other severe side-effects such as
coumarin-induced hepatitis (8).
These limitations have led to the development of direct thrombin
inhibitors (DTIs).
DTIs are agents that directly inhibit thrombin by
binding to its active catalytic site and blocking its enzymatic
activity. They have some advantages over the indirect agents: DTIs
do not require cofactors to exert their effect; they can inhibit
both soluble thrombin and fibrin-bound thrombin (9); they have an immediate onset of action
and ideally affect only the target enzyme thus their anticoagulant
effects are more predictable compared to those of heparins
(7); DTIs can treat HIT instead of
causing it and they are effective in cases where heparin treatment
fails. These are some of the reasons for the widespread use of DTIs
in the treatment of several acute vascular diseases.
2. Direct thrombin inhibitors
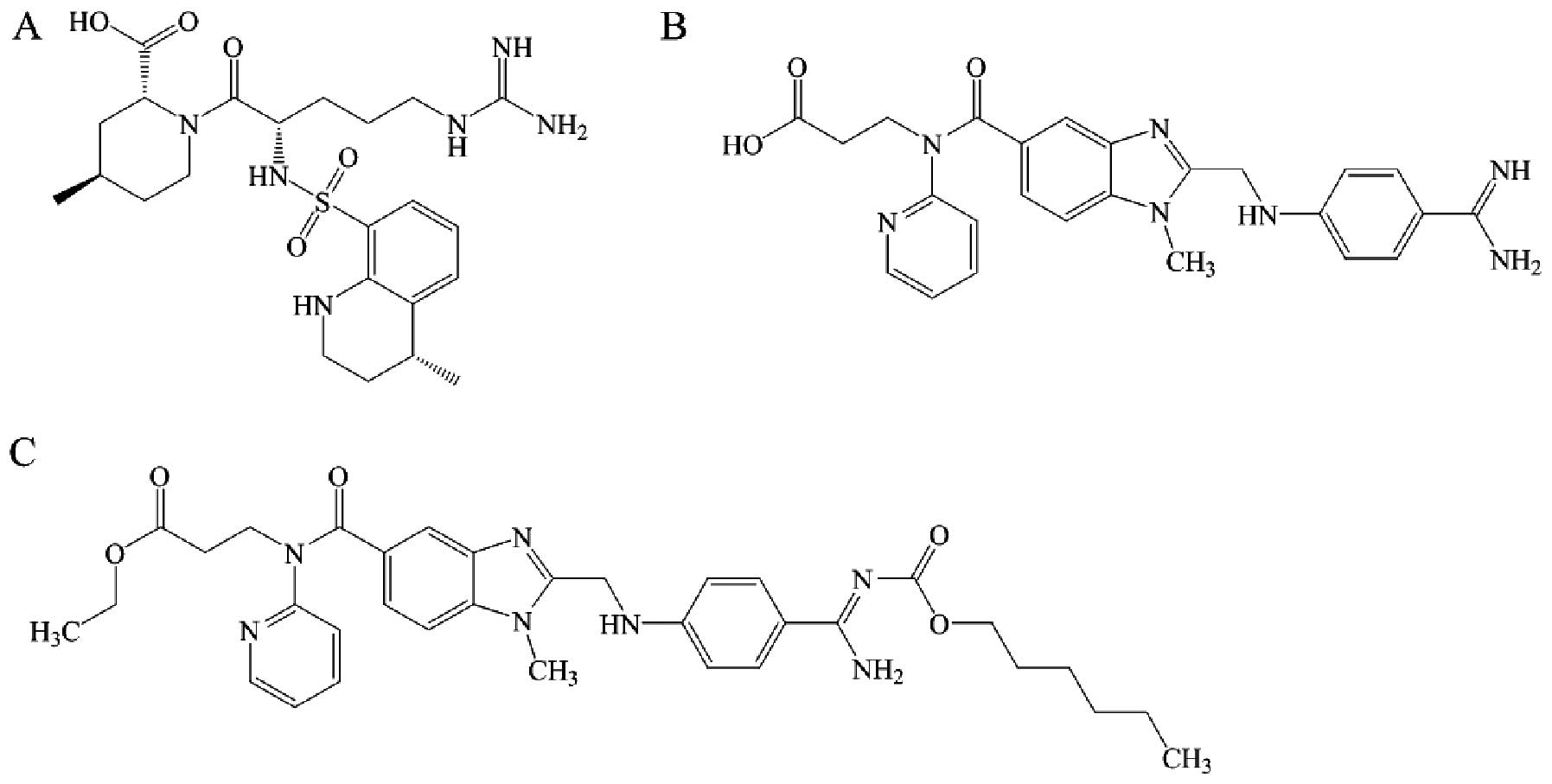
Argatroban
Argatroban (Fig.
2A) is a synthetic arginine-derived direct thrombin inhibitor
that exerts a stronger anticoagulant effect compared to heparins
and hirudins at equivalent levels. It is hepatically metabolized
and predominantly eliminated through the biliary system (7). Dose adjustments are necessary in
patients with hepatic, but not renal, impairment. The
bioavailability of orally administered argatroban is negligible and
therefore, it needs to be administered by infusion. Its half-life
is ~30 min. Argatroban can be used as a parenteral anticoagulant
for all cases where intravenous administration of heparin is
prescribed. It was approved by the Food and Drug Administration
(FDA) in 2000 for the treatment of HIT and interventional
anticoagulation during percutaneous coronary intervention (PCI). An
increased dosage can be used for a coronary artery bypass graft,
and a lower dosage can be used for DVT treatment (10). Argatroban passes through
endovascular and cellular barriers owing to its low molecular
weight. It is therefore effective for the antithrombotic treatment
of microvascular disorders (11).
Dabigatran
Dabigatran (Fig.
2B) is a synthetic benzamidine-derived direct thrombin
inhibitor that rapidly and directly inhibits both free and
fibrin-bound thrombin (7). It also
exerts an inhibitory effect on thrombin-induced platelet
aggregation and prevents the conversion of fibrinogen to fibrin.
Dabigatran is metabolized by the glycoprotein system and eliminated
through the kidneys (12).
However, the absolute bioavailability of dabigatran after oral
absorption is very low (6–7%) (13). Dabigatran was the first
anticoagulant approved by the FDA for primary prevention of
ischemic stroke and systemic thromboembolism in patients without
valvular atrial fibrillation (7).
Dabigatran etexilate
Dabigatran etexilate (Fig. 2C) is an orally administered direct
thrombin inhibitor, developed to overcome the limited oral
bioavailability of dabigatran. Once absorbed from the
gastrointestinal tract, it is rapidly converted to the active form
dabigatran. Bioconversion of dabigatran etexilate to dabigatran
occurs in enterocytes, hepatocytes and the portal vein. Dabigatran
circulates in the blood with a half-life of 12–17 h, which allows
oral administration once a day. With a low potential for drug-drug
interactions and a predictable anticoagulant effect, dabigatran
etexilate can be administered in fixed doses without need for
monitoring coagulation (14). In
2008, dabigatran etexilate was approved as a primary preventive
agent for venous thromboembolic events (VTEs) in adult patients who
underwent elective total hip or total knee replacement surgery in
Europe. In October 2010, it was approved by the FDA to reduce the
risk of stroke and systemic embolism in patients with non-valvular
atrial fibrillation. However, this inhibitor is not currently
indicated for treatment of any VTE in the USA (15).
