Introduction
Doxorubicin (DOX) remains one of the most widely
used anti-cancer drugs (1);
however, its clinical use is limited due to its severe
cardiotoxicity (2). Spallarossa
et al (3) showed that
cardiomyocyte apoptosis has an important role in DOX-induced
cardiotoxicity. Therefore, exploration of the mechanism of
DOX-induced cardiomyocyte apoptosis is required to develop
strategies to reduce the side effects of DOX by preventing
DOX-induced cardiotoxicity.
Resveratrol is a polyphenol with potent
cardioprotective, anti-inflammatory and anti-cancer activities
(4,5). Resveratrol is able to decrease the
infarct size and reduce apoptosis in ischemia-reperfusion injury
(6). Tatlidede et al
(7) revealed the protective effect
of resveratrol against DOX-induced cardiomyocyte apoptosis. In
addition, resveratrol not only improved the anti-cancer activity of
DOX, but also exerted cardioprotective effects (8,9).
Therefore, combined treatment of resveratrol with DOX may be a
feasible strategy to reduce DOX-induced cardiotoxicity (10). However, the underlying mechanisms
of how resveratrol exerts its cardioprotective effects against
DOX-induced cardiotoxicity have remained to be fully
elucidated.
Adenosine monophosphate-activated protein kinase
(AMPK) is an key regulatory protein in cellular energy metabolism,
and AMPK activation can regulate cell apoptosis (11–13).
AMPK activation results in the accumulation of pro-apoptotic
protein p53 to induce myocardial-cell apoptosis (14). Chen et al (13) reported that activation of the
AMPK/P53 signaling pathway has a crucial role in DOX-induced H9c2
cell death and apoptosis. Furthermore, Liu et al (15) confirmed that inhibition of the
AMPK/P53 signal transduction pathway can suppress DOX-stimulated
cardiomyocyte apoptosis. In the present study, H9c2 cells were
treated with DOX to establish a cell model of chemotherapy-induced
cardiotoxicity (16) and explored
whether resveratrol inhibits DOX-induced cardiomyocyte apoptosis
via the AMPK/P53 pathway.
Materials and methods
Materials
Hoechst 33258, 3-(4,
5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
DOX, resveratrol and AMPK inhibitor compound C were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The enhanced chemiluminescence
(ECL) solution was purchased from Beyotime Institute of
Biotechnology (Haimen, China). All cell culture medium components
were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA) unless stated otherwise.
Cell culture
H9c2 cardiac cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) in a humidified atmosphere containing 5% CO2
at 37°C. The H9c2 cardiac myocytes were seeded onto six-well plates
at a density of 2×106 cells/well and treated as follows
to form the different groups: Control group, H9c2 cardiac cells
were incubated in 0.5% FBS DMEM for 24 h; DOX group, treated with
DOX (5 µM) for 24 h; RES + DOX group, treated with
resveratrol (25 µM) for 30 min prior to exposure to DOX (5
µM) for 24 h; RES + DOX + compound C group, treated with
compound C (10 µM) for 60 min prior to stimulation with
resveratrol, followed by a 24-h culture with DOX.
MTT assay
An MTT assay was used to assess cell viability. H9c2
cardiac myocytes (5,000 cells/well) were seeded in 96-well
microtiter plates. After incubation with AMPK inhibitor compound C
(10 µM) for 60 min and/or resveratrol (25 µM) for 30
min, cells were cultured with 5 µM DOX for 24 h.
Subsequently, 10 µl MTT solution (5 mg/ml) was added to each
well, followed by incubation for a further 4 h at 37°C. Formazan
crystals were dissolved using 150 µl dimethyl sulfoxide and
the absorbance was measured at 470 nm (SpectraMax 190 Absorbance
Microplate Reader; Molecular Devices LLC, Sunnyvale, CA, USA) and
used to calculate the cell viability relative to that of the
control group. The assay was performed with each experimental
condition run in triplicate.
Assessment of apoptosis by Hoechst 33258
nuclear staining
Hoechst 33258 was used to assess cell apoptosis.
Following the above-mentioned treatments to form the various
groups, H9c2 cells were seeded at a density of 2×106
cells/well, incubated for 24 h and fixed in ice-cold 4%
paraformaldehyde (Beyotime Institute of Biotechnology) dissolved in
phosphate-buffered saline at 37°C for 20 min. Goat serum (5%;
Beyotime Institute of Biotechnology) was used to block non-specific
binding. Samples were then incubated with 10 µg/ml Hoechst
33258 at 37°C for 15 min. The slides were visualized under a
fluorescence microscope (BX50-FLA; Olympus, Tokyo, Japan).
Apoptotic cells were identified as those with a nucleus exhibiting
brightly stained condensed chromatin or unclear fragments, while
viable cells displayed a normal nuclear size and uniform
fluorescence.
