Introduction
Cytokines and chemokines are involved in the
development of numerous inflammatory skin disorders (1). Abnormal and dysregulated expression
of inflammatory mediators in keratinocytes is associated with the
pathogenesis of chronic inflammatory skin diseases (2). Upon stimulation by inflammatory
cytokines, including tumor necrosis factor-α (TNF-α) and
interferon-γ (IFN-γ), epidermal keratinocytes express adhesion
molecules such as intracellular adhesion molecule 1 (ICAM-1)
(3). A previous study indicated
that the serum levels of ICAM-1 are associated with the disease
progression of atopic dermatitis (AD) (4). Modulation of ICAM-1 expression in
epidermal keratinocytes therefore provides a strategy for the
development of therapeutic agents for the treatment of various
inflammatory skin diseases (5). In
addition, exposure of keratinocytes to TNF-α and IFN-γ leads to
dysregulated expression of cytokines and chemokines, and increased
infiltration of monocytes/T cells into the site of inflammation
(6). Thymus and
activation-regulated chemokine (TARC/CCL17) is constitutively
expressed in the thymus and is produced by dendritic cells,
endothelial cells, keratinocytes and fibroblasts (7). Furthermore, keratinocytes increase
TARC production in the lesional skin of AD (8). Therefore, modulation of keratinocyte
TARC production may contribute to the pathological processes of
inflammatory skin diseases including AD.
Artemisia princeps Pampanini (AP) is a
herbaceous plant that is widely used in traditional medicine in
Asia (9). Various species of
Artemisia have been demonstrated to exhibit functional properties,
including immunostimulatory (10),
anticancerous (11),
anti-inflammatory (12) and
antibacterial (13) effects. The
constituents of AP have been reported by Ryu et al (14), and include the flavonoids eupatilin
and jaceosidin. Isosecotanapartholide (ISTP), a sesquiterpene
lactone isolated from Artemisia rutifolia and Artermisia
iwayomogi, has anti-inflammatory and anticancer properties
(15). In addition, it inhibits
nitric oxide synthase (16).
However, there is limited clinical evidence to support the
anti-inflammatory effects of ISTP. Therefore, the present study
investigated the anti-inflammatory effects of ISTP isolated from
ethanol extracts of AP.
Interleukin-33 (IL-33) is associated with type 2
immune responses (17) and is
important in the pathogenesis of various type 2 helper cell
(Th2)-associated inflammatory conditions and allergic reactions
(18). Natural helper cells and
nuocytes produce abundant Th2 cytokines following stimulation by
IL-33 (19). The mature form of
IL-33 is released into the cytoplasm and stimulates keratinocytes,
T cells and mast cells. Subsequently, IL-33 may act as a
transcription factor by trafficking into the nucleus, where it
regulates various inflammatory responses (20). The present study investigated the
effect of ISTP on the production of TARC and IL-33 in TNF-α- and
IFN-γ-stimulated HaCaT keratinocytes. In addition, the mechanism of
action of ISTP was examined.
Materials and methods
Extraction and isolation of active
components
AP was purchased from a herb shop at Gyeongdong
medicinal herb market (Seoul, Korea). High-performance liquid
chromatography (HPLC)-grade methanol, and ethanol, ethyl acetate
and dichloromethane were purchased from Duksan Pure Chemicals Co.,
Ltd. (Ansan, Korea). Dimethyl sulfoxide-d6
(DMSO-d6), a common solvent used in nuclear magnetic
resonance (NMR) spectroscopy, was purchased from Sigma-Aldrich;
Merck Millipore (Darmstadt, Germany).