Lepirudin and desirudin
Hirudin is isolated from the salivary glands of
medicinal leeches, and has been used as an anticoagulant agent
since 1909. Lepirudin and desirudin are two derivatives of hirudin.
Lepirudin is composed of 65 amino acids that directly inhibit
thrombin by simultaneously binding to its active site and to
exosite 1. Lepirudin is intravenously infused and its dosage is
dependent on body weight. It is eliminated through the kidneys,
which accounts for ~90% of the systemic clearance. Lepirudin is
licensed for the treatment of thrombosis complicating HIT.
Moreover, unstable angina is an additional syndrome that lepirudin
has the potential to treat (16).
Desirudin differs from lepirudin only in the first
two N-terminal amino acids. It is also eliminated through the
kidneys, accounting for 90% of the systemic clearance. Desirudin
reaches maximal plasma concentrations 1–3 h after administration
and has a terminal half-life of 2 h. In 2010, desirudin became the
only FDA-approved fixed-dose subcutaneously administered DTI for
the post-operative prevention of VTE in patients undergoing
elective hip replacement surgery (17). Currently, desirudin is under
investigation as a potential anticoagulant for HIT patients
presenting or not thrombosis (3).
Bivalirudin
Bivalirudin is a synthetic analog of hirudin. It is
a small polypeptide comprising 20 amino acids (D-Phe-
Pro-Arg-Pro-Gly-Gly-Gly-Gly-Asn-Gly-Asp-Phe-Glu-Glu-Ile-Pro-Glu-Glu-Tyr-Leu).
The N-terminal D-Phe-Pro-Arg-Pro region binds to the active site of
thrombin with high affinity, and the C-terminal dodecapeptide
Asn-Gly-Asp-Phe-Glu-Glu-Ile-Pro-Glu-Glu-Tyr-Leu binds to exosite 1
of thrombin (18). Bivalirudin
exhibits a short half-life (25 min), predominantly non-renal
metabolism, and low immunogenicity. It achieves peak plasma
concentration within 2 min of intravenous bolus injection.
Bivalirudin is primarily used for the treatment of percutaneous
transluminal coronary angioplasty, the most frequent type of PCI.
It is also indicated for PCI when provisional therapy with the
GPIIb/IIIa antagonist is employed, and for patients with HIT or HIT
with thrombosis syndrome (HITTS) undergoing PCI (19).
3. The development of direct thrombin
inhibitors
Alhough these DTIs show specific advantages compared
to indirect thrombin inhibitors, they still have limitations such
as a narrow therapeutic window, and bleeding and anaphylaxis as
side-effects. Approximately 80% of circulating dabigatran is
eliminated through the kidneys in a manner that its plasma
concentrations increase in renal insufficiency. Consequently, it is
contraindicated in patients with renal failure (20). Argatroban and hirudins require dose
adjustment guided by monitoring of their anticoagulant effect
(8). Lepirudin may associate with
anaphylaxis, while another significant limitation of this compound
is its narrow therapeutic window (21). The main clinical disadvantage of
bivalirudin is that it has no known antidote. Desirudin is
associated with antibody formation in 10% of recipients, although
these antibodies do not appear to be inhibitory as desirudin
potency is not affected by their presence (22). Although these specific
disadvantages can be serious, the main and common disadvantage of
all DTIs is the risk of bleeding.
These limitations have prompted the search for new
anticoagulant drugs, which ideally, would be orally available,
present no bleeding complications, and have a suitable
half-life.
Low molecular weight thrombin inhibitor
candidates
Previous studies have suggested that thrombin
inhibitors that contain the tripeptide template D-Phe-Pro-Arg
(P3-P2-P1), such as argatroban and dabigatran, are the most
effective (23,24). This tripeptide can bind to multiple
amino acids at the thrombin active site. It can bind to arginines,
especially the positively-charged guanidine group interacting with
the Asp-189 residue at the bottom of the S1 pocket. Hydrophobic
amino acids, such as proline in the P2 position, can bind to the S2
pocket, and aromatic groups in the P3 position can interact with
the lipophilic and aromatic fragments of the S3 pocket (25).
Typically, DTIs possess a P1 group that fills the
specific S1 pocket. Generally, to produce potent inhibitors, the P1
ligand features a strongly basic functional group such as guanidine
(argatroban), alkylamine, amidine, benzamidine (dabigatran), or
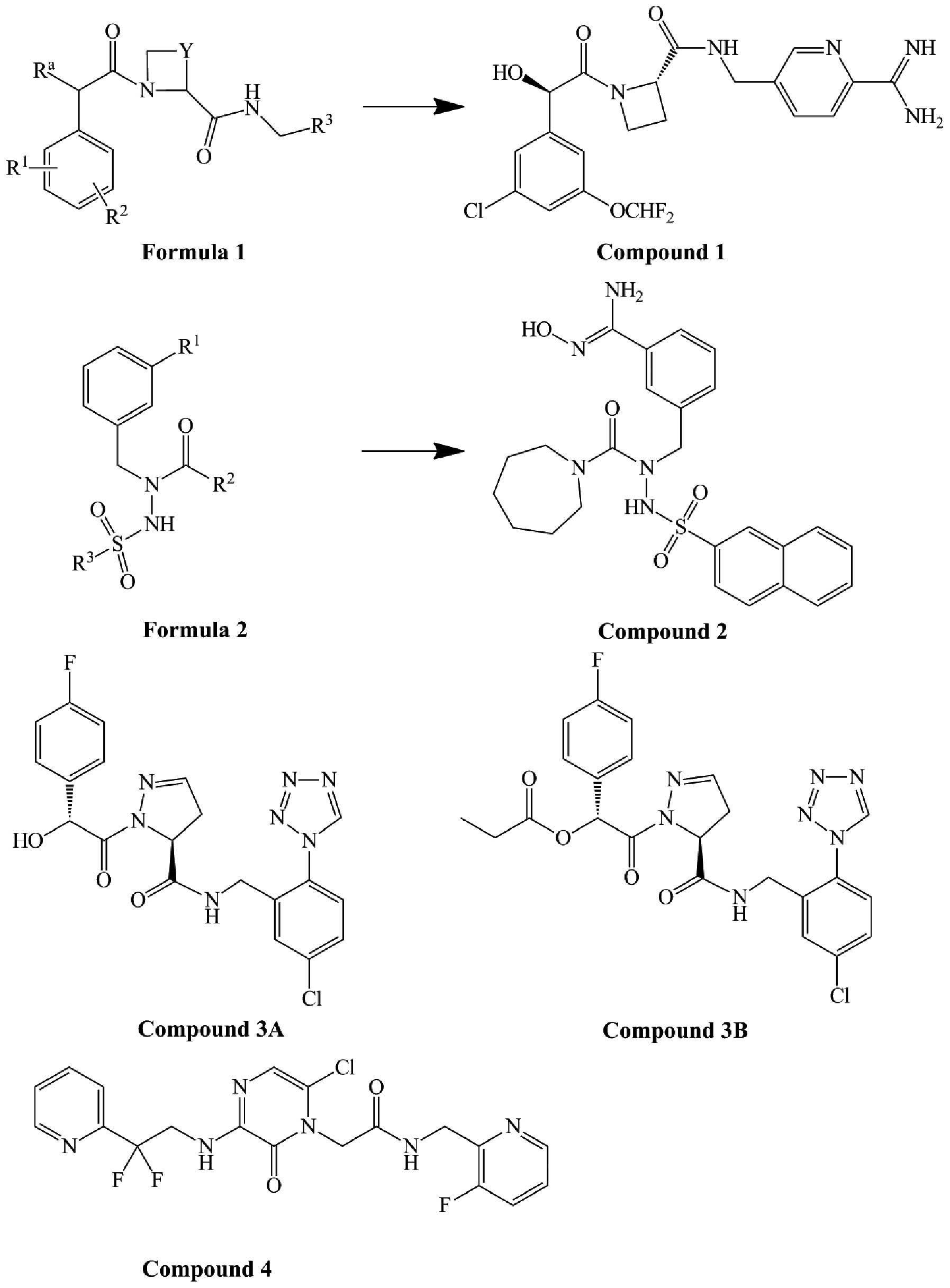
4-aminopyridine (26). Patents
WO00/42059 (27), US2007/0249578
A1 (28) and US2010/0087651 Al
(29) disclosed a series of
compounds in which the P1 position was replaced with amidino,
benzamidine, or their analogs. For example, compound 1 (Fig. 3), derived from formula 1 (Fig. 3), has an IC50 of
prothrombin time (PT) <0.02 μM, and an IC50 of
activated partial thromboplastin time (APTT) <1 μM. Compounds
containing strongly basic amines in the P1 position have poor oral
absorption properties and poor pharmacokinetics, because the basic
amines are protonated at the pH of the intestinal tract.