Western blot analysis
H9c2 cells were directly homogenized with cell lysis
buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) with
phosphatase inhibitor cocktail (Sigma-Aldrich). Protein extracts
were collected following sample centrifugation at 12,000 × g for 10
min at 4°C. Equal quantities of extracted proteins were then
separated in 10% sodium dodecyl sulfate-polyacrylamide
electrophoresis gels (Beyotime Institute of Biotechnology) and
transferred to a polyvinylidene difluoride membrane (Beyotime
Institute of Biotechnology). The membranes were incubated in 5%
non-fat dry milk at 37°C for 2 h and the blots were incubated
overnight at 4°C with the following primary antibodies: Rabbit
phosphorylated (p)-AMPK polyclonal antibody (cat. no. 2535; 1:2,000
dilution; Cell Signaling Technology, Inc.), rabbit AMPK monoclonal
antibody (cat. no. 5831; 1:1,000 dilution; Cell Signaling
Technology, Inc.), rabbit P53 monoclonal antibody (cat. no.
ab179477; 1:2,000 dilution; Abcam, Cambridge, MA, USA), rabbit
anti-B-cell lymphoma (Bcl-2)-associated X protein (Bax) polyclonal
antibody (cat. no. Ab026; 1:400 dilution; Beyotime Institute of
Biotechnology) and rabbit anti-Bcl-2 polyclonal antibody (cat. no.
AB112; 1:4,000 dilution; Beyotime Institute of Biotechnology).
Following a 30 min wash, the membranes were subsequently incubated
with the appropriate horseradish peroxidase-conjugated secondary
antibodies (cat. no. A0208; 1:1,000 dilution; Beyotime Institute of
Biotechnology) for 2 h. Protein expression was determined using an
enhanced chemiluminescence reagent kit (Beyotime Institute of
Biotechnology) and the Tanon-5500 western blotting detection system
(Tanon Science and Technology Co., Ltd., Shanghai, China), and
quantified using the Quantity One Software Package (version 4.6.2;
Bio-Rad Laboratories, Ltd., Hercules, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analysis of data was assessed using one-way
analysis of variance with SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
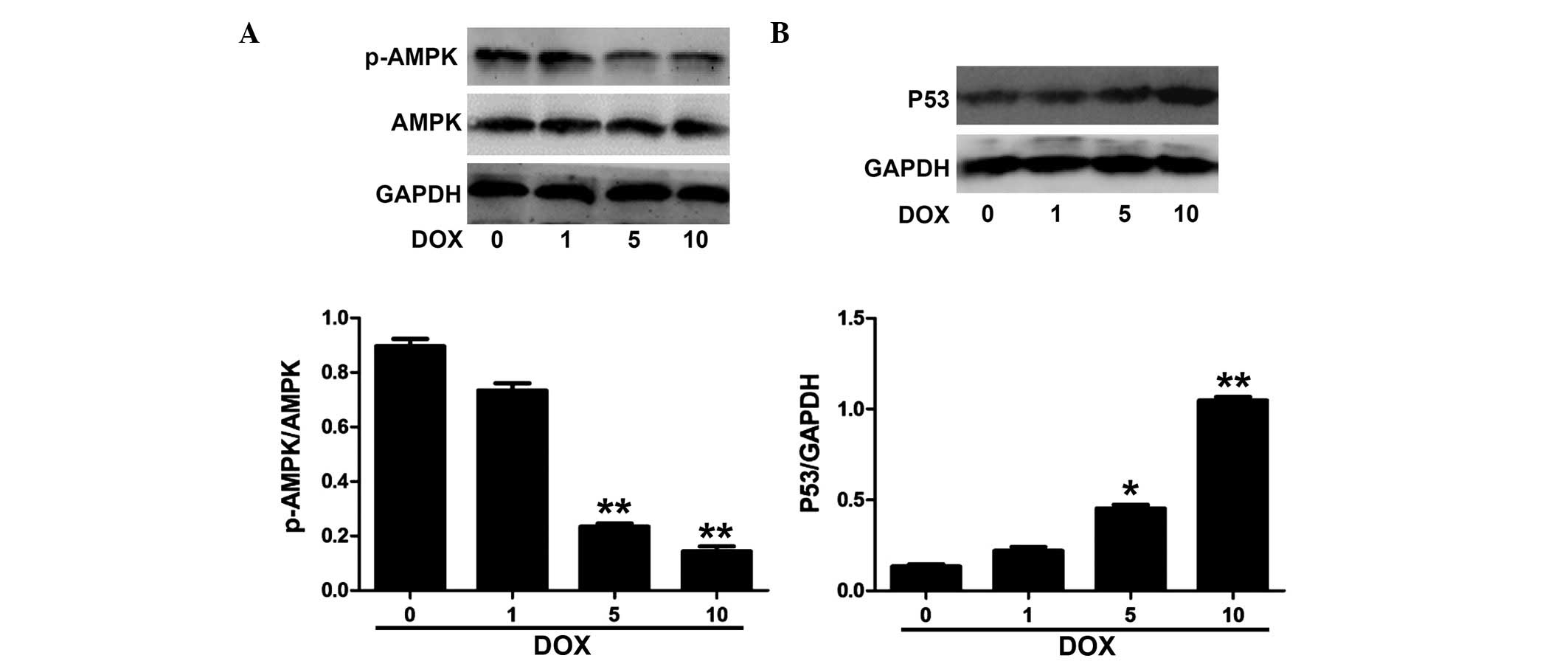
DOX decreases the phosphorylation of AMPK
and increases the expression of P53 in H9c2 cells
As shown in Fig.
