Introduction
Bone tissue engineering provides a method for repair
of bone defects (1,2). The rapid development of bone tissue
engineering in combination with neogenetic osteoblast-like cells,
bone tissues induced by seed cells, genetic carrier delivery
systems, bioscaffolds and growth factors may have the potential to
replace autogenous bone transplantation in segmental bone repair
(3,4). Previous studies have used techniques
on the nanometer scale for tissue engineering (5,6).
Bone marrow stem cells (BMSCs) may be induced to
differentiate into osteoblast-like cells during osteogenesis.
However, there is a limited number of BMSCs in the bone marrow, and
BMSCs alone are unable to induce new bone formation (7). Therefore, it is difficult to use only
BMSCs to promote the repair of bone defects. In order to induce
BMSCs to differentiate into osteoblast-like cells, bone growth
factors are required (8).
Bone morphogenetic protein 2 (BMP2) is a bone growth
factor that may be used to induce osteogenesis (9). However, it is difficult to sustain
the slow release of high concentrations of exogenous bone growth
factors in the region of the bone defect. In addition, high doses
of exogenous bone growth factors may lead to adverse side-effects
in the patient. Therefore, it is crucial to identify a method of
releasing BMP2 slowly, and at high concentrations, within the
regions of defective bone by using genetic carriers (10).
Non-viral carriers are safer alternatives to viral
carriers (11). Chitosan is a
biocompatible and bioresorbable polymer of N-acetylglucosamine and
glucosamine, and it is extensively used as a carrier for bone
growth factors (12). Favorable
characteristics of chitosan for this purpose include its easy
availability, low cytotoxicity, low immunogenicity, excellent
biocompatibility and improved biodegradability. A previous study
confirmed that chitosan may deliver exogenous BMP2 into various
seed cells, such as BMSCs (13).
In addition, chitosan nanoparticles are advantageous for delivering
bone growth factors or gene sequences into seed cells in order to
induce the formation of bone tissues with high efficiency (14) due to their excellent properties of
infiltration and absorption. In the course of delivery, DNA
combined with chitosan nanoparticles was effectively protected, and
consequently, genes and proteins remained functional for longer
periods of time.
In our previous study, chitosan nanoparticles
containing plasmid-BMP2 (pBMP2) sequences (CNPBs) were constructed
through using re-coacervation and gene recombination techniques
(15). Our previous study
determined that the average diameter of chitosan nanoparticles was
90±20 nm. CNPBs with higher enveloping ratios were able to
effectively protect BMP2 genes. Following incubation of BMSCs with
CNPBs for 12 days, cells had maintained their normal morphology and
function (15).
In the present study, CNPBs were constructed with
different concentrations of pBMP2, specifically containing 50 µg/ml
(CPB50), 100 µg/ml (CPB100) or 200 µg/ml (CPB200) pBMP2. Following
treatment with CNPB, groups were separately phagocytized by BMSCs.
The transfection efficiency of the CNPBs was confirmed, and
features of osteoblast-like cells derived from BMSCs were observed
through histological staining, including alkaline phosphatase,
Wright's and von Kasso staining. Expression levels of
osteoblast-associated molecules, such as alkaline phosphatase
(ALP), osteoprotegerin (OPG), osteocalcin (OC) and osteopontin
(OPN), were detected and analyzed in the differentiated
osteoblast-like cells. Ectopic bone formation was observed
following the integration of polyglycolic acid (PGA) scaffolds with
CNPBs and BMSCs, which were implanted into the dorsal muscles of
Sprague-Dawley rats.
Materials and methods
Ethics statement
All animals used in this study were provided by the
experimental animal center of Zhejiang University, (Zhejiang,
China). The animal use and care protocol was approved by the
Institutional Animal Use and Care Committee of Zhejiang University.
Experimenters were approved through the Health Department of
Zhejiang Province for experiments with animals (certificate no.
×0901616).
Culture of BMSCs and construction of
CNPBs
A total of 10 female Sprague Dawley rats were
anesthetized with pentobarbital sodium (50 mg/kg, Sigma-Aldrich
Merck Millipore, Darmstadt, Germany) and sacrificed by cervical
dislocation (6-weeks old; weight, 180–220 g; sanitary degree). The
animals were kept in air-circulated housing a 14 h light/10 h dark
cycle at 22±2°C. Bones were dissected from the rats using a
surgical knife. Bone marrow cavities of tibias and femoral bones
were rinsed using phosphate buffered saline (PBS). Cells were
centrifuged at 300 × g for 5 min at 37°C and cultured in
Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life
Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Next,
BMSCs were subjected to digestion with 0.25% trypsin (Gibco; Thermo
Fisher Scientific, Inc.), cells were cultured until passage 3 and
used in the subsequent experiments. In our previous study (15), CNPBs were constructed using
re-coacervation and gene recombination techniques. Escherichia coli
bacteria were transfected with plasmids (cat. no. 6085-1; Addgene,
Cambridge, MA, USA) that included an insertion of BMP2 cDNA, and
extracted plasmid-DNA was subjected to restriction enzyme analysis.
The successful insertion of BMP2 cDNA fragments was confirmed
through DNA sequencing, performed by the laboratory in Zhejiang
University (Hangzhou, China). Purified pBMP2 was dissolved into
Na2SO4 solution. The concentrations of pBMP2
used were 50, 100 or 200 µg/ml. Subsequently, chitosan was mixed
with the different concentrations of pBMP2 to form CNPBs (CPB50,
CPB100 and CPB200, respectively). Chitosan was purchased from
Sigma-Aldrich; Merck Millipore (50 g; cat. no. 448877) (15).
Transfection efficiency of CNPBs
Experimental groups were established as follows: i)
Blank control (untreated BMSCs); ii) positive control (200 µg/ml
pBMP2); iii) CPB50 (chitosan + 50 µg/ml pBMP2); iv) CPB100
(chitosan + 100 µg/ml pBMP2); and v) CPB200 (chitosan + 200 µg/ml
pBMP2). BMSCs were seeded into 6-well culture plates (5.0×105
cells/well) for 24 h. Following the removal of DMEM, BMSCs were
rinsed using 1 ml DMEM. Next, pBMP2 and the different CNPB
concentrations were added separately into the experimental wells
for a 6-h incubation with Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Green fluorescent protein (Guduo
Corporation, Shanghai, China) was used as reporter. Following the
removal of the transfection solution, BMSCs were continuously
cultured with DMEM containing 1 ml 10% FBS for 24 h. BMSCs
transfected with CNPBs were photographed using an inverted
fluorescence microscope (XD30-RFL; Sunny Instruments Co., Ltd.,
Ningbo, China). The number of, and total area occupied by, the
cells in the micrographs were analyzed using ImageJ version 1.42
(National Institutes of Health, Bethesda, MD, USA).