Strategies to overcome these shortcomings include
the use of a prodrug with a masked, weakly basic or non-basic, P1
group, such as dabigatran etexilate (30). N-hydroxylated derivatives such as
amidoxime and benzamidoxime, are less basic because of the
introduced oxygen atom. They are not protonated under physiological
conditions and are expected to have sufficient oral absorption, and
therefore, improved bioavailability (31). Patent US2008/0004256 A1 (32) disclosed a series of compounds
derived from formula 2 (Fig. 3).
Compound 2 (Fig. 3), where the P1
position is replaced by benzamidoxime, is the most bioavailable of
these compounds. The inhibition constant (Ki) values for thrombin
and trypsin are 0.59 and 32.23 μM, respectively, for this compound.
Azoles (26,33) (such as imidazoles, aminothiazoles
and N-acetamidoimidazole) and aryl heterocycles (pyridines,
pyrazinones, piperidines and pyridinones) are also weakly basic
groups. When they are incorporated into the P1 position, the
resulting peptides exhibit very good selectivity for thrombin vs.
trypsin and lower in vivo toxicity.
A recent study (25) demonstrated that the P1 fragment of
the inhibitor does not need to contain a highly basic functional
group to efficiently inhibit thrombin. The chlorophenyl fragment in
the P1 position can be deeply inserted into the S1 pocket of the
thrombin active site (25). In
addition, heterocycle-substituted chlorophenyl incorporated into
the P1 group was shown to provide more potent inhibitors (34). Patent US8119673 B2 (35) disclosed and described 57
D-Phe-Pro-Arg derivatives with heterocycle-substituted chlorophenyl
incorporated into the P1 position, along with their synthetic
routes. Compounds 3A and 3B (Fig.
3) were claimed in this patent. Their IC50 values
are 4 and 120 nM, respectively, according to a chromogenic robotic
assay.
The P2 position of the tripeptide is important, not
only in relation to its thrombin inhibitory activity, but also in
relation to the oral bioavailability. In previous studies, it was
found that using dehydroproline to replace the proline of the
tripeptide slightly increases the in vitro potency, in vivo
activity, and oral bioavailability (36), replacing it with 4-fluoroproline
increased the oral bioavailability (34) and replacing it with a pyrazinone
ring increased both activity and oral bioavailability (37). For example, compound 4 (Fig. 3), first described in patent
US6455532 B1 (38), is a
potentially new thrombin inhibitor with 3-aminopyrazinone in the P2
position. It inhibited thrombin activity with a Ki of 5.2 nM. The
3-aminopyrazinone in the P2 position of compound 3 played a crucial
role in thrombin inhibition by forming an hydrogen bond with
Gly-216 of thrombin. However, it was less resistant to oxidative
metabolism. Researchers from Merck Research Laboratories found that
2-aminopyridine N-oxide in the P2 position confers reduced
metabolic liability compared to that of pyrazinones (39).
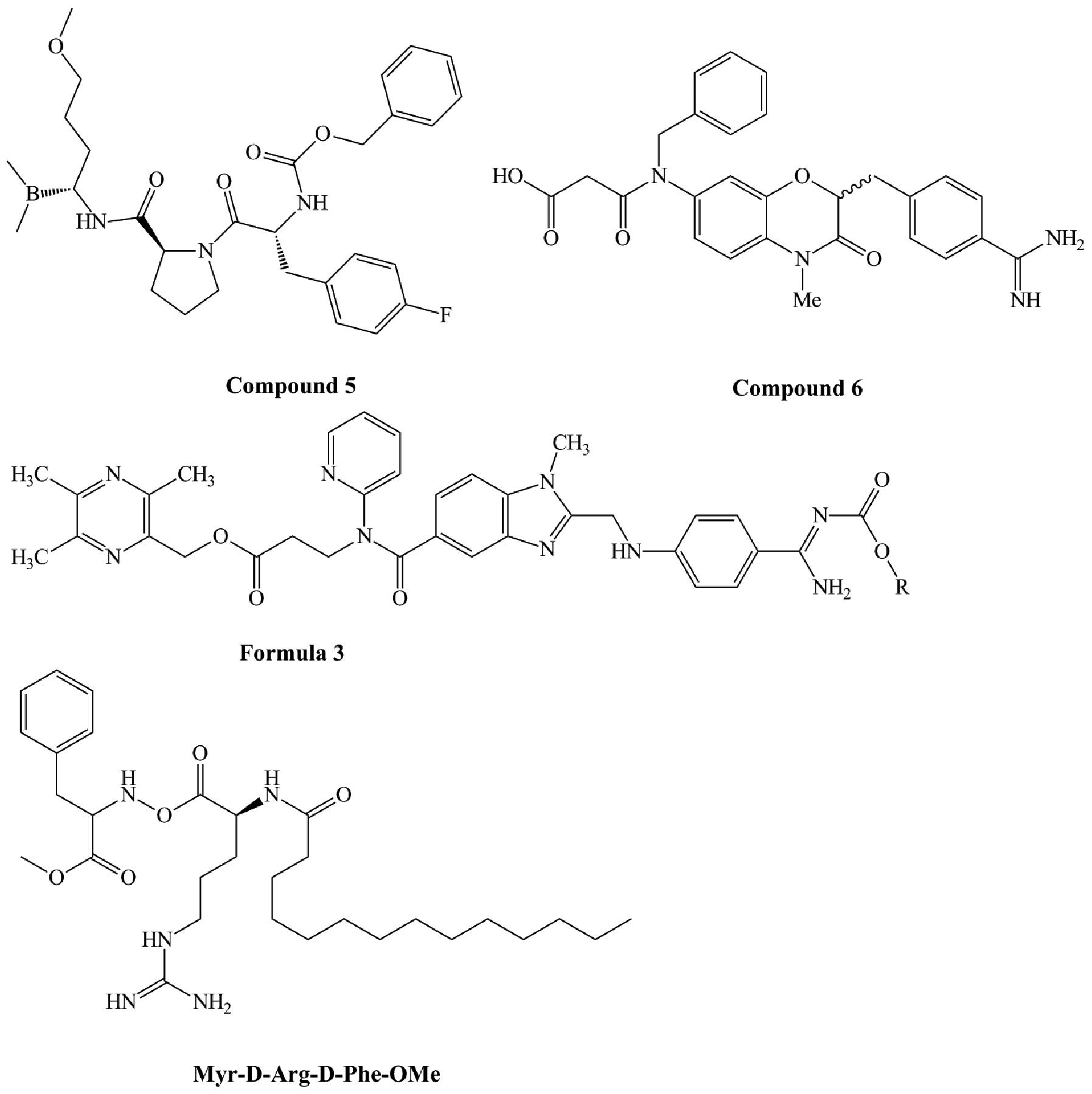
Derivatives of organoboronic acids exhibit thrombin
inhibitory activity. Patent US20070185060 A1 (40) disclosed a series of organoboronic
acid compounds such as compound 5 (Fig. 4). This compound has thrombin
inhibitory activity with an IC50 <0.1 μm. It has a
neutral aminoboronic acid residue, which can bind thrombin S1,
linked to a hydrophobic moiety, which can bind thrombin S2 and S3.