1A, DOX decreased the phosphorylation of AMPK in H9c2 cells in
a concentration-dependent manner at the tested concentrations of
1–10 µM. Fig. 1B shows that
DOX significantly induced the expression of P53 at 5 µM,
which was further increased at 10 µM.
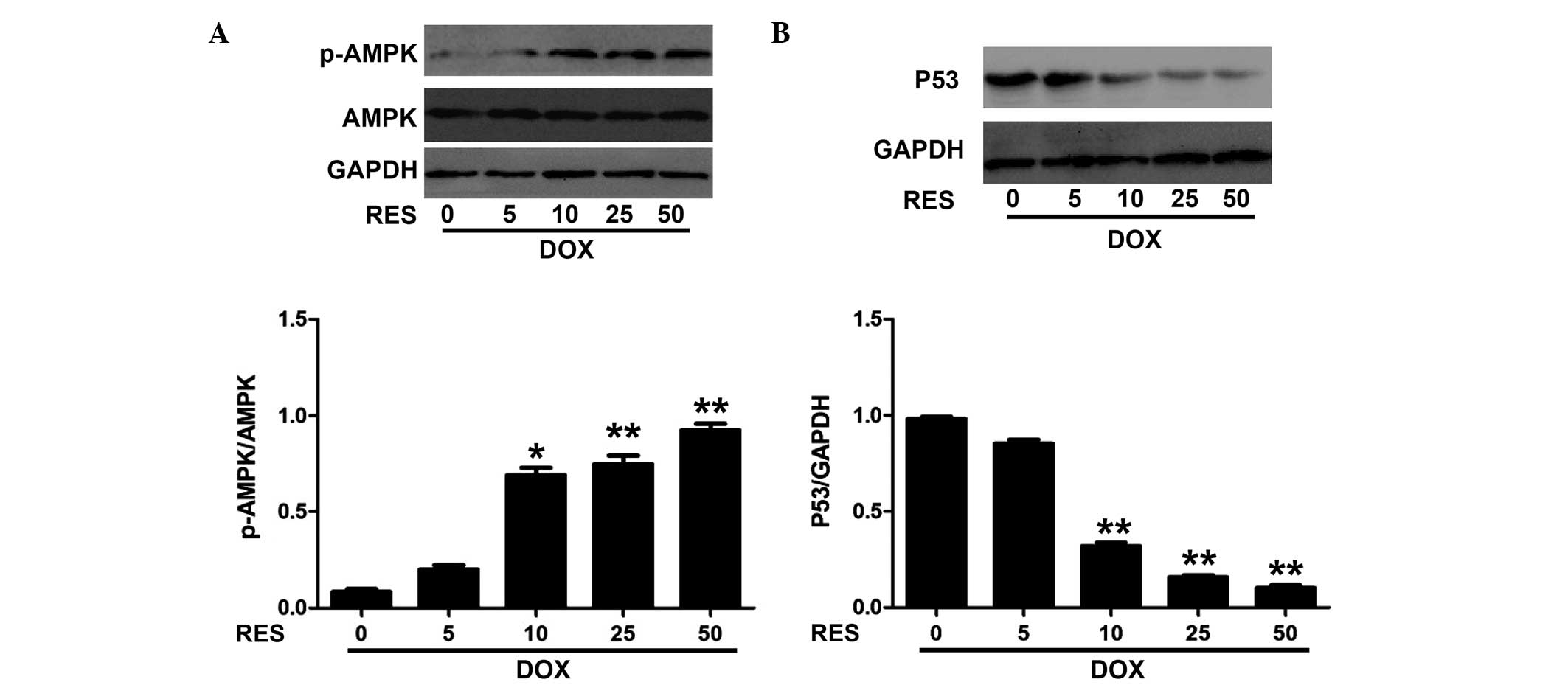
Resveratrol increases the phosphorylation
of AMPK and decreases the expression of P53 in DOX-induced H9c2
cells
To investigate the effects of resveratrol against
DOX-induced toxicity, the phosphorylation of AMPK and the
expression of P53 in H9c2 cells were assessed following DOX and
resveratrol treatment. Fig. 2
shows that resveratrol increased the phosphorylation of AMPK and
decreased the expression of P53 in a concentration-dependent manner
in DOX-induced H9c2 cells. The phosphorylation of AMPK was
significantly increased by resveratrol at the concentration of 10
µM and above.