Cellular staining
Alkaline phosphatase staining, Wright's staining,
and von Kossa staining were used for identification of
osteoblast-like cells differentiated from the transfected BMSCs.
Experimental groups included: i) Blank control; ii) CPB50; iii)
CPB100; and iv) CPB200. Initially, BMSCs were seeded into 6-well
culture plates (1.0×105 cells/well) and maintained overnight. Next,
DMEM was removed, cells were rinsed using 1 ml DMEM, the various
concentrations of CNPB were added for the aforementioned specific
treatment groups, and the cells were cultured for 24 h at 37°C.
For alkaline phosphatase staining, the transfected
BMSCs were fixed for 10 min in 4% paraformaldehyde and then rinsed
using distilled water for 5–10 min. Cells were immersed in alkaline
phosphatase solution for 25 min at 37°C. Finally, cells were rinsed
using distilled water for 5–10 min.
For Wright's staining, the transfected BMSCs were
rinsed with PBS and fixed in methanol for 3–5 min. Cells were
treated with Wright's staining solution for 2 min. Subsequently,
transfected BMSCs were treated with Wright's phosphate buffer
solution for 4–10 min and rinsed using distilled water.
Von Kossa staining was performed after transfected
BMSCs were fixed in 4% paraformaldehyde for 15 min. The cells were
then rinsed with distilled water. Then, cells were treated in 1%
silver nitrate solution and incubated under an ultraviolet lamp for
15 min. Finally, the cells were stained with 5% natrium
hyposulfurosum solution (Shengrui Transmission Corporation Ltd.,
Xian, China) for 2 min.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Experimental groups included: i) Blank control; ii)
CPB50; iii) CPB100; and iv) CPB200. BMSCs were seeded into 6-well
culture plates (1.0×105 cells/well) for 24 h culture, pBMP2 was
added, and each of the CNPB treatment groups were set up in
separate experimental wells for a 48 h transfection. RT-PCR was
used to determine the mRNA expression levels of ALP, OPG and OC in
osteoblast-like cells. mRNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The primers used for
RT-PCR of ALP, OPG and OC are presented in Table I. A PCR kit with DNA-free sensitive
Taq DNA polymerase (Amresco, LLC, Solon, OH, USA) was used.
The RT conditions were: 37°C for 1 h and 95°C for 5 min in order to
deactivate M-MLV reverse transcriptase. The thermocycling
conditions were as follows: 95°C for 15 min, 30 cycles at 95°C for
15 sec, 52°C 30 sec, 72°C for 30 sec and 72°C for 2 min.
 | Table I.Primers of ALP, OPG and OC. |
Table I.
Primers of ALP, OPG and OC.
| Primers | Sequences
(5′-3′) | Length (bp) |
|---|
| Actin | F:
CTAAGGCCAACCGTGAAA; | 724 |
|
| R:
TGGAAGGTGGACAGTGAG |
|
| ALP | F:
GGTGGACGCAAAAATTTCAT; | 379 |
|
| R:
ATGCCTTGATCGGTTTGTTC |
|
| OPG | F:
CGACTGGAGAGCGAAGAC; | 364 |
|
| R:
CTAAGCAATGTTGGCGTA |
|
| OC | F:
GAGGACCCTCTCTCTGCTCA; | 405 |
|
| R:
AGCTGTGCCGTCCATACTTT |
|
| OPN | F:
CATCAGAGCCACCACTTTCA | 273 |
|
| R:
TCAGGGCCCAAAACACTATC |
|
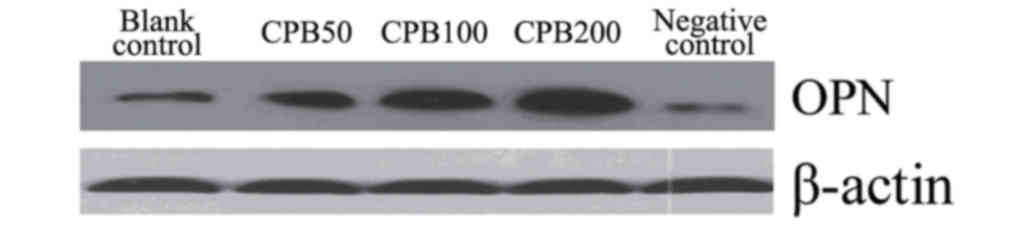
Western blotting
Protein expression levels of OPN in cells was
detected using western blot analysis. Cells were rinsed twice using
PBS. Cell lysis buffer (100 µl) was added into each culture well
(cat. no. P0013; Beyotime Institute of Biotechnology, Beijing,
China). Cells were centrifuged for 5 min at 300 × g at 37°C.
Polyvinylidene fluoride membranes (Merck Millipore) were rinsed
using PBS with Tween-20 (PBST). The membranes were incubated with
the rabbit anti-OPN primary antibody (cat. no. 000019-R; 1:500;
CellChip Biotechnology Co., Ltd., Beijing, China) for 1 h at 37°C.
PBST was used to wash the membranes 4 times for 10 min each. The
membranes were then incubated for 1 h with a secondary
biotin-labeled rabbit anti-goat IgG antibody (cat. no. E030330;
1:5,000; EarthOx Life Sciences, Millbrae, CA, USA). PBST was used
to wash the membranes 4 times for 10 min each. The protein was then
visualized using a Developer and fixer kit (cat. no. P0019,
Beyotime Institute of Biotechnology), and the development time was
1–2 min.
Ectopic bone formation
Polyglycolic acid scaffold materials (Dexon,
Shanghai, China) were sterilized using 75% alcohol and then cut
into 0.5×0.5×0.5 cm2 pieces. The PGA scaffolds were soaked in DMEM
for 2 h, then 2 ml BMSCs (5.0×107 cells/ml) were seeded into the
scaffolds as the negative control group. The following treatment
groups were seeded onto the scaffolds: i) BMSCs + PGA + CPB50; ii)
BMSCs + PGA + CPB100; and iii) BMSCs + PGA + CPB200. The PGA
scaffolds were incubated for 5 days to ensure that cells and
carriers had adhered successfully. The scaffolds were then
implanted into the dorsal muscles of rats in order to determine the
ectopic bone formation. Three scaffolds were implanted per
treatment group. After 2 months, the 4 rats were euthanized by
cervical dislocation and the regions with the implanted scaffolds
were dissected. The tissues were then subjected to hematoxylin and
eosin staining.
Statistical analysis
Data are presented as the mean ± standard error.
One-way analysis of variance was performed using SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate statistically significant difference.