1,4-Benzoxazine-3(4H)-one was found to be a suitable scaffold for a
thrombin inhibitor. Compound 6 (Fig.
4), which was derived from this scaffold, is a highly potent
and selective thrombin inhibitor with Ki of 2.6 nM for thrombin and
112,400-fold selectivity for trypsin. Due to the presence of the
benzamidine moiety, it has considerable potential to show improved
bioavailability and be developed as an orally active prodrug
(41). Compounds derived from
formula 3 (Fig. 4) are a series of
non-peptide thrombin inhibitors that are described in patent
WO2012/174865 A1 (42). These
compounds have higher thrombin inhibitory activities compared to
dabigatran etexilate. Non-classic tripeptide templates such as
Tos-Phe-Arg-OCH3 and X-D-Arg-D-Phe-OMe can also act as
thrombin inhibitors. When X is replaced by myristic acid, the
compound (Fig. 4) has high
activity in thrombin inhibition (Ki=0.17 μM) and high selectivity
towards thrombin in comparison to factor X, plasmin, and trypsin
(>600-, 900- and 5,000-fold, respectively) (43).
Aptamers are 15–40 nucleotide, single-stranded DNA
or RNA molecules forming three-dimensional structures that bind to
their molecular targets with affinity and specificity (44,45).
Due to their high binding affinities for target macromolecules,
aptamers can act as inhibitors (46). ARC-183, or HD1, is a 15-nucleotide
DNA molecule discovered in the 1990s. It is a strong anticoagulant
in vitro, and inhibits the thrombin-catalyzed activation of
fibrinogen and thrombin-induced platelet aggregation by binding to
exosite 1. Despite its high affinity (Kd=30 nM), it has a short
half-life (2.5 min). In order to overcome this disadvantage,
another DNA aptamer (NU172) was developed. This aptamer binds to
thrombin with a Kd of 100 pM and has a half-life of 25 min when
tested in pigs. Toggle-25 is a 25-nucleotide RNA aptamer that
contains 2′-fluoropyrimidine nucleotides selected to bind exosite 2
of thrombin with high specificity and affinity (Kd=2nM) (47). Other thrombin inhibitors, such as
the DNA aptamer RE31 (48), the
RNA aptamer HD22 (49), and R9D-14
(50) were also identified. These
aptamers possess properties that render them especially suitable
for use as thrombin inhibitors: they typically exhibit high
affinity and specific binding to the target protein, and
demonstrate little to no toxicity or immunogenicity (51).
Derivatives of natural substances
The widely used in the clinic anticoagulant drugs
such as hirudin and its analog lepirudin were isolated from
blood-sucking leeches. For these animals to feed on blood from
mammalian hosts, they need to prevent local clotting of the host’s
blood. These parasites have developed various anti-clotting
mechanisms, including the specific inhibition of thrombin (52). In addition to hirudin, which was
first described in 1884 (53),
other thrombin inhibitors such as haemadin, bufrudin and theromin
have been isolated from various species of leeches (54). Haemadin is a slow, tight-binding
thrombin inhibitor isolated from the species Haemadipsa
sylvestris with an apparent molecular mass of 5 kDa. It
exhibits a Ki as low as 100 fM (55). Bufrudin was isolated from the
species Hirudinaria manillensis and has 66% sequence
homology with hirudin, indicating that it acts as a similar
inhibitor (56). Theromin,
isolated from the gut leech Theromyzon tessulatum, is the
most potent inhibitor (Ki of 12 vs. 21 fM for hirudin). It is a
homodimer of 67 amino acid residues with 16 cysteines engaged in
eight disulfide bridges (57).
Additional agents targeting thrombin were obtained
from various hematophagous animals such as mosquitoes, ticks, and
insects. Mosquito saliva carries a high number of factors with
anticoagulant activities. Anophelin is a peptide with a molecular
weight of 6.5 kDa, isolated from the salivary glands of the
mosquito species Anopheles albimanus. It inhibits thrombin
with an IC50 of 45 nM and binds to both the active site
and exosite 1 (58). An additional
peptide of 45 kDa was identified and isolated from the salivary
glands of the malarial vector mosquito Anopheles stephensi.
It inhibits thrombin only by binding to the active site (59). AaTI is another peptide from the
mosquito Aedes aegypti, which inhibits thrombin by binding
to exosite 2 (60).
A few potential thrombin inhibitors were also
isolated from ticks. Boophilin is a novel 20 kDa thrombin inhibitor
isolated from the cattle tick Boophilus microplus. It
inhibits thrombin with a Ki of 57 pM by binding to the active site
and exosite 1 (61). Boophilin is
composed of two canonical Kunitz domains, which inhibit not only
thrombin, but also, other serine proteases (62). Microphilin is the smallest salivary
thrombin inhibitor peptide known to date, with a molecular weight
of 1.7 kDa. It was also isolated from the cattle tick Boophilus
microplus. Microphilin inhibits thrombin in a dose-dependent
manner with an IC50 of 5.5 μM (63). From the midgut of the
parthenogenetic tick species Haemaphysalis longicornis
(Ixodidae), another thrombin inhibitor was isolated, known as
hemalin. It contains two Kunitz domains and has high homology to
boophilin. Hemalin inhibits thrombin in a dose-dependent manner
with a Ki of 0.25 μM. It also inhibited both thrombin-induced
fibrinogen clotting and thrombin-induced platelet aggregation
(64). From the same species, two
additional proteins that inhibit thrombin by binding to exosite 1
were identified, madanin 1 and 2 (65).
