Spandidos Publications style
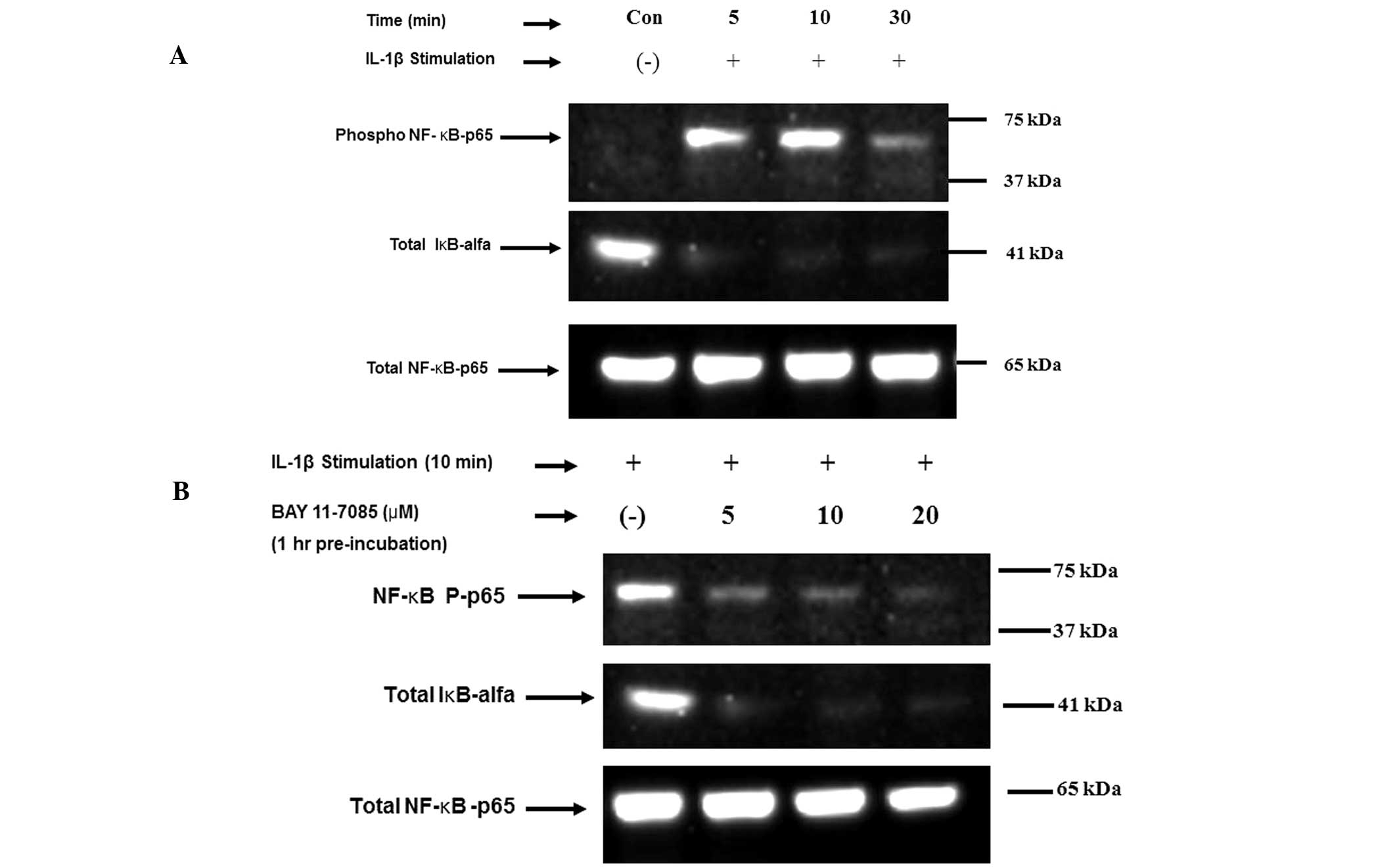
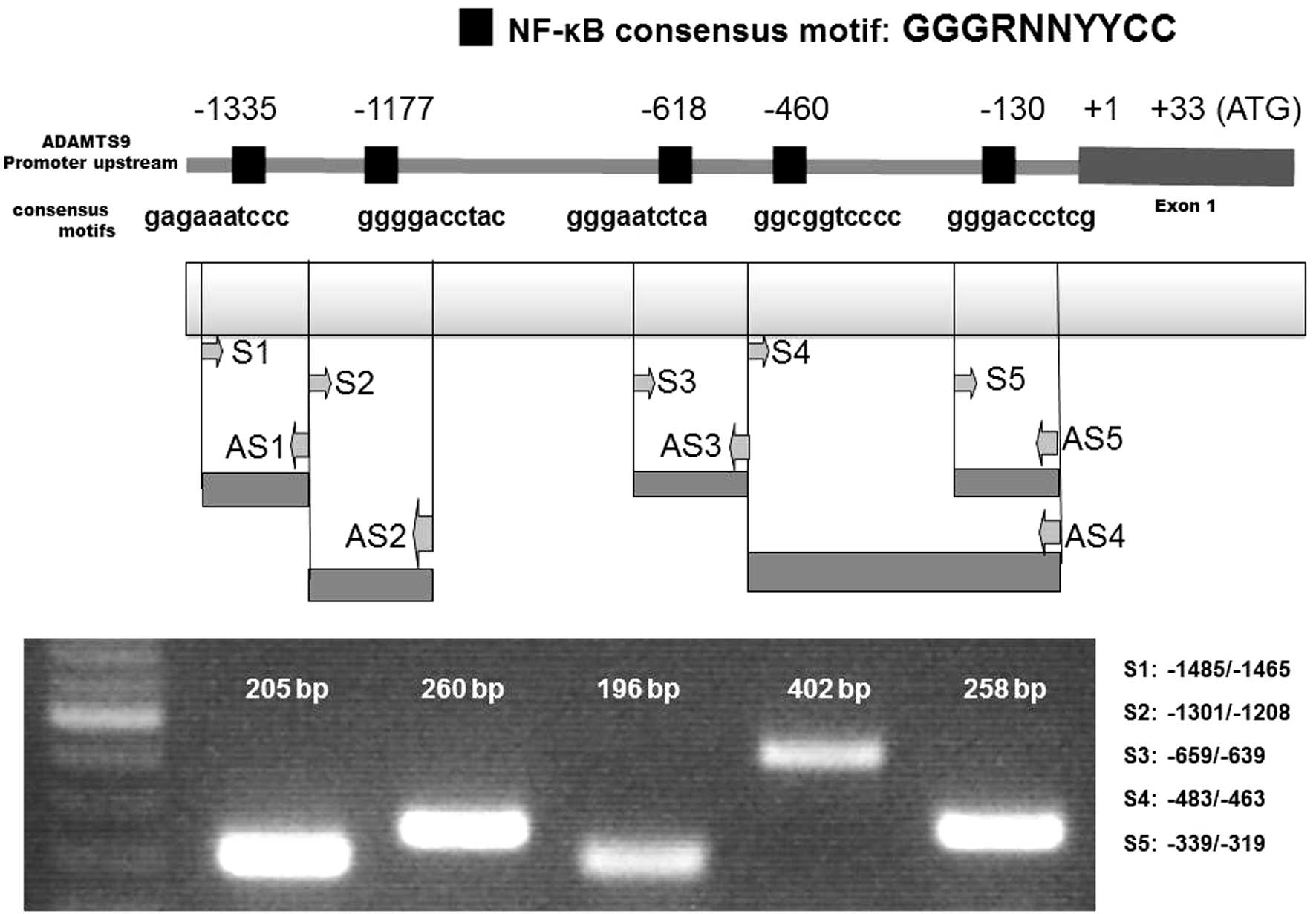
Altuntas A, Halacli SO, Cakmak O, Erden G, Akyol S, Ugurcu V, Hirohata S and Demircan K: Interleukin-1β induced nuclear factor-κB binds to a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 9 promoter in human chondrosarcoma cells. Mol Med Rep 12: 595-600, 2015.
APA
Altuntas, A., Halacli, S.O., Cakmak, O., Erden, G., Akyol, S., Ugurcu, V. ... Demircan, K. (2015). Interleukin-1β induced nuclear factor-κB binds to a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 9 promoter in human chondrosarcoma cells. Molecular Medicine Reports, 12, 595-600. https://doi.org/10.3892/mmr.2015.3444
MLA
Altuntas, A., Halacli, S. O., Cakmak, O., Erden, G., Akyol, S., Ugurcu, V., Hirohata, S., Demircan, K."Interleukin-1β induced nuclear factor-κB binds to a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 9 promoter in human chondrosarcoma cells". Molecular Medicine Reports 12.1 (2015): 595-600.
Chicago
Altuntas, A., Halacli, S. O., Cakmak, O., Erden, G., Akyol, S., Ugurcu, V., Hirohata, S., Demircan, K."Interleukin-1β induced nuclear factor-κB binds to a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 9 promoter in human chondrosarcoma cells". Molecular Medicine Reports 12, no. 1 (2015): 595-600. https://doi.org/10.3892/mmr.2015.3444