Dried AP (5 kg) was extracted with ethanol (95%) for
3 or 4 days at room temperature. Following filtration through a
400-mesh filter, the product was passed through filter paper
(Whatman® Grade 5) and concentrated under reduced
pressure by rotary evaporation (EYELA N-1000; Tokyo Rikakikai Co.,
Ltd., Tokyo, Japan). The ethanol extract of AP (279.4 g) was
suspended in H2O and extracted with ethyl acetate to
obtain an ethyl acetate soluble layer (97.8 g). The ethyl acetate
soluble layer (97.8 g) was mixed to a silica column (97.8 g) and
concentrated, followed by oven drying to obtain a coating loading
powder. Then 20 g of powder was loaded into a silica open column
(4.5×40 cm) filled with 100 g silica. The solvent was developed by
gradient elution with methanol in dichloromethane (80:1, 50:1,
30:1, 20:1, 15:1, 5:1, 1:1). Sub-fractions (n=6; APEA-1 to APEA-6)
were collected, and the concentrate (22.5 g) of APEA-3 containing
the active ingredient was dissolved in 20 ml of 30% (v/v) methanol
and filtered through a 0.45 µm filter. Then, in order to purify the
active ingredient, a Shim-Pack Prep-ODS column (20×250 mm; 5 µm),
packed with silica gel at a flow rate of 10 ml/min and an injection
volume of 50 µl was dissolved in a methanol-H2O solution
(50:50). Following purification using a Prep LC column (Shimadzu
Co., Kyoto Japan) and vacuum drying, pure ISTP was obtained (111
mg). This active component was identified by 1H-NMR and
13C-NMR (16,21). Nuclear magnetic resonance (NMR)
spectroscopy was used to analyze the structure of the compounds
separated by H1-NMR for Varian (GEMINI, 400 MHz) and C13-NMR for
Varian (GEMINI, 100 MHz). DMSO (Sigma Aldrich) was used as a
solvent and the NMR results were consistent with the previous
studies (data not shown). ISTP: syrup, 1H-NMR
(DMSO-d6, 400 MHz): δ1.70 (1H, m, H-8), 1.85 (1H, m, H-8′),
2.05 (3H, s, H-14), 2.08 (1H, d, H-2), 2.11 (3H, s, H-15), 2.45
(2H, m, H-9), 2.66 (1H, dd, J=18.2, 6.2 Hz, H-2′), 3.05 (1H, m,
H-7), 4.55 (1H, s, H-6), 5.02 (1H, d, J=5.6 Hz, H-3), 5.61 (1H,
brs, -OH), 5.73 (1H, d, J=2.5 Hz, H-13), 6.11 (1H, d, J=2.9 Hz,
H-13′), 13C-NMR (DMSO-d6, 100 MHz): δ13.4 (C-15),
26.8 (C-8), 29.7(C-14), 39.1(C-9), 41.3(C-7), 44.3(C-2), 69.7(C-3),
75.4(C-6), 121.6(C-13), 135.7(C-5), 139.1(C-11), 169.6(C-12),
175.0(C-4), 203.4(C-1), 207.6(C-10).
HPLC analysis
A modular Shimadzu LC-20A System was utilized. A
Capcell Pak C-18 Column (250×4.6 mm internal diameter×5 µm;
Shiseido Co., Ltd., Tokyo, Japan) was used at 30°C. Isocratic
elution [mobile phase, solvent mixture of methanol (15%)] was
performed for 1 h at a flow rate of 1 ml min−1 with an
injection volume of 20 µl. The UV detector was set at a wavelength
of 220 nm.
Human keratinocyte cultures
HaCaT cells (immortalized human keratinocyte cell
line, obtained from American Type Culture Collection, Manassas, VA,
USA) were cultured in Dulbecco's modified Eagle's medium (DMEM;
Welgene Biotech, Taipei, Taiwan) supplemented with 10% fetal bovine
serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100
U/ml penicillin/streptomycin at 37°C and 5% CO2. Cells
(5×105 cells/dish in 60 mm dishes) were pretreated with
10 ng/ml TNF-α and 10 ng/ml IFN-γ at 37°C and 5% CO2 for
30 min and subsequently incubated with 2.5, 5 or 10 µg/ml ISTP or
125, 250 or 500 µg/ml AP extract (APE) for 30 min at 37°C and 5%
CO2. For induction of IL-33, HaCaT cells were treated
with 20 ng/ml TNF-α and 20 ng/ml IFN-γ at 37°C and 5%
CO2 for 30 min (22).
Cell Counting kit-8 (CCK-8) assay
HaCaT cells (2.5×104 cells/well) were
seeded into 96-well plates and their proliferation was measured
using a CCK-8 assay (Dojindo Molecular Technologies, Inc.,
Rockville, MD, USA). Cells were treated with 62.5, 125, 250 and 500
µg/ml APE or 1.25, 2.5, 5 and 10 µg/ml ISTP for 24 h. Cells were
subsequently incubated with TNF-α (20 ng/ml) and IFN-γ (20 ng/ml)
for 30 min at 37°C and 5% CO2. CCK-8 solution (10 µl) was added to
the cells in 1 ml DMEM and incubated for 2 h at 37°C. Absorbance
was measured at a wavelength of 450 nm using a microplate reader
(SpectraMax 340; Molecular Devices, LLC, Sunnyvale, CA, USA).