Results
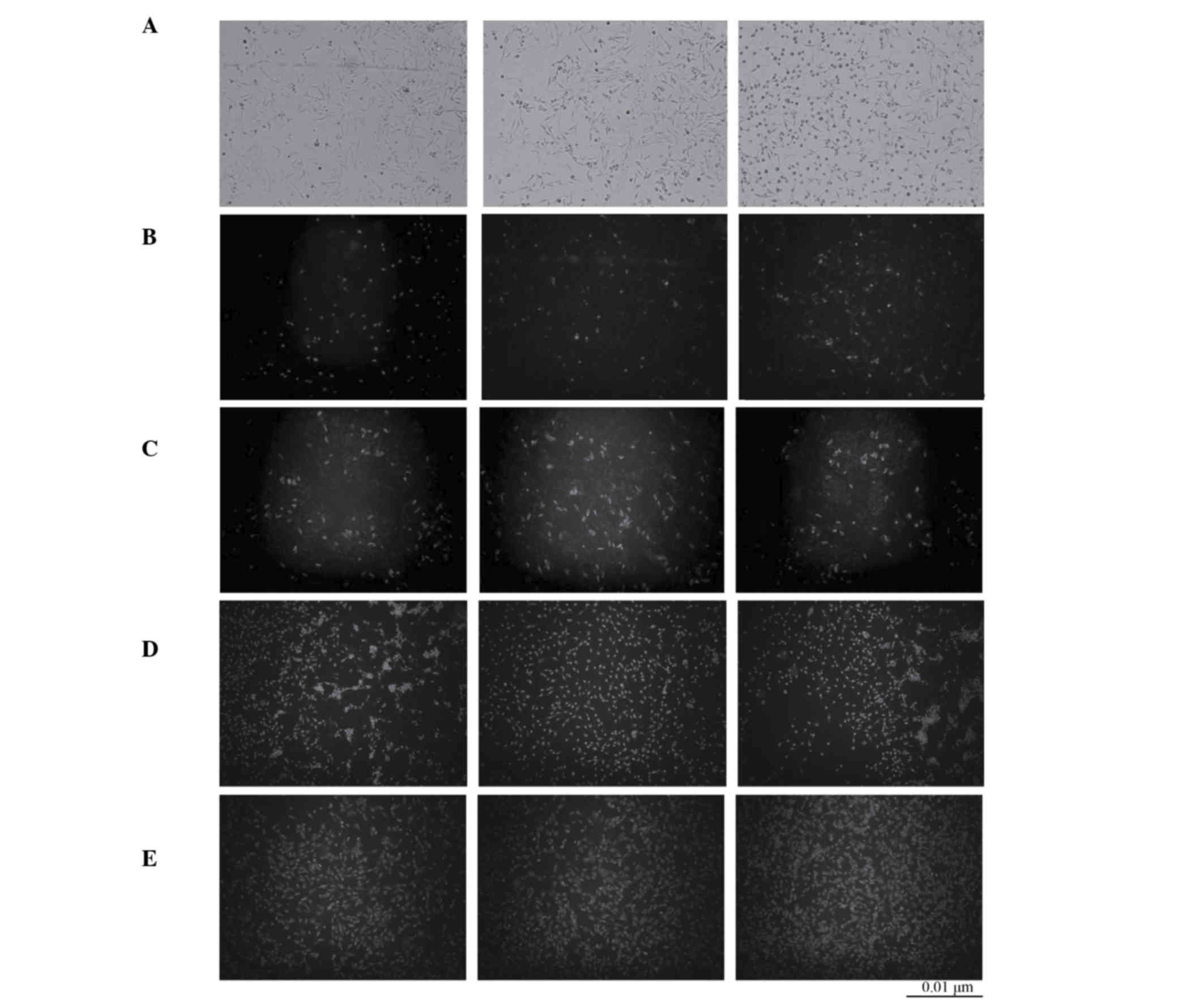
Transfection efficiency of CNPBs
Following 24 h transfection of BMSCs no green
fluorescence was observed in the blank control BMSC (untreated)
group. However, green fluorescence was observed in the pBMP2
(positive control) and CNPB at different concentrations groups.
This indicated that chitosan nanoparticles delivered the BMP2 gene
into BMSCs and successfully displayed green fluorescence. The
fluorescence of the pBMP2 and CPB50 groups was weaker compared with
the CPB100 and CPB200 groups and particle analysis revealed that
there was a greater quantity of fluorescence particles and in
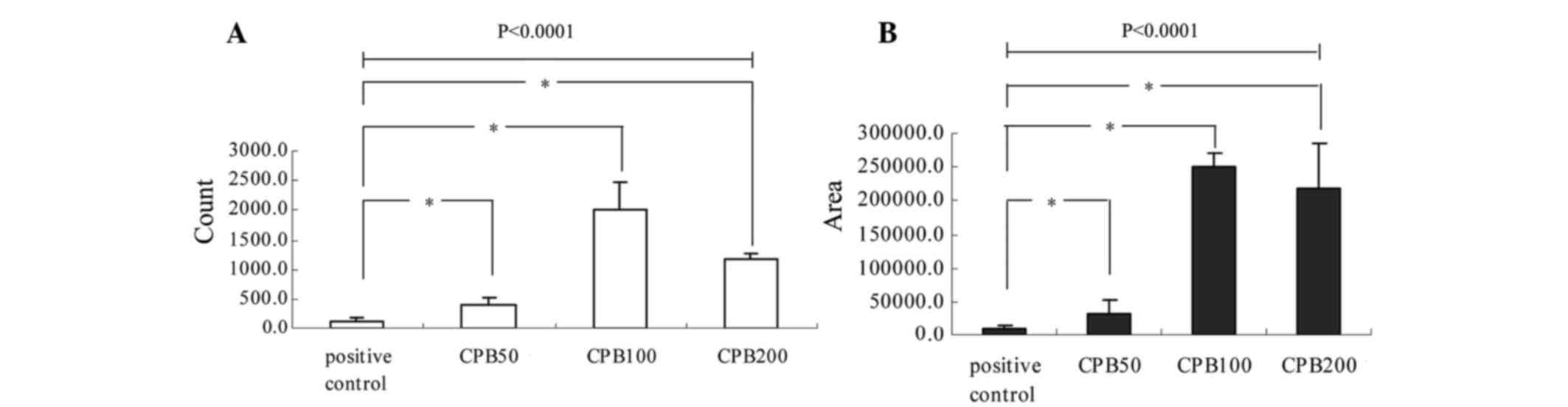
greater total areas in the CPB100 and CPB200 groups (Table II, and Figs. 1 and 2). Statistical analysis revealed that
there was a significant difference in particle quantity among the
groups (P<0.0001). Pairwise comparisons determined that there
was a significant difference in particle quantity between any two
groups, with the exception of the CPB50 and pBMP2 groups (P=0.243).
There was also a significant difference in total areas with
fluorescence particles among the groups (P<0.0001). The
difference in total areas between any two groups was significant,
except between the CPB100 and CPB200 treatment groups (P=0.321;
Table II, Figs. 1 and 2).
 | Table II.Quantity and total area of
fluorescence particles in every group following transfection of
bone marrow stem cells with chitosan nanoparticles containing
plasmid-bone morphogenetic protein 2 sequences. |
Table II.