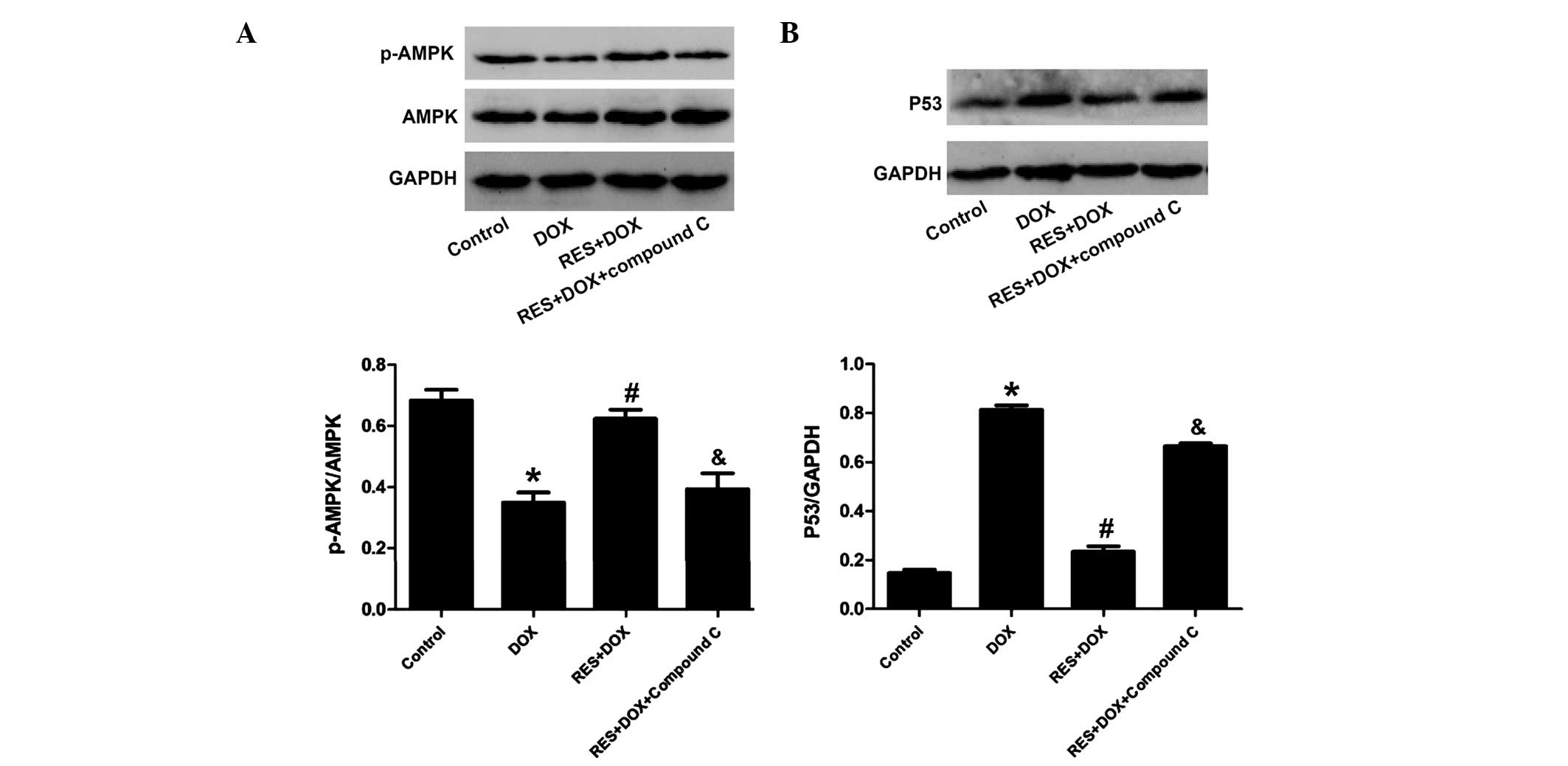
Compound C inhibits the cardioprotective
effects of resveratrol against DOX-mediated decreases in the
phosphorylation of AMPK and increases in the expression of P53 in
H9c2 cells
To explore the implication of the AMPK/P53 pathway
in the protective effects of resveratrol, H9c2 cells were
pre-treated with AMPK inhibitor compound C followed by treatment
with DOX and resveratrol. The results showed that compound C
significantly attenuated the inhibitory effects of resveratrol on
the DOX-mediated reduction of AMPK phosphorylation and increase of
P53 expression (Fig. 3). These
results further indicated that the AMPK/P53 pathway was involved in
the protective effects of resveratrol.