Western blot analysis
Western blot analysis was performed as described
previously (23). Proteins were
quantified using a Bio-Rad DC Protein assay kit II (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equal amounts of protein
were separated by SDS-PAGE, transferred to polyvinylidene
difluoride membranes and incubated with the following primary
antibodies: Rabbit anti-phosphorylated (p)-STAT-1 phosphorylated at
tyrosine 701 (pY-STAT-1; 1:1,000; cat. no. 9167; Cell Signaling
Technology, Inc., Danvers, MA, USA); rabbit anti-p-STAT-1
phosphorylated at serine 727 (pS-STAT-1; 1:1,000; cat. no. 9177;
Cell Signaling Technology, Inc.); rabbit anti-total STAT-1
(1:1,000; cat. no. 9172; Cell Signaling Technology, Inc.); mouse
anti-ICAM-1 (1:1,000; cat. no. ab2213; Abcam, Cambridge, MA, USA);
rabbit anti-β-actin (1:1,000; cat. no. 4967; Cell Signaling
Technology); and anti-IL-33 (1:1,000; cat. no. sc-98659; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA).
Measurement of chemokines
The concentration of six cytokines and chemokines
[IL-1β, IL-6, monocyte chemoattractant protein-1 (MCP-1)/CCL2,
TARC, soluble ICAM-1 (sICAM-1) and IL-33] were measured in cell
supernatants using human ELISA kits as follows: Human IL-1β (ELISA
ready-SET-GO; cat. no. 88-7261, eBioscience, Inc., San Diego, CA,
USA), human CCL2 (MCP-1) (ELISA ready-SET-GO; cat. no. 88-7399,
eBioscience, Inc.), human sICAM-1 (Platinum ELISA; cat. no.
BMS201CE; eBioscience, Inc.), human IL-33 (Platinum ELISA; cat. no.
BMS2048, eBioscience, Inc.), and human CCL17/TARC (Quantikine ELISA
kit; cat. no. DDN00, R&D Systems, Inc., Minneapolis, MN, USA).
ELISA was performed according to the manufacturer's protocol.
RNA extraction and gene
expression
RNA was isolated from HaCaT cells using an RNeasy
Plus Mini kit (Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's protocol. Reverse transcription was performed using
the RevertAid First Strand cDNA synthesis kit (cat. no. #K1622;
Thermo Fisher Scientific, Inc.). Quantitative polymerase chain
reaction (qPCR) was performed using the EmeraldAmp GT PCR Master
Mix (cat. no. RR310A; Takara Biotechnology Co., Ltd., Dalian,
China). The primers for the qPCR were as follows: Human GAPDH, 5′
AGG GCT GCT TTT AAC TCT GGT 3′ (sense) and 5′ CCC CAC TTG ATT TTG
GAG GGA 3′ (antisense); ICAM-1, 5′ CAC CCT AGA GCC AAG GTG AC 3′
(sense) and 5′ CAT TGG AGT CTG CTG GGA AT 3′ (antisense); TARC, 5′
CTT CTC TGC AGC ACA TCC 3′ (sense) and 5′ AAG ACC TCT CAA GGC TTTG
3′ (antisense); IL-33, 5′ AGC CTT GTG TTT CAA GCT GG 3′ (sense) and
5′ ATG GAG CTC CAC AGA GTG TTC 3′ (antisense). The thermocycling
conditions were as follows: An initial denaturation step at 94°C
for 2–10 min, followed by 30–35 cycles of denaturation at 94°C for
30 sec to 3 min, annealing at 50–58°C for 30 sec to 1 min and
extension at 72°C for 30 sec to 1 min, and a final extension step
at 72°C for 4–7 min. The digitized gel images were analyzed using
Quantity One 1-D Analysis software version 4.6.5 (Bio-Rad
Laboratories, Inc.).
Immunocytochemistry
HaCaT cells (1.5×104 cells/well) were
seeded onto a 4-well chamber slide and treated with TNF-α (20
ng/ml), IFN-γ (20 ng/ml), ISTP (10 µg/ml) or APE (500 µg/ml) for 24
h at 37°C incubation. Following fixation in 100% methanol (chilled
at −20°C) at room temperature for 5 min and permeabilization, cells
were incubated with 1% BSA/ 22.52 mg/ml glycine in PBS/0.1%
Tween-20 (PBST) for 30 min to block unspecific binding of the
antibodies. Then, cells were incubated overnight at 4°C with an
anti-IL-33 primary antibody (1:100; cat. no. sc-98659, Santa Cruz
Biotechnology, Inc.), followed by a fluorescein
isothiocyanate-labeled goat anti-rabbit IgG (1:1,000; cat. no.