Other thrombin inhibitors such as triabin, infestin,
brasiliensin and dipetalogastin were identified and isolated from
blood-sucking insects. Triabin was purified from the saliva of
Triatoma pallidipennis (66). Infestin was isolated from midguts
of Triatoma infestans (67). Brasiliensin was identified and
isolated from Triatoma brasiliensis (68). Dipetalogastin was found and
isolated from Dipetalogaster maximus (69).
Besides these blood-sucking animals, other natural
sources of compounds targeting thrombin include snake and wasp
venom, and skin secretions of toads. TTI-Nh is a new 14.3-kDa
protein isolated from venom of the cobra species Naja haje,
and consisting of a single polypeptide chain with 14 cysteine
residues that form seven intramolecular disulfide bridges. It
inhibits thrombin with an IC50 of 73 nM without any
cytotoxic side-effects (70).
Bothrojaracin is a 27-kDa anticoagulant protein isolated from the
venom of the viper Bothrops jararaca. It inhibits thrombin
by binding to exosites 1 and 2 (71). Bicolin was purified and
characterized from the venom of wasps. It showed inhibitory
activity against trypsin and thrombin (72). BMSI-1 is a serine protease
inhibitor identified from skin secretions of the toad Bombina
microdeladigitora. It inhibits trypsin and thrombin with a Ki
of 0.02 and 0.15 μM, respectively. Furthermore, the activity of
BMSI-1 was not lost after incubation in boiling water for 10 min,
indicating that BMSI-1 is a thermally highly stable inhibitor
(73).
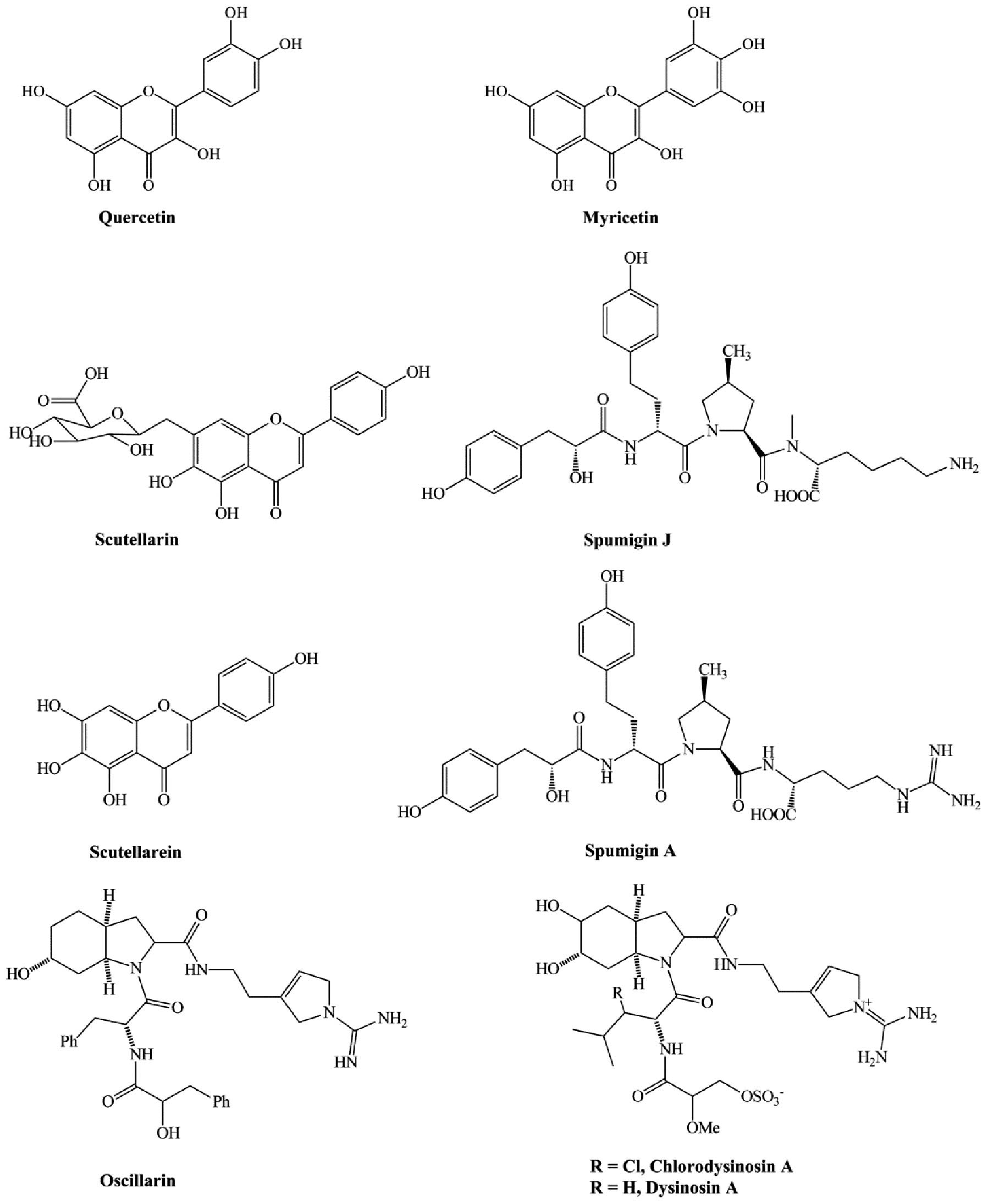
Natural flavonoids such as myricetin and quercetin
(Fig. 5) were discovered to act as
thrombin inhibitors, but with low activity. The IC50 of
TT is 0.006 and 0.035 mM for myricetin and quercetin, respectively
(74). Glycerolipids, especially
the diacylglycerophospholipids isolated from the fungus Stereum
hirsutum, can inhibit thrombin by binding to its active site
(75). Scutellarin (Fig. 5), the major anti-oxidant in
breviscapine extracted from the plant species Erigeron
breviscapus, was clinically effective in treating acute
cerebral infarction and paralysis induced by cerebrovascular
diseases. However, it has certain disadvantages, such as low
solubility in water and low bioavailability. Scutellarein (Fig. 5), the hydrolyzed product and
metabolic form of scutellarin, was used to design and synthesize
derivatives with improved biological activity and water solubility.
Many of these derivatives, especially the morpholinyl
aminomethylene substituent, showed stronger anticoagulant activity
and higher solubility in water (76). A recent study identified thrombin
inhibitors isolated from the freshwater cyanobacterium Anabaena
compacta. These molecules, known as spumigin J and A (Fig. 5), inhibited thrombin with
EC50 values of 4.9 and 2.1 μM, respectively. In
addition, the two compounds showed higher inhibitory activity
against cathepsin B, with EC50 values of 0.7 and 0.2 μM,
respectively (77).
Chlorodysinosin A, dysinosin A and oscillarin (Fig. 5), three natural products that
belong to the aeruginosin family of serine protease inhibitors,
exhibit low inhibitory activity against thrombin in vitro
(IC50=5.8, 46 and 28 nM, respectively). These molecules
inhibit thrombin owing to their hydrophobic interactions with the
S3 site of thrombin (78).