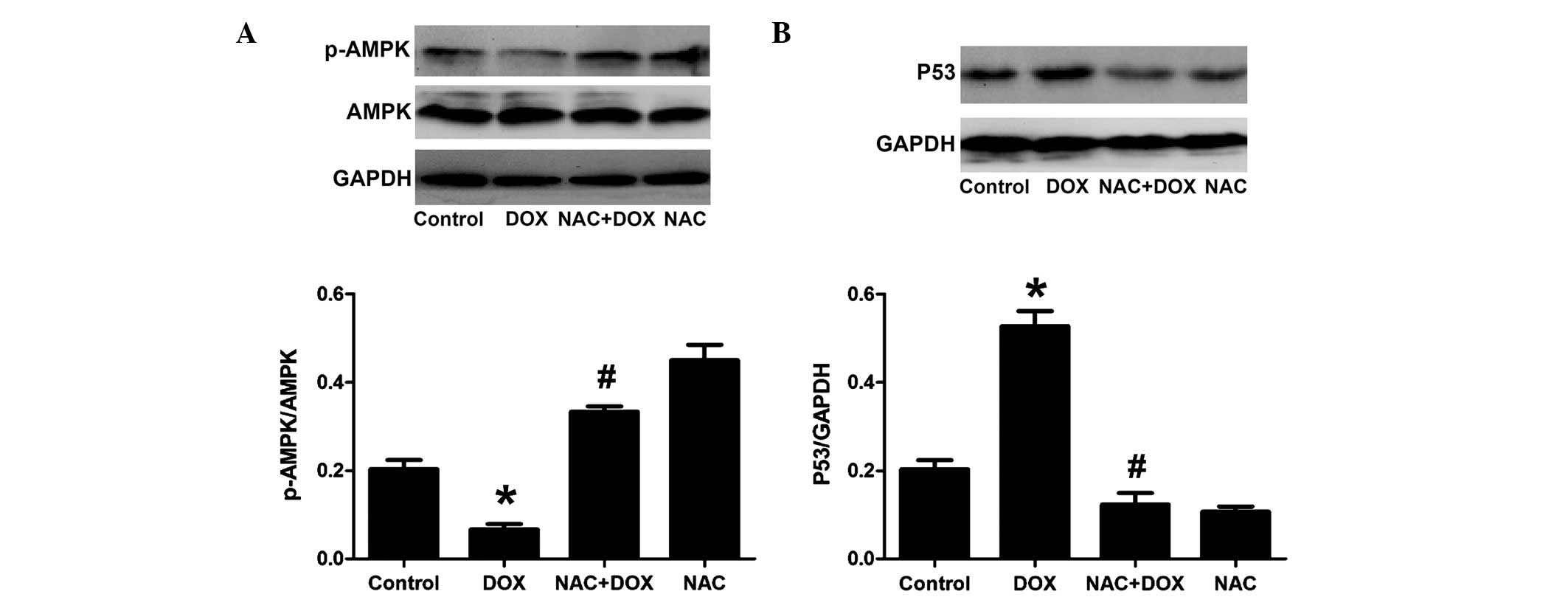 | Figure 3Compound C inhibits the effect of RES
on p-AMPK and P53 in H9c2 cells. Control group, H9c2 cardiac cells
incubated in 0.5% FBS DMEM for 24 h; DOX group, treated with DOX (5
µM) for 24 h; RES + DOX group, treated with RES (25
µM) for 30 min prior to exposure to DOX (5 µM) for 24
h; RES + DOX + compound C group, treated with compound C (10
µM) for 60 min prior to stimulation with RES, followed by a
24-h culture with DOX. (A) AMPK phosphorylation and (B) P53
expression were analyzed by immunoblotting. Relative levels of
p-AMPK vs. total AMPK and P53 in each sample as determined by
densitometry. Values are expressed as the mean ± standard error
(n=3). *P<0.05, compared with the control group;
#P<0.05, compared with the DOX-treated group. DOX,
doxorubicin; RES, resveratrol; p-AMPK, phosphorylated adenosine
monophosphate-activated protein kinase. |
Resveratrol attenuates DOX-induced
reduction of Bcl-2 and enhancement of Bax expression in
cardiomyocytes
To further investigate the protective effects of
resveratrol against DOX-mediated toxicity in H9c2 cells, the
expression of Bcl-2 and Bax was examined. As illustrated in
Fig. 4, DOX markedly decreased the
expression of Bcl-2 and increased the expression of Bax. Of note,
following pre-treatment with resveratrol, the levels of Bax were
decreased, whereas Bcl-2 levels increased, indicating that
resveratrol exerted anti-apoptotic effects on DOX-treated H9c2
cells. However, compound C abolished the protective effects of
resveratrol.