NB730-F; Novus Biologicals, LLC, Littleton, CO, USA) in 1% BSA/
PBST for 1 h at room temperature in the dark. DAPI was used to
counterstain the nuclei. Stained cells were visualized using a
confocal microscope (Olympus FluoView FV10i; Olympus Corporation,
Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation
(n=3). Statistical significance was calculated by one-way analysis
of variance followed by Duncan's multiple range test using PASW
Statistics software version 18.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of ISTP on the viability of
HaCaT cells
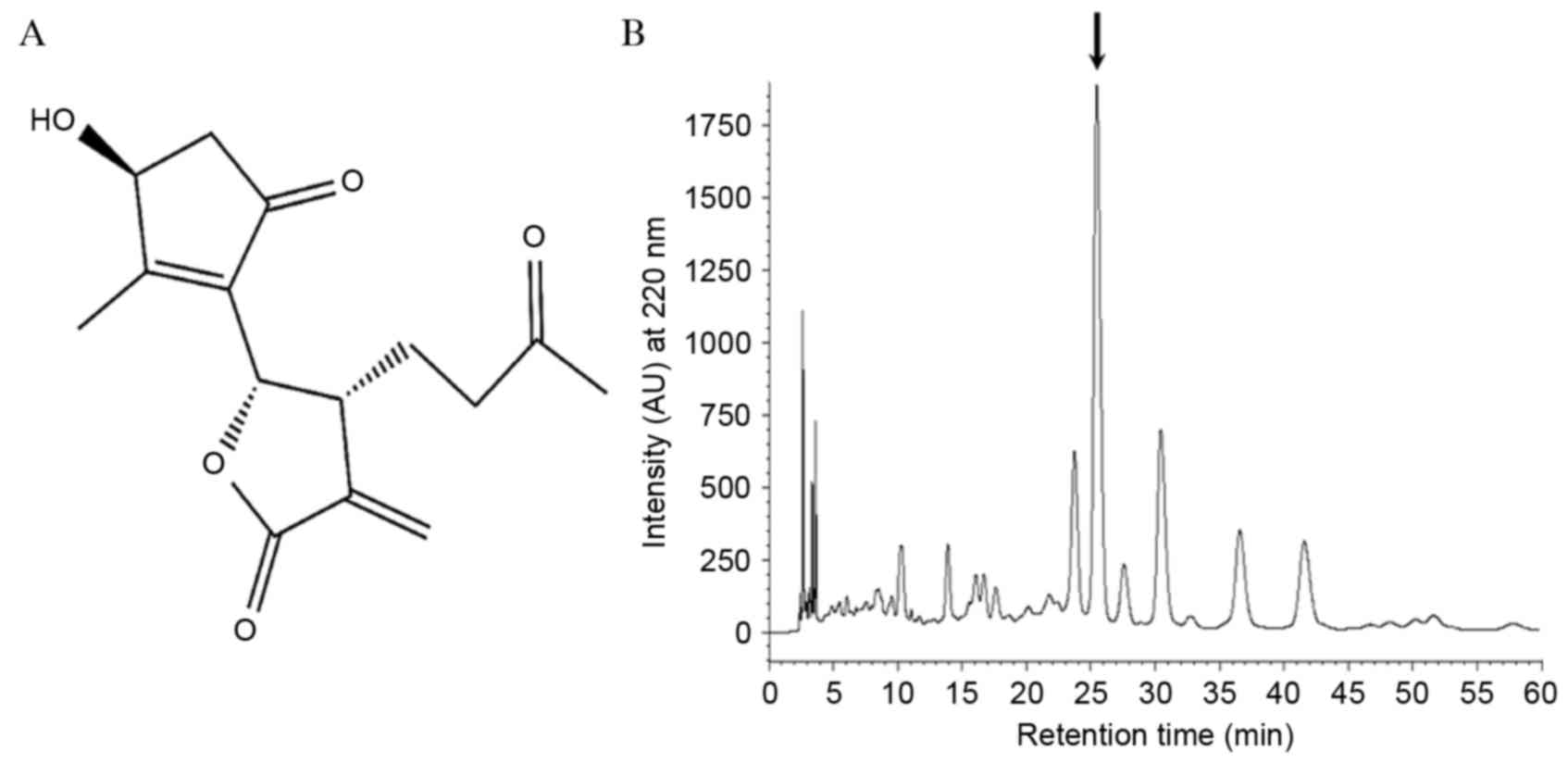
The structure of ISTP is presented in Fig. 1A. ISTP, isolated from Artermisia
rutifolia and Artermisia iwayomogi, has a sesquiterpene lactone
structure and has been demonstrated to inhibit nitric oxide
synthase. The present study investigated whether APE contained
other primary compounds, including ISTP, eupatilin (24) and jaceosidin (25). Compounds were extracted from APE by
HPLC and various other peaks were detected (Fig. 1B). To exclude the possibility that
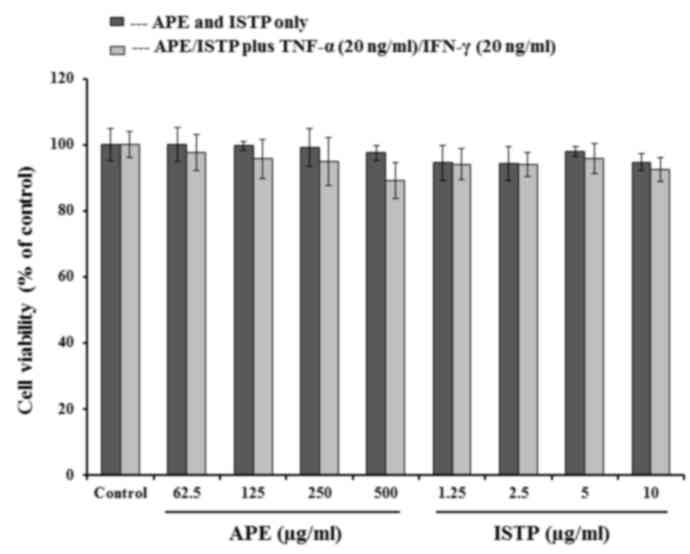
the cytotoxicity of ISTP may contribute to its suppressive effects,
cell viability was determined using a CCK-8 assay. HaCaT cells were
stimulated with TNF-α and IFN-γ in the absence or presence of ISTP
or APE. As presented in Fig. 2,
ISTP and APE exhibited no significant cytotoxic effect on HaCaT
cells at the concentrations assessed.