4. Discussion
Thrombotic diseases are major causes of mortality
and morbidity in the industrial world. Thrombin, which plays an
important role in blood coagulation, is a key cause of thrombotic
diseases. Indirect thrombin inhibitors such as heparins and VKAs
are widely used in the treatment of thrombotic diseases despite
their serious disadvantages. DTIs were developed as potential drugs
allowing to overcome these disadvantages while still maintaining
pharmacological activity.
In this review, we briefly summarized the advantages
and disadvantages of DTIs. These agents have several advantages
over indirect ones, such as direct inhibition of free and
clot-bound thrombin, lack of required cofactors, more predictable
anticoagulant response, and the fact that they do not cause
immune-mediated thrombocytopenia. Despite their effective clinical
use, they have some adverse effects. Bleeding is the side-effect of
almost all anti-thrombotic agents. Eliminating or reducing the
bleeding may be the aim of future studies. There are currently no
DTI-specific antidotes that show potential to reduce bleeding,
therefore developing such drugs is also a desired direction for
future research. For example, an aptamer termed JPB5 was shown to
act as an antidote, thus providing a superior safety profile for
bivalirudin (79). In addition to
bleeding, some DTIs have a narrow therapeutic window, making it is
necessary to adjust the dose by monitoring their anticoagulant
effect. This is another issue that needs to be solved in the
future. Additionally, future exploration of additional effects of
these new agents is necessary. For example, dabigatran etexilate
can be also used to treat cerebral venous thrombosis (80).
In the search for new direct thrombin inhibitors,
many candidate compounds were discovered. Compounds based on the
amino acid sequence Phe-Val-Arg (P3-P2-P1) were confirmed to be
effective inhibitors. Argatroban, the derivative of arginine, and
dabigatran, the derivative of benzamidine, are used effectively in
the clinic. However, these derivatives were found to have low oral
bioavailability and cytotoxicity. New direct thrombin inhibitors
with improved properties need thus to be discovered or
developed.
Aptamers have a few advantages such as high affinity
and complete absence of toxicity and immunogenicity, which renders
their use in pharmacological therapy an attractive approach. For
example, pegaptanib is an aptamer that binds to the vascular
endothelial growth factor (VEGF) and inhibits its biological
activity. This aptamer was recently approved for therapeutic use
for the treatment of age-related macular degeneration (81). Aptamers that inhibit thrombin in
different developmental stages may also represent a powerful
approach in antithrombotic therapies.
Hirudin is a well-known thrombin-inhibitory agent,
first isolated from a blood-sucking animal. The finding of this
compound inspired the search for new thrombin inhibitors, and
numerous active agents against thrombin have been identified
thereafter, isolated from blood-sucking animals such as mosquitos,
ticks and bugs. These animals are so small in size that it is
difficult to directly obtain active compounds from these, therefore
genetic engineering is required to produce active
thrombin-inhibitory agents. Some analogs and derivatives of natural
compounds also exhibit inhibitory activity, such as the derivatives
of hirudin, lepirudin and desirudin. High numbers of analogs or
derivatives are expected to emerge from ongoing investigations. In
addition, a few small molecular compounds isolated from plants and
bacteria showed thrombin-inhibitory activity. Traditional Chinese
medicine (TCM) has been employed for decades in the treatment of
thrombotic diseases. Previous studies (82,83)
have identified a number of compounds with thrombin-inhibitory
activity isolated from medicine administered in TCM. Future studies
on these TCM drugs may lead to the finding of new thrombin
inhibitors.
Acknowledgements
This project was sponsored by grants from the
National Natural Science Foundation of China (nos. 30972822,
81273274, 81273375 and 81273593), the Priority Academic Program
Development of Jiangsu Higher Education Institutions, National
Major Scientific, Technological Special Project for ‘Significant
New Drugs Development’ (no. 2011ZX09302-003-02), the Jiangsu
Province Major Scientific and Technological Special Project (no.
BM2011017) and the Jiangsu Provincial Qing Lan Project.
References
|
1
|
Baldo L, Plent S, Skerjanec S and Meanwell
C: Novel use of bivalirudin in the treatment of acute coronary
syndrome US Patent 20090247465 A1. Filed March 27, 2009; issued
October 1, 2009.
|
|
2
|
Di Cera E: Thrombin. Mol Aspects Med.
29:203–254. 2008.
|
|
3
|
Nar H: The role of structural information
in the discovery of direct thrombin and factor Xa inhibitors.
Trends Pharmacol Sci. 33:279–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bock PE, Panizzi P and Verhamme I:
Exosites in the substrate specificity of blood coagulation
reactions. J Thromb Haemost. 5:81–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gustafsson D: Combinations comprising a
low molecular weight thrombin inhibitor and a prodrug of a low
molecular weight thrombin inhibitor US Patent 20080113960 A1. Filed
November 9, 2007; issued May 15, 2008.
|
|
6
|
Robert S, Ghiotto J, Pirotte B, et al: Is
thrombin generation the new rapid, reliable and relevant
pharmacological tool for the development of anticoagulant drugs?
Pharmacol Res. 59:160–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee CJ and Ansell JE: Direct thrombin
inhibitors. Br J Clin Pharmacol. 72:581–592. 2011. View Article : Google Scholar
|
|
8
|
Harenberg J, Marx S, Krejczy M and Wehling
M: New anticoagulants-promising and failed developments. Br J
Pharmacol. 165:363–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nutescu EA and Wittkowsky AK: Direct
thrombin inhibitors for anticoagulation. Ann Pharmacother.
38:99–109. 2004.PubMed/NCBI
|
|
10
|
Fareed J and Jeske WP: Small-molecule
direct antithrombins: argatroban. Best Pract Res Clin Haematol.
17:127–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Owoo G and Burgos RA: Argatroban
formulations and methods for making and using same US Patent
7915290 B2. Filed February 29, 2008; issued March 29, 2011.
|
|
12
|
Blech S, Ebner T, Ludwig-Schwellinger E,
Stangier J and Roth W: The metabolism and disposition of the oral
direct thrombin inhibitor, dabigatran, in humans. Drug Metab
Dispos. 36:386–399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fugate JE, Rabinstein AA, McBane RD and
Lanzino G: Dabigatran: a primer for neurosurgeons. World Neurosurg.
79:154–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Franchini M and Mannucci PM: A new era for
anticoagulants. Eur J Intern Med. 20:562–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eriksson BI, Smith H, Yasothan U and
Kirkpatrick P: Dabigatran etexilate. Nat Rev Drug Discov.
7:557–558. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greinacher A and Lubenow N: Recombinant
hirudin in clinical practice: focus on lepirudin. Circulation.
103:1479–1484. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frame JN, Rice L, Bartholomew JR and
Whelton A: Rationale and design of the PREVENT-HIT study: a
randomized, open-label pilot study to compare desirudin and
argatroban in patients with suspected heparin-induced
thrombocytopenia with or without thrombosis. Clin Ther. 32:626–636.