Quantity and total area of
fluorescence particles in every group following transfection of
bone marrow stem cells with chitosan nanoparticles containing
plasmid-bone morphogenetic protein 2 sequences.
| Treatment
Groups | Count | Total area
(µm2) |
|---|
| Positive
control | 133.3±45.0 |
9,189.3±5,632.1 |
| CPB50 |
401.3±132.8 |
33,494.0±19,076.8 |
| CPB100 | 1,997.3±490.7 |
250,702.6±20,510.9 |
| CPB200 | 1,162.0±105.1 |
219,652.0±65,961.3 |
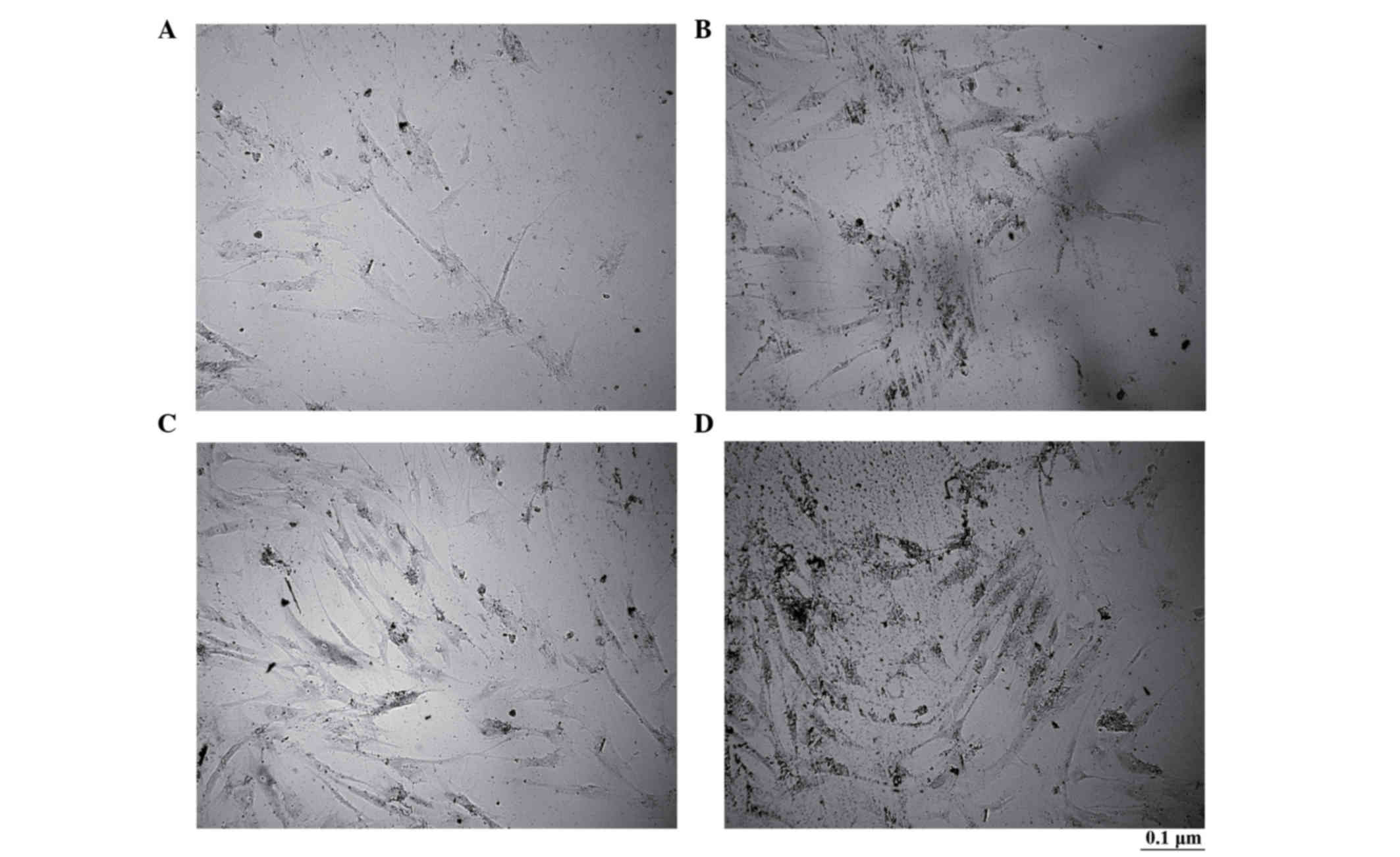
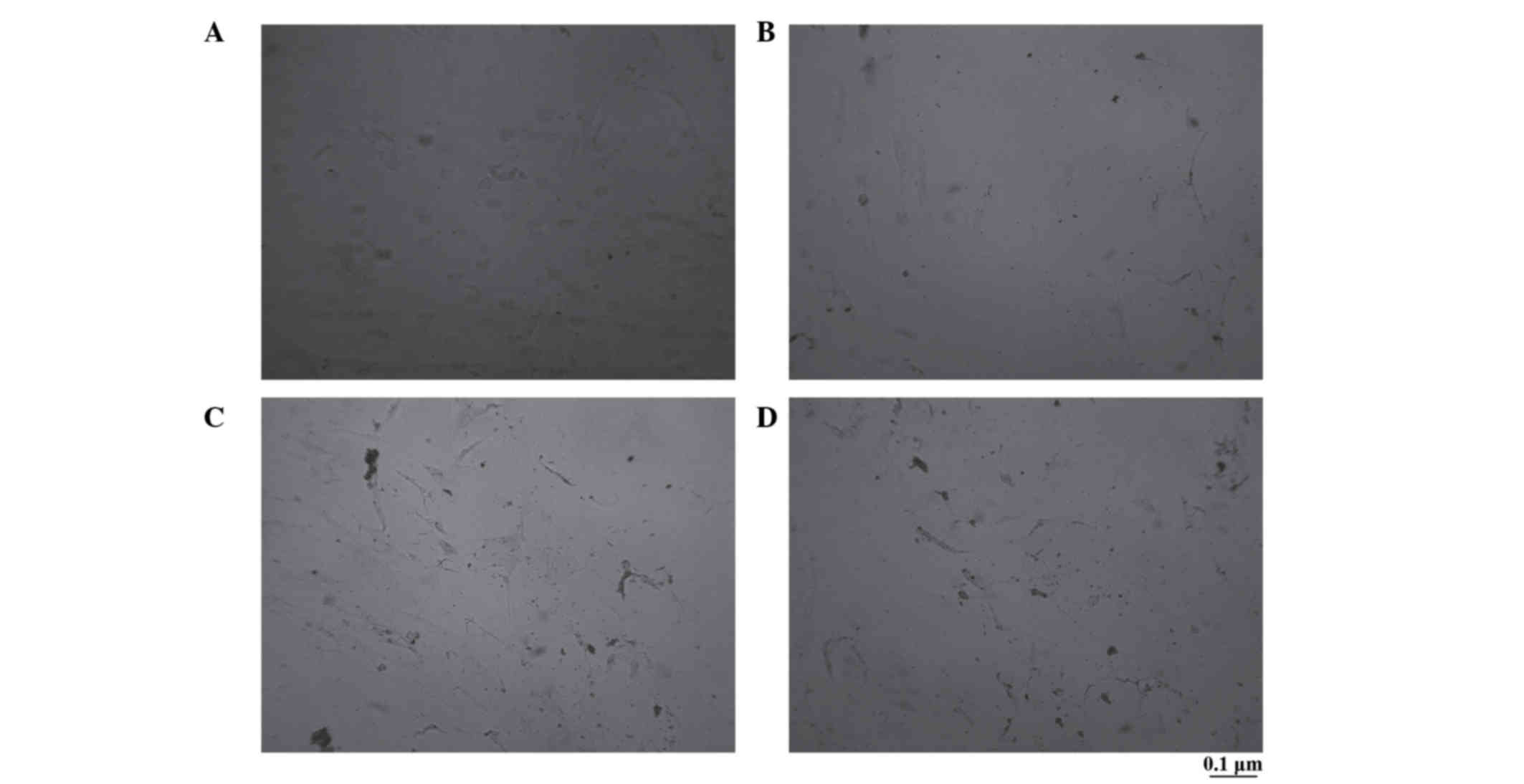
Alkaline phosphatase staining of
osteoblast-like cells differentiated from transfected BMSCs with
CNPBs
There were no obvious brownish-black particles
observed in the control group; however, there were numerous
brownish-black particles in the CPB50, CPB100 and CPB200 groups. In
addition, more particles were observed in the CPB200 group compared
with the CPB50 and CPB100 groups (Fig.