 | Figure 4Effects of RES on the changes of Bcl-2
and Bax expression induced by DOX in H9c2 cells. Control group,
H9c2 cardiac cells incubated in 0.5% FBS DMEM for 24 h; DOX group,
treated with DOX (5 µM) for 24 h; RES + DOX group, treated
with RES (25 µM) for 30 min prior to exposure to DOX (5
µM) for 24 h; RES + DOX + compound C group, treated with
compound C (10 µM) for 60 min prior to stimulation with RES,
followed by a 24-h culture with DOX. (A) Western blots demonstrate
the expression changes of Bcl-2 and Bax protein. (B) Quantification
of data from A by densitometric analysis. Values are expressed as
the mean ± standard error (n=3). *P<0.05, compared
with the control group; #P<0.05, compared with the
DOX-treated group; &P<0.05, compared with the RES
+ DOX group. DOX, doxorubicin; RES, resveratrol; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein. |
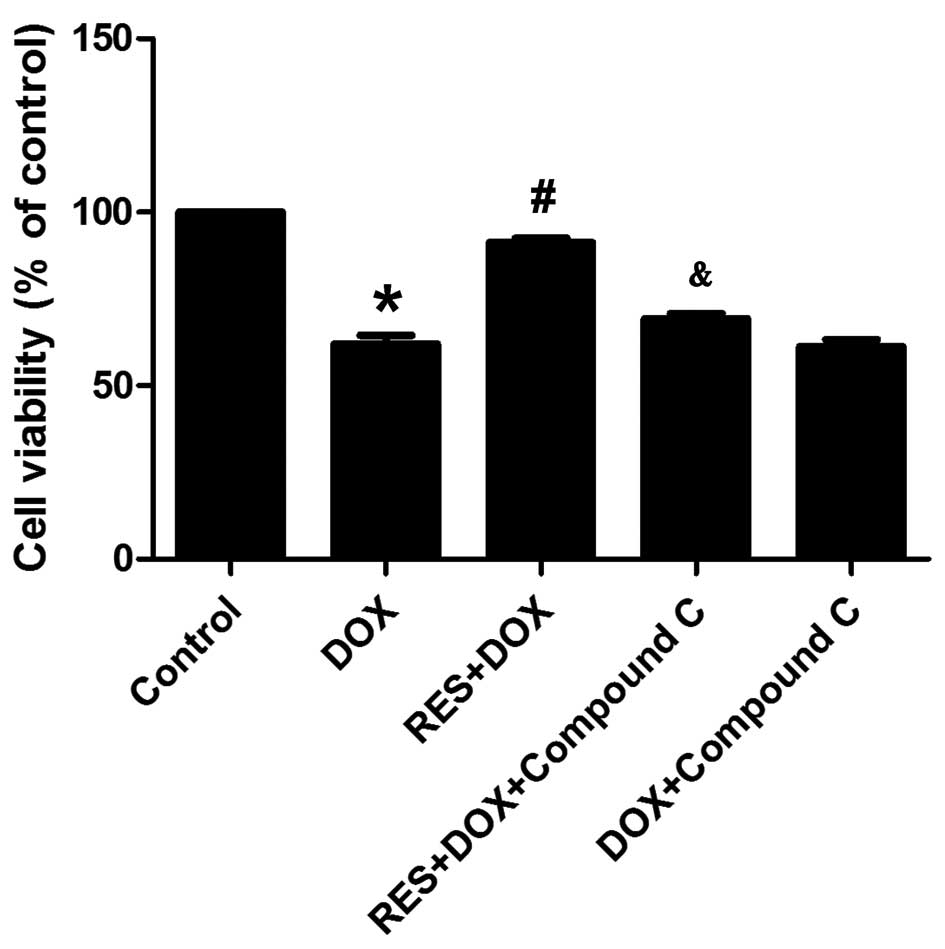
Resveratrol inhibits DOX-induced
cytotoxicity
Fig. 5 shows that
DOX treatment significantly decreased the viability of H9c2 cells
and induced marked cytotoxicity. However, pre-treatment with
resveratrol significantly increased the cell viability and
ameliorated the DOX-induced cytotoxicity. In addition, compound C
abolished the protective effects of resveratrol as indicated by
decreased cell viability and the induction of marked
cytotoxicity.
Resveratrol reduces DOX-induced apoptosis
in H9c2 cells
Fig. 6 shows that
DOX induced apoptosis in a large percentage of H9c2 cells. However,
resveratrol inhibited DOX-induced H9c2-cell apoptosis.
Pre-treatment of the H9c2 cells with compound C abolished the
protective effects of resveratrol.
Discussion
Although studies on DOX-derived cardiotoxicity have
been performed for decades (17),
the underlying mechanisms have remained to be fully elucidated. It
is known that oxidative stress-induced cardiomyocyte apoptosis and
death is an major molecular mechanism involved in DOX-induced
cardiotoxicity (18,19). The present study observed that cell
viability was markedly decreased and cell apoptosis was
significantly increased following DOX-induced cellular injury.