2010. View Article : Google Scholar
|
|
18
|
Gosselin RC, Dager WE, King JH, et al:
Effect of direct thrombin inhibitors, bivalirudin, lepirudin, and
argatroban, on prothrombin time and INR values. Am J Clin Pathol.
121:593–599. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Warkentin TE: Bivalent direct thrombin
inhibitors: hirudin and bivalirudin. Best Pract Res Clin Haematol.
17:105–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gross PL and Weitz JI: New anticoagulants
for treatment of venous thromboembolism. Arterioscler Thromb Vasc
Biol. 28:380–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greinacher A, Lubenow N and Eichler P:
Anaphylactic and anaphylactoid reactions associated with lepirudin
in patients with heparin-induced thrombocytopenia. Circulation.
108:2062–2065. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewis CM and Deschler DG: Desirudin
reduces the rate of microvenous thrombosis in a rat model.
Laryngoscope. 118:1149–1152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blombäck B, Blombäck M, Olsson P, Svendsen
L and Aberg G: Synthetic peptides with anticoagulant and
vasodilating activity. Scand J Clin Lab Invest Suppl. 107:59–61.
1969.PubMed/NCBI
|
|
24
|
Costanzo MJ, Maryanoff BE, Hecker LR, et
al: Potent thrombin inhibitors that probe the S1 subsite:
tripeptide transition state analogues based on a
heterocycle-activated carbonyl group. J Med Chem. 39:3039–3043.
1996. View Article : Google Scholar
|
|
25
|
Kudryavtsev K, Shulga D, Chupakhin V, et
al: Design of small-molecule thrombin inhibitors based on the
cis-5-phenylproline scaffold. Russ Chem Bull Int Ed. 60:685–693.
2011. View Article : Google Scholar
|
|
26
|
Isaacs RC, Solinsky MG, Cutrona KJ, et al:
Structure-based design of novel groups for use in the P1 position
of thrombin inhibitor scaffolds. Part 1: Weakly basic azoles.
Bioorg Med Chem Lett. 16:338–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inghardt T and Nystrm JE:
Amidinobenzylamine derivatives and their use as thrombin inhibitors
US Patent 6599894. PCT Pub No. WO00/42059. Filed January 13, 2000;
issued July 29, 2003.
|
|
28
|
Inghardt T, Karlsson O, Linschoten M and
Nystrom JE: New amidino derivatives and their use as thrombin
inhibitors US Patent 20070249578 A1. Filed June 6, 2007; issued
October 25, 2007.
|
|
29
|
Inghardt T, Johansson A and Svensson A:
New mandelic acid derivatives and their use as thrombin inhibitors
US Patent 20100087651 Al. Filed June 25, 2009; issued April 8,
2010.
|
|
30
|
Kreutter KD, Lu T, Lee L, et al: Orally
efficacious thrombin inhibitors with cyanofluorophenylacetamide as
the P2 motif. Bioorg Med Chem Lett. 18:2865–2870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zega A, Mlinšek G, Šolmajer T,
Trampuš-Bakija A, Stegnar M and Urleb U: Thrombin inhibitors built
on an azaphenylalanine scaffold. Bioorg Med Chem Lett.
14:1563–1567. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Urleb U, Zega A, Stegnar M, Trampus BA,
Solmajer T and Mlinsek G: Amidinophenylalanine derivatives as
thrombin inhibitors US Patent 20080004256 A1. Filed September 12,
2007; issued January 3, 2008.
|
|
33
|
Isaacs RC, Solinsky MG, Cutrona KJ, et al:
Structure-based design of novel groups for use in the P1 position
of thrombin inhibitor scaffolds. Part 2: N-acetamidoimidazoles
Bioorg Med Chem Lett. 18:2062–2066. 2008.
|
|
34
|
Staas DD, Savage KL, Sherman VL, et al:
Discovery of potent, selective 4-fluoroproline-based thrombin
inhibitors with improved metabolic stability. Bioorg Med Chem Lett.
14:6900–6916. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brånalt J, Gustafsson D, Nilsson I and
Polla M: Compounds 148 US Patent 8119673 B2. Filed June 23, 2009;
issued February 21, 2012.
|
|
36
|
Lange UE, Baucke D, Hornberger W, Mack H,
Seitz W and Höffken HW: Orally active thrombin inhibitors. Part 2:
Optimization of the P2-moiety. Bioorg Med Chem Lett. 16:2648–2653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Young MB, Barrow JC, Kristen L, et al:
Discovery and evaluation of potent P1 aryl heterocycle-based
thrombin inhibitors. J Med Chem. 47:2995–3008. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Burgey CS, Robinson KA, Williams PD,
Coburn C, Lyle TA and Sanderson PE: Pyrazinone thrombin inhibitors
US Patent 6455532 B1. Filed June 1, 2000; issued September 24,
2002.
|
|
39
|
Nantermet PG, Burgey CS, Robinson KA, et
al: P2 pyridine N-oxide thrombin inhibitors: a novel peptidomimetic
scaffold. Bioorg Med Chem Lett. 15:2771–2775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S: Boronic acid thrombin inhibitors
US Patent 20070185060 A1. Filed March 9, 2005; issued August 9,
2007.
|
|
41
|
Ilaš J, Tomašić T and Kikelj D: Novel
potent and selective thrombin inhibitors based on a central
1,4-benzoxazin-3(4H)-one scaffold. J Med Chem. 51:2863–2867.
2008.PubMed/NCBI
|
|
42
|
Xu Y, Yang X, Gong G, Yang W, He G and Li
F: Oral thrombin inhibitor and preparation method and medical use
thereof WIPO Patent 2012174856 A1. Filed June 15, 2012; issued
December 27, 2012.
|
|
43
|
Poyarkov AA, Poyarkova SA, Smirnova IV and
Kukhar VP: Liporetro-D-peptides-a novel class of highly selective
thrombin inhibitors. Thromb Res. 129:97–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Burke JM and Berzal-Herranz A: In vitro
selection and evolution of RNA: applications for catalytic RNA,
molecular recognition, and drug discovery. FASEB J. 7:106–112.
1993.PubMed/NCBI
|
|
45
|
Bock LC, Griffin LC, Latham JA, Vermaas EH
and Toole JJ: Selection of single-stranded DNA molecules that bind
and inhibit human thrombin. Nature. 355:564–566. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lancellotti S and De Cristofaro R:
Nucleotide-derived thrombin inhibitors: a new tool for an old
issue. Cardiovasc Hematol Agents Med Chem. 7:19–28. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
White R, Rusconi C, Scardino E, et al:
Generation of species cross-reactive aptamers using ‘toggle’ SELEX.