ISTP and APE suppress
TNF-α/IFN-γ-induced TARC/CCL17 production and ICAM-1/STAT1
activation in human keratinocytes
The mechanism of action of ISTP inhibition of
chemokine and cytokine release from TNF-α/IFN-γ-stimulated HaCaT
cells was investigated. Previous studies reported that TNF-α/IFN-γ
stimulation activates signaling molecules, including STAT-1,
extracellular signal-regulated kinase, c-Jun N-terminal kinase, p38
mitogen-activated protein kinases (MAPKs) and nuclear factor
(NF)-κB in HaCaT cells (26–30).
Thus, the present study evaluated whether ISTP affects the STAT
signaling pathway in TNF-α/IFN-γ-stimulated HaCaT cells using
western blot analysis. HaCaT cells were pretreated with APE or
ISTP, followed by incubation with TNF-α and IFN-γ. Treatment with
ISTP or APE reduced ICAM-1 protein expression levels and decreased
STAT-1 phosphorylation in a dose-dependent manner (Fig. 3A). In addition, the ability of ISTP
to inhibit ICAM-1 and TARC mRNA expression levels was investigated.
RT-PCR demonstrated that the expression levels of TARC and ICAM-1
mRNA were increased by TNF-α/IFN-γ (Fig. 3B and D); this increase was
abrogated by ISTP or APE treatment (Fig. 3B and D). ELISA was performed to
determine the inhibitory effects of ISTP and APE on TARC
production. ISTP and APE significantly inhibited
TNF-α/IFN-γ-induced TARC production in HaCaT cells compared with
TNF-α/IFN-γ treatment only (P<0.05; Fig. 3C), in a dose-dependent manner. The
results indicated that ISTP may inhibit TNF-α/IFN-γ-induced TARC
expression by suppression of ICAM-1 and STAT-1 activation.
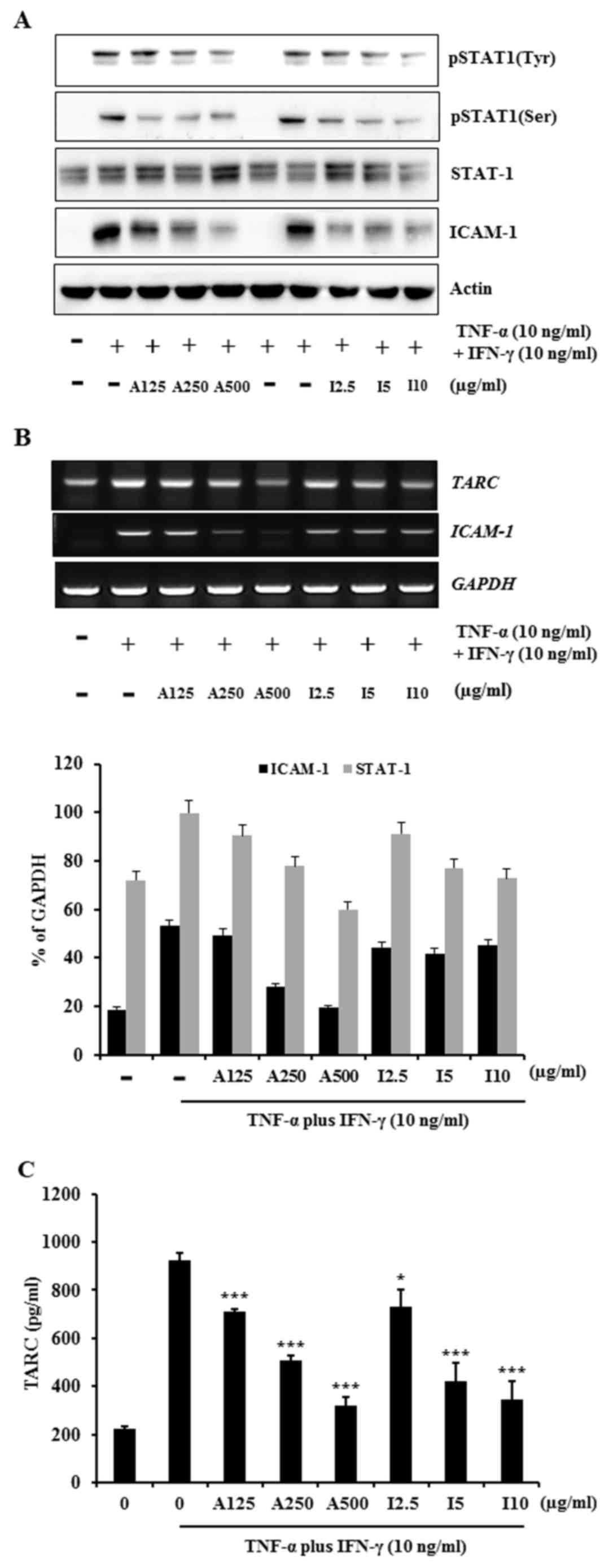 | Figure 3.Effects of ISTP and APE on
TNF-α/IFN-γ-induced ICAM-1/STAT-1 activation and TARC production in
HaCaT cells. (A) Cells were pretreated with APE (125, 250 or 500