3).
Wright's staining of osteoblast-like
cells differentiated from BMSCs transfected with CNPBs
The staining revealed that BMSCs of a smaller size
were fibriform in the control group (Fig. 4A). However, osteoblast-like cells
that differentiated from BMSCs were observed to be larger in size,
with inflated cell bodies and nucleoli stained dark blue in the
CBP50, CBP100 and CBP200 treatment groups. In addition,
osteoblast-like cells also had characteristic stick-like
prominences (Fig. 4).
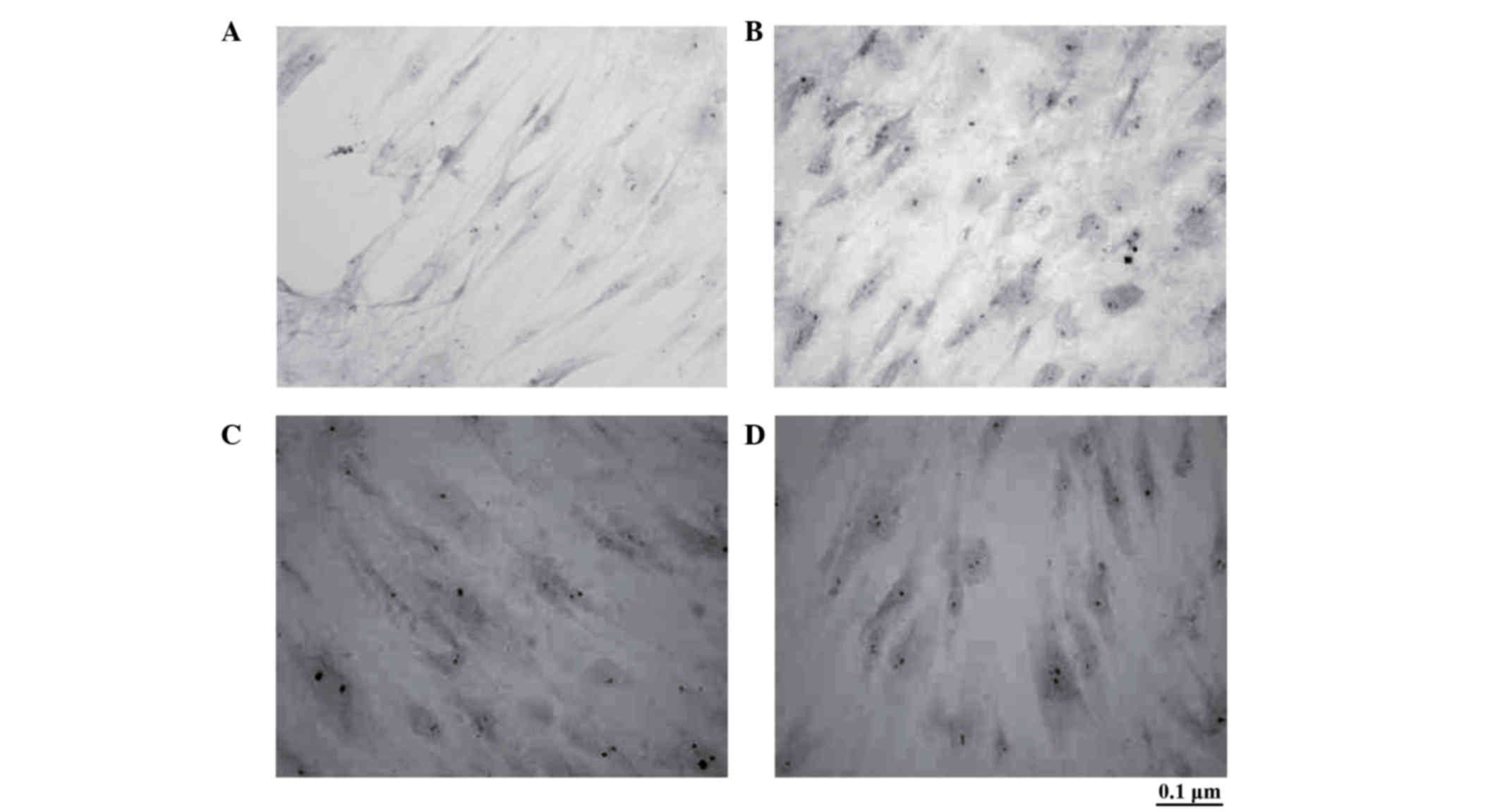
Von Kossa staining of osteoblast-like
cells differentiated from BMSCs transfected with CNPBs
No staining was observed in the control group
(Fig. 5A). Black particles,
indicating a positive reaction of calcium phosphate in the
mineralized extracellular matrix of osteoblasts, were observed in
the CPB50, CPB100 and CPB200 treatment groups (Fig. 5B-D).