Resveratrol potently protects cardiomyocytes from
apoptosis and reduces the risk of cardiovascular disease (20). Oktem et al (21) reported that resveratrol protects
cardiomyocytes from DOX-induced apoptosis. In addition, resveratrol
was shown to enhance the anti-cancer effects of DOX and to
simultaneously protect against DOX-induced cardiotoxicity (9). However, the underlying mechanism of
the cardioprotective effects of resveratrol has remained to be
fully elucidated.
Shirwany et al (22) have reported that AMPK inhibits the
proliferation of non-malignant cells. P53 is a member of the P53
tumor suppressor family and acts as a critical regulator of
numerous cellular processes, including cell-cycle arrest and
apoptosis (23). Studies have
reported that the AMPK/P53 pathway has an important role in
DOX-induced cardiomyocyte death (24,25).
The present study found that the phosphorylation of AMPK protein
was significantly decreased, while the expression of P53 protein
was significantly increased in DOX-treated H9c2 cells. In addition,
the expression of Bax protein was significantly increased, while
the expression of Bcl-2 protein was markedly reduced in DOX-treated
H9c2 cells. These results supported that the AMPK/P53 pathway was
involved in DOX-induced cardiomyocyte apoptosis.
To further elucidate the molecular mechanism of the
cardioprotective effects of resveratrol, its effect on the AMPK/P53
pathway was assessed in DOX-treated H9c2 cells. The results
demonstrated that resveratrol significantly attenuated DOX-induced
decreases in AMPK activation and increases of P53 expression. In
addition, resveratrol significantly reduced the DOX-induced
enhancement of Bax and the decrease of Bcl-2 protein levels in H9c2
cells. Furthermore, the present study indicated an association of
ROS and the deactivation of AMPK in DOX-treated H9c2 cells.
Oxidative stress is defined as an imbalanced redox state in which
pro-oxidants overwhelm antioxidant capacity, resulting in increased
ROS production. ROS has been implicated in the pathogenesis of
DOX-induced H9c2 cardiomyocyte apoptosis. AMPK, which is a cellular
energy sensor and regulator, as well as a pressure sensor,
regulates ROS/redox balance, cell apoptosis and cell proliferation
to maintain cellular homeostasis. Thus, it was hypothesized in the
present study that resveratrol protects against DOX-induced H9c2
cardiomyocyte apoptosis via reduce generation of ROS, which
activates AMPK and the expression of p53 protein. In addition,
compound C, an inhibitor of AMPK phosphorylation, reversed the
protective effects of resveratrol by significantly increasing
apoptosis of H9c2 cells, inhibiting the phosphorylation of AMPK and
increasing the expression of P53. These results indicated that
resveratrol inhibited the generation of ROS and thereby activated
AMPK to prevent DOX-induced cardiotoxicity.
In conclusion, the present study showed that
resveratrol suppressed DOX-induced cardiomyocyte apoptosis via
increasing AMPK phosphorylation and inhibiting P53 expression, as
well as inducing Bcl-2 and downregulating Bax expression. These
results suggested that resveratrol represents a promising, novel
drug candidate for the treatment and prevention of DOX-induced
cardiotoxicity.
Acknowledgments
This work was supported by a grant from the Graduate
Student Research Innovation Project of Hunan province (grant no.
CX2013B397).
References
|
1
|
Menna P, Recalcati S, Cairo G and Minotti
G: An introduction to the metabolic determinants of anthracycline
cardiotoxicity. Cardiovasc Toxicol. 7:80–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lipshultz SE, Karnik R, Sambatakos P,
Franco VI, Ross SW and Miller TL: Anthracycline-related
cardiotoxicity in childhood cancer survivors. Curr Opin Cardiol.
29:103–112. 2014. View Article : Google Scholar
|
|
3
|
Spallarossa P, Garibaldi S, Altieri P,
Fabbi P, Manca V, Nasti S, Rossettin P, Ghigliotti G, Ballestrero
A, Patrone F, et al: Carvedilol prevents doxorubicin-induced free
radical release and apoptosis in cardiomyocytes in vitro. J Mol
Cell Cardiol. 37:837–846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Renaud J, Bournival J, Zottig X and
Martinoli MG: Resveratrol protects DAergic PC12 cells from high
glucose-induced oxidative stress and apoptosis: Effect on p53 and
GRP75 localization. Neurotox Res. 25:110–123. 2014. View Article : Google Scholar :
|
|
5
|
Liu MH, Yuan C, He J, Tan TP, Wu SJ, Fu
HY, Liu J, Yu S, Chen YD, Le QF, et al: Resveratrol protects PC12
cells from high glucose-induced neurotoxicity Via PI3K/Akt/FoxO3a
pathway. Cell Mol Neurobiol. 35:513–522. 2015. View Article : Google Scholar
|
|
6
|
Das S, Falchi M, Bertelli A, Maulik N and
Das DK: Attenuation of ischemia/reperfusion injury in rats by the
anti-inflammatory action of resveratrol. Arzneimittelforschung.