µg/ml) or ISTP (2.5, 5 or 10 µg/ml) for 30 min and subsequently
incubated with TNF-α (10 ng/ml) and IFN-γ (10 ng/ml) for 24 h. Cell
lysates were subjected to western blot analysis for pSTAT-1 (Tyr),
p-STAT-1 (Ser), total STAT-1, ICAM-1 and β-actin. (B)
Representative gel images and quantification from reverse
transcription-polymerase chain reaction analysis of ICAM-1 and TARC
mRNA expression levels. (C) Production of TARC was measured by
ELISA performed on cell supernatants. Data are presented as the
mean ± standard deviation (n=3). *P<0.05, **P<0.01 and
***P<0.001 vs. untreated cells control. ISTP,
isosecotanapartholide; APE, Artemisia princeps Pampanini extract;
TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; ICAM-1,
intracellular adhesion molecule 1; STAT-1, signal transducer and
activator of transcription-1; TARC, thymus and activation-regulated
chemokine; p, phosphorylated; Tyr, tyrosine; Ser, serine. |
Effects of ISTP and APE on
TNF-α/IFN-γ-induced chemokine/cytokine production in HaCaT
cells
Subsequently, the present study investigated whether
ISTP inhibits inflammatory cytokine and chemokine production in
TNF-α/IFN-γ-stimulated HaCaT cells. The STAT family serves an
important role in cytokine production. The production of the
majority of cytokines and chemokines are primarily regulated at the
transcriptional level through activation of specific sets of
transcription factors, which are controlled by NF-κB and MAPKs. The
results demonstrated that pretreatment with ISTP or APE inhibited
the production of IL-1β (Fig. 4A),
IL-6 (Fig. 4B), MCP-1/CCL-2
(Fig. 4C) and sICAM-1 (Fig. 4D) by HaCaT cells, in a
dose-dependent manner. The results indicated that ISTP inhibits the
release of pro-inflammatory cytokines.