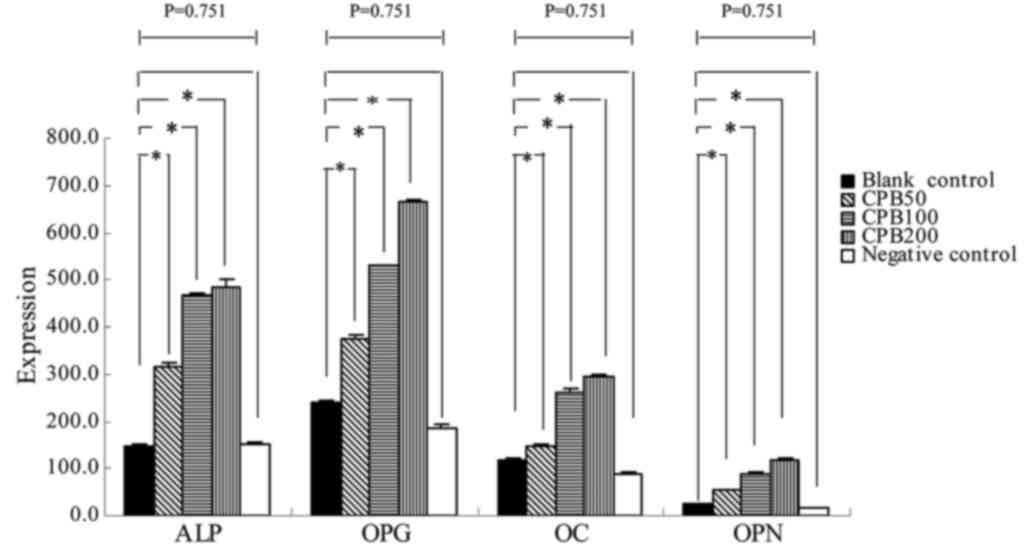
Expression levels of ALP, OPG, OC and
OPN in osteoblast-like cells differentiated from BMSCs transfected
with CNPBs
The RT-PCR determined that the mRNA expression
levels of ALP, OPG, OC and OPN were increased in the CNPB treatment
groups compared with the blank and negative control groups
(Fig. 6). The highest mRNA
expression levels of ALP, OPG, OC and OPN were identified in the
CPB200 treatment group (Table
III; Figs. 6–8). Statistical analysis revealed that
there was a significant difference between any two groups
(P<0.0001), except between the blank and negative control groups
(P=0.751).
 | Table III.Expression levels of ALP, OPG, OC,
and OPN in osteoblast-like cells differentiated from BMSCs
transfected with CNPBs. |
Table III.
Expression levels of ALP, OPG, OC,
and OPN in osteoblast-like cells differentiated from BMSCs
transfected with CNPBs.
|
| mRNA
expression | Protein
expression |
|---|
|
|
|
|
|---|
| Groups Objective
genes | ALP | OPG | OC | OPN |
|---|
| Blank control | 148.8±4.5 | 238.1±7.1 | 116.0±4.3 | 24.2±2.2 |
| CPB50 | 314.0±9.5 | 372.7±9.8 | 147.7±4.3 | 53.3±2.4 |
| CPB100 | 467.0±6.3 | 529.3±2.5 | 262.2±5.4 | 88.7±1.8 |
| CPB200 | 485.2±15.5 | 666.0±3.9 | 292.8±6.6 | 117.6±5.2 |
| Negative
control | 151.3±6.2 | 186.2±6.3 | 88.7±2.1 | 16.6±0.7 |
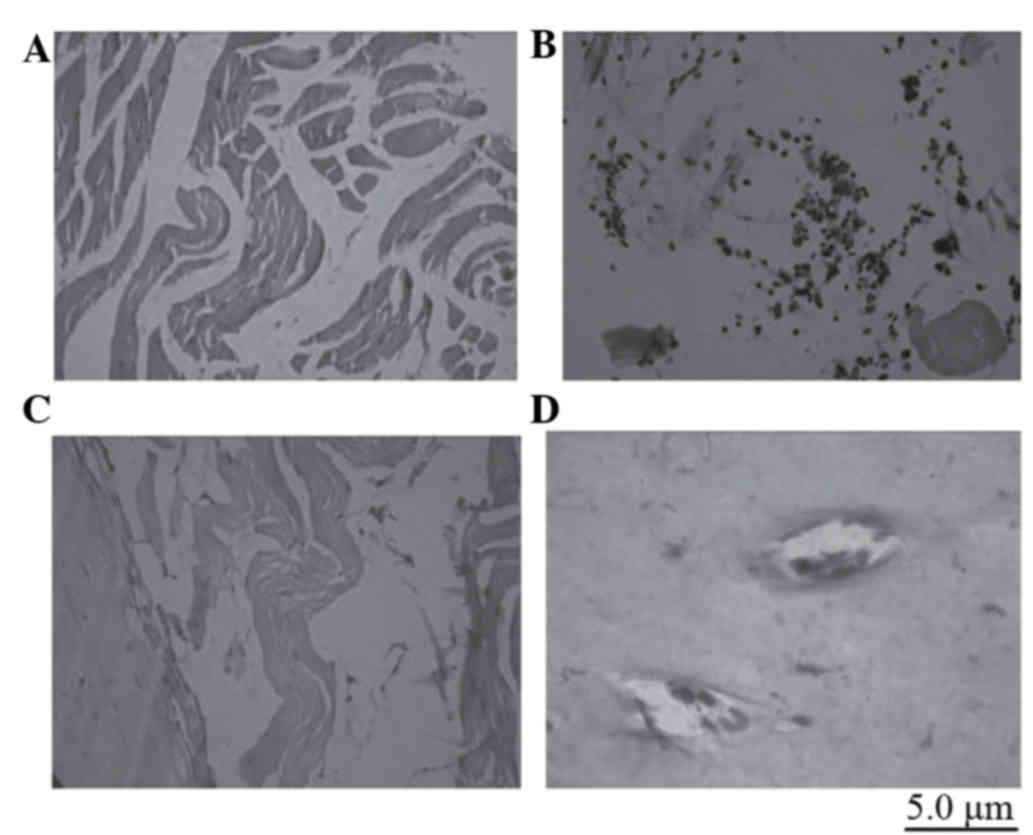
Ectopic bone formation
Two months after the PGA scaffolds that were
integrated with BMSCs transfected with CNPBs were implanted into
the muscles of rats, by touch, the sensation of a ‘string-like’
object in the subdermal implanted regions of the control and CPB50
groups was identified. However, a node-like object was identified
in the subdermal implanted regions of the CPB100 and CPB200 groups.
After the implanted regions were excised, numerous muscle fibers
were observed in the control and CPB50 groups. In the CPB100 and
CPB200 groups, a dark-red node with white spots was identified,
which was surrounded by fibrous tissues. Histological staining
revealed that there was novel bone formation had occurred in the
CPB100 and CPB200 treatment groups (Fig. 9). This bone formation was more
evident in the CBP200 group. In the positions of white spots were
determined to be cartilage islands (Fig. 9). The PGA scaffolds were absorbed
in all treatment groups.