56:700–706. 2006.
|
|
7
|
Tatlidede E, Sehirli O, Velioğlu-Oğünc A,
Cetinel S, Yeğen BC, Yarat A, Süleymanoğlu S and Sener G:
Resveratrol treatment protects against doxorubicin-induced
cardiotoxicity by alleviating oxidative damage. Free Radic Res.
43:195–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shankar S, Singh G and Srivastava RK:
Chemoprevention by resveratrol: Molecular mechanisms and
therapeutic potential. Front Biosci. 12:4839–4854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rezk YA, Balulad SS, Keller RS and Bennett
JA: Use of resveratrol to improve the effectiveness of cisplatin
and doxorubicin: Study in human gynecologic cancer cell lines and
in rodent heart. Am J Obstet Gynecol. 194:e23–e26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park DG: Antichemosensitizing effect of
resveratrol in cotreatment with oxaliplatin in HCT116 colon cancer
cell. Ann Surg Treat Res. 86:68–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H,
Chen W, Shen T, Han X and Huang S: Hydrogen peroxide inhibits mTOR
signaling by activation of AMPKalpha leading to apoptosis of
neuronal cells. Lab Invest. 90:762–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Towler MC and Hardie DG: AMP-activated
protein kinase in metabolic control and insulin signaling. Circ
Res. 100:328–341. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen MB, Wu XY, Gu JH, Guo QT, Shen WX and
Lu PH: Activation of AMP-activated protein kinase contributes to
doxorubicin-induced cell death and apoptosis in cultured myocardial
H9c2 cells. Cell Biochem Biophys. 60:311–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okoshi R, Ozaki T, Yamamoto H, Ando K,
Koida N, Ono S, Koda T, Kamijo T, Nakagawara A and Kizaki H:
Activation of AMP-activated protein kinase induces p53-dependent
apoptotic cell death in response to energetic stress. J Biol Chem.
283:3979–3987. 2008. View Article : Google Scholar
|
|
15
|
Liu J, Mao W, Ding B and Liang CS:
ERKs/p53 signal transduction pathway is involved in
doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am
J Physiol Heart Circ Physiol. 295:H1956–H1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo R, Lin J, Xu W, Shen N, Mo L, Zhang C
and Feng J: Hydrogen sulfide attenuates doxorubicin-induced
cardiotoxicity by inhibition of the p38 MAPK pathway in H9c2 cells.
Int J Mol Med. 31:644–650. 2013.PubMed/NCBI
|
|
17
|
Gu J, Hu W and Zhang DD: Resveratrol, a
polyphenol phytoalexin, protects against doxorubicin-induced
cardiotoxicity. J Cell Mol Med. 19:2324–2328. PubMed/NCBI
|
|
18
|
Wang X, Wang XL, Chen HL, Wu D, Chen JX,
Wang XX, Li RL, He JH, Mo L, Cen X, et al: Ghrelin inhibits
doxorubicin cardiotoxicity by inhibiting excessive autophagy
through AMPK and p38-MAPK. Biochem Pharmacol. 88:334–350. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo R, Wu K, Chen J, Mo L, Hua X, Zheng D,
Chen P, Chen G, Xu W and Feng J: Exogenous hydrogen sulfide
protects against doxorubicin-induced inflammation and cytotoxicity
by inhibiting p38MAPK/NFkB pathway in H9c2 cardiac cells. Cell
Physiol Biochem. 32:1668–1680. 2013.
|
|
20
|
Das DK, Mukherjee S and Ray D: Erratum to:
Resveratrol and red wine, healthy heart and longevity. Heart Fail
Rev. 16:425–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oktem G, Uysal A, Oral O, Sezer ED,
Olukman M, Erol A, Akgur SA and Bilir A: Resveratrol attenuates
doxorubicin-induced cellular damage by modulating nitric oxide and
apoptosis. Exp Toxicol Pathol. 64:471–479. 2012. View Article : Google Scholar
|
|
22
|
Shirwany NA and Zou MH: AMPK: A cellular
metabolic and redox sensor. A minireview. Front Biosci (Landmark
Ed). 19:447–474. 2014. View
Article : Google Scholar
|
|
23
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang S, Song P and Zou MH: Inhibition of
AMP-activated protein kinase alpha (AMPKα) by doxorubicin
accentuates genotoxic stress and cell death in mouse embryonic
fibroblasts and cardiomyocytes: Role of p53 and SIRT1. J Biol Chem.
287:8001–8012. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones RG, Plas DR, Kubek S, Buzzai M, Mu
J, Xu Y, Birnbaum MJ and Thompson CB: AMP-activated protein kinase
induces a p53-dependent metabolic checkpoint. Mol Cell. 18:283–293.
2005. View Article : Google Scholar : PubMed/NCBI
|