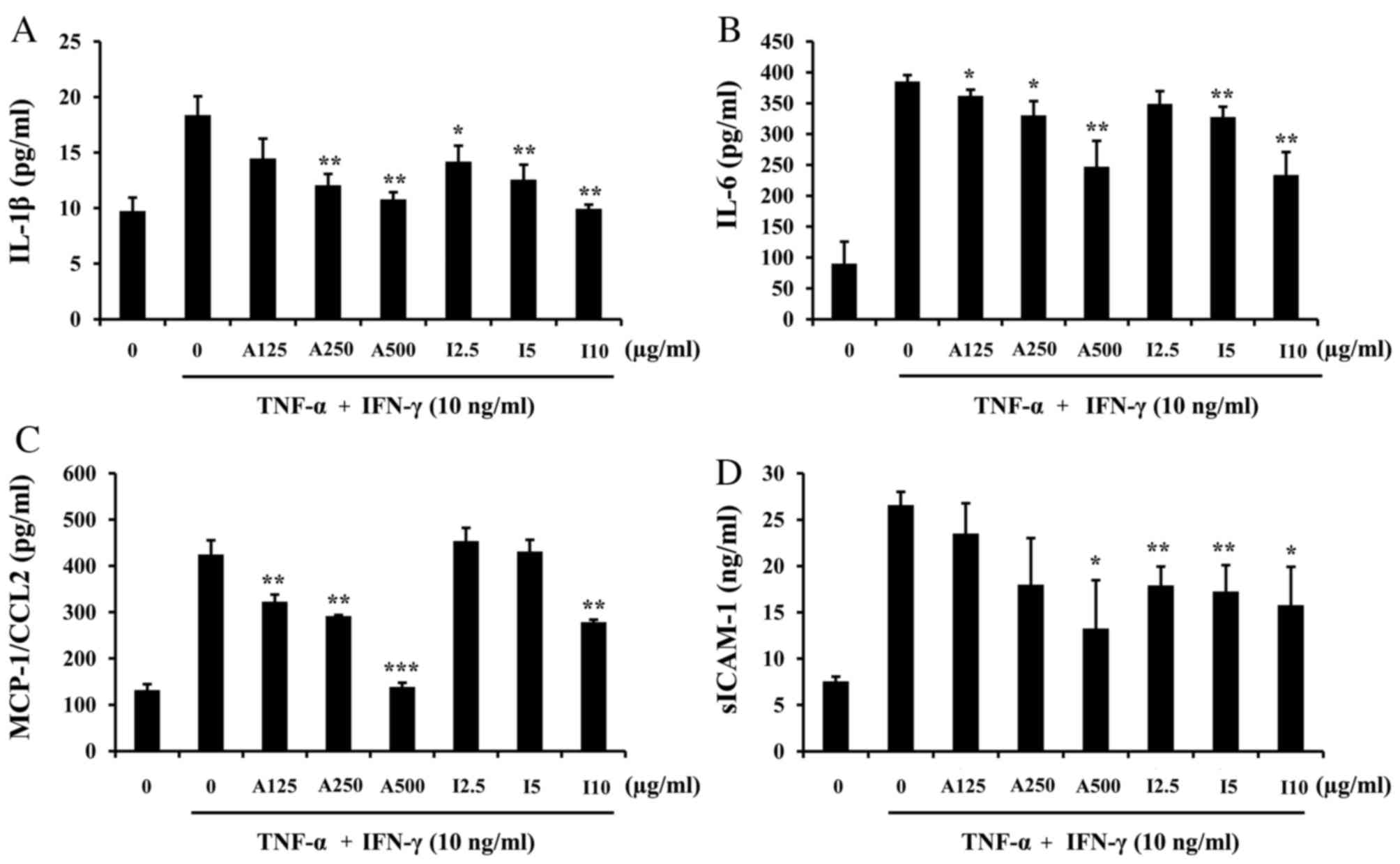 | Figure 4.Effects of ISTP and APE on
TNF-α/IFN-γ-induced chemokine/cytokine production in HaCaT cells.
Cells were pretreated with APE (125, 250 or 500 µg/ml) or ISTP
(2.5, 5 or 10 µg/ml) for 30 min and subsequently incubated with
TNF-α (10 ng/ml) and IFN-γ (10 ng/ml) for 24 h. Production of (A)
IL-1β, (B) IL-6, (C) MCP-1/CCL2 and (D) sICAM-1 were measured by
ELISA. Data are presented as the mean + standard deviation (n=3).
*P<0.05, **P<0.01 and ***P<0.001 vs. TNF-α/IFN-γ treatment
alone. ISTP, isosecotanapartholide; APE, Artemisia princeps
Pampanini extract; TNF-α, tumor necrosis factor-α; interferon-γ;
IL, interleukin; MCP-1, monocyte chemoattractant protein-1;
sICAM-1, soluble intracellular adhesion molecule 1. |
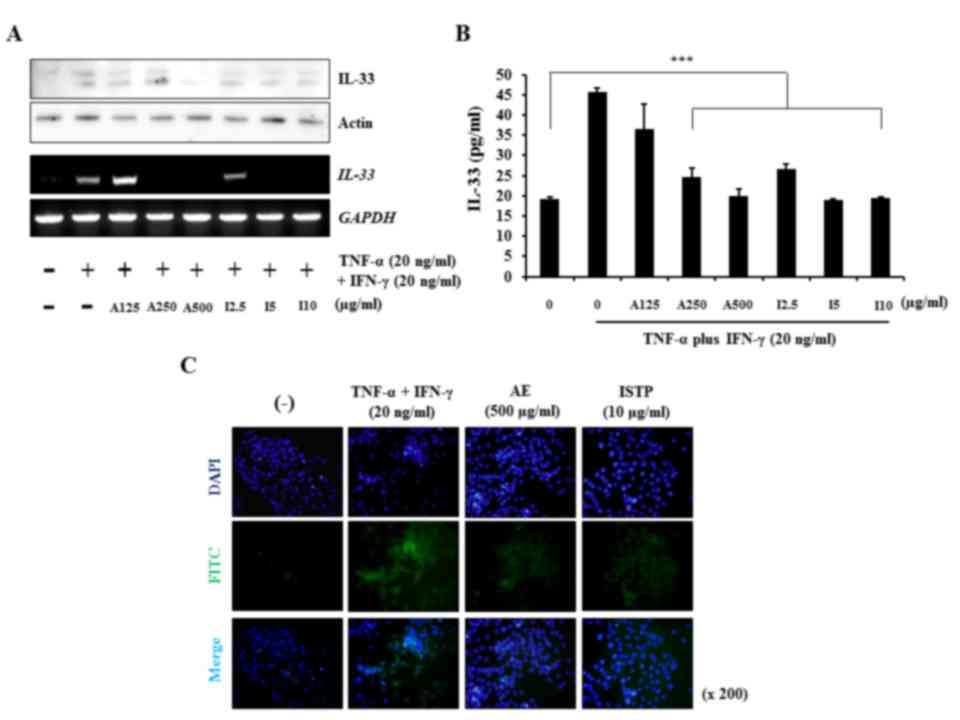
ISTP and APE markedly suppress IL-33
production
It has previously been reported that IL-33 is
upregulated when keratinocytes are exposed to pro-inflammatory
stimuli such as TNF-α/IFN-γ, and may therefore be important in the
pathogenesis of chronic inflammatory skin disorders, including AD
and psoriasis (31). Therefore,
the present study evaluated the effect of ISTP on IL-33 expression
in TNF-α/IFN-γ-treated HaCaT keratinocytes and detected a high
level of IL-33 at 20 ng/ml TNF-α/IFN-γ. As presented in Fig. 5A, protein and mRNA expression
levels of IL-33 were increased following TNF-α/IFN-γ stimulation,
compared with cells that were not treated with TNF-α/IFN-γ.
Conversely, ISTP or APE treatment appeared to reduce IL-33
expression (Fig. 5A). In addition,
pretreatment with ISTP or APE inhibited IL-33 production
dose-dependently in supernatants from cultured HaCaT cells
(P<0.001; Fig. 5B).
Immunocytochemistry indicated that stimulation of HaCaT
keratinocytes with TNF-α/IFN-γ led to an increase in IL-33 compared
with untreated cells. However, IL-33 staining reduced following
pretreatment of HaCaT cells with 10 µg/ml ISTP or 500 µg/ml APE
(Fig. 5C). The results indicated
that ISTP inhibits IL-33 production and may be important in the
crosstalk between pro-inflammatory cytokines.
Discussion