Mol Ther. 4:567–573. 2001.PubMed/NCBI
|
|
48
|
Mazurov AV, Titaeva EV, Khaspekova SG, et
al: Characteristics of a new DNA aptamer, direct inhibitor of
thrombin. Bull Exp Biol Med. 150:422–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tasset DM, Kubik MF and Steiner W:
Oligonucleotide inhibitors of human thrombin that bind distinct
epitopes. J Mol Biol. 272:688–698. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bompiani KM, Monroe DM, Church FC and
Sullenger BA: A high affinity, antidote-controllable prothrombin
and thrombin-binding RNA aptamer inhibits thrombin generation and
thrombin activity. J Thromb Haemost. 10:870–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nimjee SM, Rusconi CP, Harrington RA and
Sullenger BA: The potential of aptamers as anticoagulants. Trends
Cardiovasc Med. 15:41–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
van de Locht A, Lamba D, Bauer M, et al:
Two heads are better than one: crystal structure of the insect
derived double domain Kazal inhibitor rhodniin in complex with
thrombin. EMBO J. 14:5149–5157. 1995.PubMed/NCBI
|
|
53
|
Markwardt F: The comeback of hirudin - an
old-established anticoagulant agent. Folia Haematol Int Mag Klin
Morphol Blutforsch. 115:10–23. 1988.PubMed/NCBI
|
|
54
|
Salzet M: Leech thrombin inhibitors. Curr
Pharm Des. 8:493–503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Strube KH, Kröger B, Bialojan S, Otte M
and Dodt J: Isolation, sequence analysis and cloning of haemadin.
An anticoagulant peptide from the Indian leech. J Biol Chem.
268:8590–8595. 1993.PubMed/NCBI
|
|
56
|
Markwardt F: State-of-the-Art Review:
antithrombotic agents from hematophagous animals. Clin Appl Thromb
Hemost. 2:75–82. 1996. View Article : Google Scholar
|
|
57
|
Salzet M, Chopin V, Baert J, Matias I and
Malecha J: Theromin, a novel leech thrombin inhibitor. J Biol Chem.
275:30774–30780. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Francischetti IM, Valenzuela JG and
Ribeiro JM: Anophelin: kinetics and mechanism of thrombin
inhibition. Biochemistry. 38:16678–16685. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Waidhet-Kouadio P, Yuda M, Ando K and
Chinzei Y: Purification and characterization of a thrombin
inhibitor from the salivary glands of a malarial vector mosquito,
Anopheles stephensi. Biochim Biophys Acta. 1381:227–233.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Watanabe RM, Soares TS, Morais-Zani K, et
al: A novel trypsin Kazal-type inhibitor from Aedes aegypti
with thrombin coagulant inhibitory activity. Biochimie. 92:933–939.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Soares TS, Watanabe RM, Tanaka-Azevedo AM,
et al: Expression and functional characterization of boophilin, a
thrombin inhibitor from Rhipicephalus (Boophilus) microplus
midgut. Vet Parasitol. 521–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Macedo-Ribeiro S, Almeida C, Calisto BM,
et al: Isolation, cloning and structural characterisation of
boophilin, a multifunctional Kunitz-type proteinase inhibitor from
the cattle tick. PLoS One. 3:e16242008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ciprandi A, de Oliveira SK, Masuda A, Horn
F and Termignoni C: Boophilus microplus: Its saliva contains
microphilin, a small thrombin inhibitor. Exp Parasitol. 114:40–46.
2006. View Article : Google Scholar
|
|
64
|
Liao M, Zhou J, Gong H, et al: Hemalin, a
thrombin inhibitor isolated from a midgut cDNA library from the
hard tick Haemaphysalis longicornis. J Insect Physiol.
55:165–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Iwanaga S, Okada M, Isawa H, Morita A,
Yuda M and Chinzei Y: Identification and characterization of novel
salivary thrombin inhibitors from the ixodidae tick,
Haemaphysalis longicornis. Eur J Biochem. 270:1926–1934.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Noeske-Jungblut C, Haendler B, Donner P,
Alagon A, Possani L and Schleuning WD: Triabin, a highly potent
exosite inhibitor of thrombin. J Biol Chem. 270:28629–28634. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Campos I, Amino R, Sampaio C, et al:
Infestin, a thrombin inhibitor present in Triatoma infestans
midgut, a Chagas’ disease vector: gene cloning, expression and
characterization of the inhibitor. Insect Biochem Mol Biol.
32:991–997. 2002.PubMed/NCBI
|
|
68
|
Araujo R, Campos I, Tanaka A, et al:
Brasiliensin: a novel intestinal thrombin inhibitor from
Triatoma brasiliensis (Hemiptera: Reduviidae) with an
important role in blood intake. Int J Parasitol. 37:1351–1358.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mende K, Petoukhova O, Koulitchkova V, et
al: Dipetalogastin, a potent thrombin inhibitor from the
blood-sucking insect Dipetalogaster maximus. Eur J Biochem.
266:583–590. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Osipov AV, Filkin SY, Makarova YV, Tsetlin
VI and Utkin YN: A new type of thrombin inhibitor, noncytotoxic
phospholipase A2, from the Naja haje cobra venom. Toxicon.
55:186–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Arocas V, Zingali RB, Guillin MC, Bon C
and Jandrot-Perrus M: Bothrojaracin: a potent two-site-directed
thrombin inhibitor. Biochemistry. 35:9083–9089. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang X, Wang Y, Lu Z, et al: A novel
serine protease inhibitor from the venom of Vespa bicolor
Fabricius. Comp Biochem Physiol B Biochem Mol Biol. 153:116–120.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lu X, Ma Y, Wu J and Lai R: Two serine
protease inhibitors from the skin secretions of the toad,
Bombina microdeladigitora. Comp Biochem Physiol B Biochem
Mol Biol. 149:608–612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu L, Ma H, Yang N, et al: A series of
natural flavonoids as thrombin inhibitors: structure-activity
relationships. Thromb Res. 126:e365–e378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Doljak B, Cateni F, Anderluh M, Procida G,
Zilic J and Zacchigna M: Glycerolipids as selective thrombin
inhibitors from the fungus Stereum hirsutum. Drug Dev Ind
Pharm. 32:635–643. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li NG, Song SL, Shen MZ, et al: Mannich
bases of scutellarein as thrombin-inhibitors: design, synthesis,
biological activity and solubility. Bioorg Med Chem. 20:6919–1923.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Anas AR, Kisugi T, Umezawa T, et al:
Thrombin inhibitors from the freshwater cyanobacterium Anabaena
compacta. J Nat Prod. 75:1546–1552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hanessian S, Therrien E, Zhang J, et al:
From natural products to achiral drug prototypes: potent thrombin
inhibitors based on P2/P3 dihydropyrid-2-one core motifs. Bioorg
Med Chem Lett. 19:5429–5432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Martin JA, Parekh P, Kim Y, et al:
Selection of an aptamer antidote to the anticoagulant drug
bivalirudin. PLoS One. 8:e573412013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hon SF, Li HL and Cheng PW: Use of direct
thrombin inhibitor for treatment of cerebral venous thrombosis. J
Stroke Cerebrovasc Dis. 21:e11–e15. 2012.PubMed/NCBI
|
|
81
|
Moshfeghi AA and Puliafito CA: Pegaptanib
sodium for the treatment of neovascular age-related macular
degeneration. Expert Opin Investig Drugs. 14:671–682. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ku SK, Kim TH and Bae JS: Anticoagulant
activities of persicarin and isorhamnetin. Vascul Pharmacol.
58:272–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Robert S, Baccelli C, Devel P, Dogné JM
and Quetin-Leclercq J: Effects of leaf extracts from Croton
zambesicus Müell. Arg on hemostasis. J Ethnopharmacol. 128:641–648.
2010.
|