Discussion
Chitosan is considered to be an effective non-viral
carrier and is extensively used for tissue engineering (13,16).
In our previous study (15),
chitosan nanoparticles had excellent biocompatibility. On the basis
of these results, the present study used chitosan nanoparticles as
carriers of genetic material. CNPBs had excellent transfection
efficiency to BMSCs and induced the differentiation and
proliferation of osteoblast-like cells. In addition, CNPBs induced
ectopic formation of new bone in rats. Therefore, the present study
determined that CNPBs are promising carriers of genetic
material.
BMSCs have been used extensively in bone tissue
engineering. As BMSCs are capable of multi-directional
differentiation, bone growth factors and osteogenesis are important
factors to consider for successful bone tissue engineering. Bone
growth factors primarily include the BMP family, transforming
growth factor-β, dexamethasone, the active form of vitamin D
[1,25-(OH) 2D3], vitamin C and sodium glycerophosphate. The
exogenous BMP family is a group of acid polypeptides with low
molecular weights. BMP2 may promote cell proliferation and induce
seed cells to differentiate into osteoblast-like cells, which may
result in the formation of new bone. Hou et al (16) determined that BMP2 had potent
osteoinductive properties in bone regeneration (16). The composite scaffold that Hou
et al (16) constructed of
recombinant human BMP2 (rhBMP2)-loaded collagen/chitosan
microspheres bridged bone defects and recanalized the bone-marrow
cavity. The results of a previous study indicated that BMP2 alone
had a positive effect on bone regeneration (17). Yilgor et al (18) revealed that treatment with BMP2
alone resulted in a higher ALP activity compared with treatment
with BMP7 (18).
In the present study, BMSCs were transfected with
CNPBs. BMP2 was released continuously during osteogenesis to
promote formation of new bone. Therefore, the present study
determined that BMSCs transfected with CNPBs at a higher
efficiency. The phenotype of osteoblast-like cells was confirmed
using alkaline phosphatase, Wright's, von Kossa staining. In
addition, the mRNA and protein expression levels of ALP, OPG, OC
and OPN in osteoblast-like cells differentiated from the
transfected BMSCs were recorded at higher levels compared with the
control group, which had untreated cells. In order to determine the
extent of ectopic bone formation, CNPBs were attached to PGA
scaffolds to induce new bone formation following implantation into
the dorsal muscles of rats.
Chitosan gels loaded with BMP2 enhanced ALP activity
in BMSCs by 3.6-fold, and increased the calcium mineral deposition
of mesenchymal cells by 2.8-fold (19). In addition, chitosan gels loaded
with BMP2 induced synthesis of OC in BMSCs (19). The present study was consistent
with the findings of previous studies (20–22).
Zhao et al (20) determined
that a calcium phosphate-chitosan fibrous scaffold delivery system
with BMP2 promoted osteo-differentiation and resulted in increased
gene expression levels of ALP and OC (20). Shi et al (21) demonstrated that carboxymethyl
chitosan-BMP2 modified substrates significantly promoted ALP
activity and calcium mineral deposition of osteoblasts and human
bone marrow-derived mesenchymal stem cells (21). Additionally, bone formation was
observed in the quadriceps muscles of rats following the
implantation of rhBMP2-loaded chitosan carriers (22).
The BMP2-induced differentiation of BMSCs into
osteoblast-like cells ensures that BMP2 is able to recruit BMSCs
and promote their proliferation. During bone formation, BMP2
promotes cellular differentiation and proliferation through
autocrine and paracrine secretion. Following BMP2 binding to the
BMP2-specific receptor on the cell surface, Smad protein becomes
activated through signal transmission. Subsequently, Smad protein
is transported into the cell nuclei. Next, the nuclear factor,
runt-related transcription factor 2 (Runx2; also known as
core-binding factor subunit-α1), is activated through the
mitogen-activated protein kinase pathway. Following the combination
of Runx2 with Smad, specific phenotypes in osteoblast-like cells
are activated sequentially (16–22).
Finally, expression levels of ALP, OPG, OC and OPN were
upregulated, and BMSCs were differentiated into osteoblast-like
cells.
Scaffold materials may primarily include natural and
synthesis polymer and bioceramics (23). Natural polymers such as collagen,
chitosan-alginate gel and hyaluronic acid may be used. Synthesized
polymers that are biodegradable include polyactide, polyviol,
polyacrylic acid and polyethylene glycol. The primary bioceramics
used are calcium phosphate ceramics, hydroxyapatite and calcium
carbonate (24,25). Chen et al (26) used bilayered integrated scaffolds
in their study. Their findings revealed that mesenchymal stem cells
seeded in each layer of the bilayered gene-activated osteochondral
scaffold had a higher expression of BMP2 protein (26). Park et al (27) determined that chitosan-alginate
gel/mesenchymal stem cell/BMP2 composites were able to stimulate
novel bone formation. The primary aim of tissue engineering is to
construct a three-dimensional complex composed of seed cells and
biomaterials. Therefore, PGA scaffolds with the same excellent
biocompatibility and biodegradability have been used extensively in
bone tissue engineering. The present study involved the
transfection of BMSCs with CNPBs, and they were subsequently
incubated with PGA scaffolds. Cells adhered to, and proliferated
on, the PGA scaffolds. The histological staining revealed that the
PGA scaffolds had successfully degraded, and novel bone formation
was observed. The present study determined that PGA scaffolds with
three-dimensional structures provided a larger surface area, and
simulated the formation of natural bone structures. This allowed
for the slow release of BMP2 in the CNPBs, providing an excellent
‘shelter’ for osteoblast-like cells differentiated from BMSCs. This
promoted the osteogenic functions of osteoblast-like cells.
In conclusion, in the present study CNPBs were
successfully transfected into BMSCs with a high efficiency, and
BMSCs were promoted to differentiate into osteoblast-like cells
in vitro. Additionally, CNPBs upregulated the expression
levels of ALP, OPG, OC and OPN in osteoblast-like cells and induced
the formation of new ectopic bone in vivo.
Acknowledgements
The present study was supported by the Department of
Education Foundation of Zhejiang Province of China (grant nos.
20080180, Y201018976 and N20110143), Zhejiang Provincial Natural
Science Foundation of China (grant nos. Y2080340 and LY12H14004)
and Zhejiang Provincial and Ministry Joint Project (grant no.
WKJ2011-2-009).
Glossary
Abbreviations
Abbreviations:
|
BMP2
|
bone morphogenetic protein 2
|
|
CNPBs
|
chitosan nanoparticles containing
plasmid-bone morphogenetic protein 2 sequences
|
|
BMSC
|
bone marrow stem cells
|
|
pBMP2s
|
plasmid-BMP2s
|
|
PGA
|
polyglycolic acid
|
|
GFP
|
green fluorescent protein
|
References
|
1
|
Amini AR, Laurencin CT and Nukavarapu SP:
Differential analysis of peripheral blood- and bone marrow-derived
endothelial progenitor cells for enhanced vascularization in bone
tissue engineering. J Orthop Res. 30:1507–1515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takekawa M, Matsuda M and Ohotubo S:
Effect of irradiation on autogenous bone transplantation in rat
parietal bone. Histol Histopathol. 15:7–19. 2000.PubMed/NCBI
|
|
3
|
Erlwein O and McClure M: Gene delivery the
key to gene therapy: The case for foamy viruses. Ther Deliv.
2:681–684. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moawad HM and Jain H: Fabrication of
nano-macroporous glass-ceramic bioscaffold with a water soluble
pore former. J Mater Sci Mater Med. 23:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fazil M, Md S, Haque S, Kumar M, Baboota
S, Sahni JK and Ali J: Development and evaluation of rivastigmine
loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci.
47:6–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yadav A, Lomash V, Samim M and Flora SJ:
Curcumin encapsulated in chitosan nanoparticles: A novel strategy
for the treatment of arsenic toxicity. Chem Biol Interact.
199:49–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al Faqeh H, Nor Hamdan BM, Chen HC,
Aminuddin BS and Ruszymah BH: The potential of intra-articular
injection of chondrogenic-induced bone marrow stem cells to retard
the progression of osteoarthritis in a sheep model. Exp Gerontol.
47:458–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zou D, Han W, You S, Ye D, Wang L, Wang S,
Zhao J, Zhang W, Jiang X, Zhang X and Huang Y: In vitro
study of enhanced osteogenesis induced by HIF-1α-transduced bone
marrow stem cells. Cell Prolif. 44:234–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhakta G, Rai B, Lim ZX, Hui JH, Stein GS,
van Wijnen AJ, Nurcombe V, Prestwich GD and Cool SM: Hyaluronic
acid-based hydrogels functionalized with heparin that support
controlled release of bioactive BMP-2. Biomaterials. 33:6113–6122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hunziker EB, Enggist L, Küffer A, Buser D
and Liu Y: Osseointegration: The slow delivery of BMP-2 enhances
osteoinductivity. Bone. 51:98–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santos JL, Pandita D, Rodrigues J, Pêgo
AP, Granja PL and Tomás H: Non-viral gene delivery to mesenchymal
stem cells: Methods, strategies and application in bone tissue
engineering and regeneration. Curr Gene Ther. 11:46–57. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernández MS, Arias JI, Martínez MJ, Saenz
L, Neira-Carrillo A, Yazdani-Pedram M and Arias JL: Evaluation of a
multilayered chitosan-hydroxy-apatite porous composite enriched
with fibronectin or an in vitro-generated bone-like extracellular
matrix on proliferation and diferentiation of osteoblasts. J Tissue
Eng Regen Med. 6:497–504. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peschel D, Zhang K, Fischer S and Groth T:
Modulation of osteogenic activity of BMP-2 by cellulose and
chitosan derivatives. Acta Biomater. 8:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee D and Mohapatra SS: Chitosan
nanoparticle-mediated gene transfer. Methods Mol Biol. 433:127–140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu MT, Zhang QH, Zhai JJ and Liang X:
Construction and analysis of one kind of chitosan-coated BMP-2
nanoparticles as genetic carrier. Sichuan Da Xue Xue Bao Yi Xue
Ban. 42:485–489. 2011.PubMed/NCBI
|
|
16
|
Hou J, Wang J, Cao L, Qian X, Xing W, Lu J
and Liu C: Segmental bone regeneration using rhBMP-2-loaded
collagen/chitosan microspheres composite scaffold in a rabbit
model. Biomed Mater. 7:0350022012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Canter HI, Vargel I, Korkusuz P, Oner F,
Gungorduk DB, Cil B, Karabulut E, Sargon MF and Erk Y: Effect of
use of slow release of bone morphogenetic protein-2 and
transforming growth factor-Beta-2 in a chitosan gel matrix on
cranial bone graft survival in experimental cranial critical size
defect model. Ann Plast Surg. 64:342–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N
and Hasirci V: Incorporation of a sequential BMP-2/BMP-7 delivery
system into chitosan-based scaffolds for bone tissue engineering.
Biomaterials. 30:3551–3559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim S, Tsao H, Kang Y, Young DA, Sen M,
Wenke JC and Yang Y: In vitro evaluation of an injectable
chitosan gel for sustained local delivery of BMP-2 for osteoblastic
differentiation. J Biomed Mater Res B Appl Biomater. 99:380–390.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Tang M, Weir MD, Detamore MS and
Xu HH: Osteogenic media and rhBMP-2-induced differentiation of
umbilical cord mesenchymal stem cells encapsulated in alginate
microbeads and integrated in an injectable calcium
phosphate-chitosan fibrous scaffold. Tissue Eng Part A. 17:969–979.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi Z, Neoh KG, Kang ET, Poh CK and Wang
W: Surface functionalization of titanium with carboxymethyl
chitosan and immobilized bone morphogenetic protein-2 for enhanced
osseointegration. Biomacromolecules. 10:1603–1611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luca L, Rougemont AL, Walpoth BH, Gurny R
and Jordan O: The effects of carrier nature and pH on
rhBMP-2-induced ectopic bone formation. J Control Release.
147:38–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mishra S, Rajyalakshmi A and
Balasubramanian K: Compositional dependence of hematopoietic stem
cells expansion on bioceramic composite scaffolds for bone tissue
engineering. J Biomed Mater Res A. 100:2483–2491. 2012.PubMed/NCBI
|
|
24
|
Do TN, Lee WH, Loo CY, Zavgorodniy AV and
Rohanizadeh R: Hydroxyapatite nanoparticles as vectors for gene
delivery. Ther Deliv. 3:623–632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee GS, Park JH, Shin US and Kim HW:
Direct deposited porous scaffolds of calcium phosphate cement with
alginate for drug delivery and bone tissue engineering. Acta
Biomater. 7:3178–3186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Chen H, Li P, Diao H, Zhu S, Dong
L, Wang R, Guo T, Zhao J and Zhang J: Simultaneous regeneration of
articular cartilage and subchondral bone in vivo using MSCs induced
by a spatially controlled gene delivery system in bilayered
integrated scaffolds. Biomaterials. 32:4793–4805. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park DJ, Choi BH, Zhu SJ, Huh JY, Kim BY
and Lee SH: Injectable bone using chitosan-alginate gel/mesenchymal
stem cells/BMP-2 composites. J Craniomaxillofac Surg. 33:50–54.
2005. View Article : Google Scholar : PubMed/NCBI
|