Certain herbal medicines have been considered as
potential novel anti-inflammatory drugs (32). Natural products have been used
extensively in the treatment of chronic skin diseases, including AD
and psoriasis (33).
Anti-inflammatory drugs developed from natural sources have been
widely investigated. There are >400 classes of Artemisia
identified (34). One of them, AP,
has previously been demonstrated to exert various biological
activities in vitro (35)
and in vivo (36). ISTP, an
active component of APE, suppressed LPS-induced nitric oxide
production in murine macrophage RAW 264.7 cells (16). However, the anti-atopic activity
and mechanism of action of ISTP remain to be elucidated. Therefore,
the present study investigated the anti-inflammatory properties of
APE and ISTP. In addition, the inhibitory effect of ISTP on
AD-associated factors was examined. The specific inhibition of
cytokine production by ISTP may be an alternative approach for the
treatment of AD.
TARC/CCL17 is a useful clinical biomarker of AD
(7). In addition, TARC is
associated with AD immunopathology, TNF-α and IFN-γ (37). Therefore, the present study
investigated the inhibitory activity of ISTP on the inflammatory
chemokine TARC. ISTP and APE inhibited the mRNA expression levels
of TARC in a dose-dependent manner and exhibited no cytotoxicity in
HaCaT cells. Consequently, the current study examined the effect of
ISTP and APE on TNF-α/IFN-γ signaling in HaCaT cells. STAT-1
regulates the expression of numerous genes underlying various
cellular processes, including the immune response, antiviral
protection and apoptosis (38).
Various plant extracts and compounds have been demonstrated to
inhibit the activities of inflammatory chemokines via the
regulation of signaling pathways stimulated by TNF-α and IFN-γ,
including STAT1 (39,40), thus implicating STAT-1 in
inflammatory processes. The present study demonstrated that
treatment of HaCaT cells with ISTP or APE reduced ICAM-1 expression
and STAT-1 phosphorylation.
A number of studies have identified a panel of
pro-inflammatory cytokines with important roles in the induction
and maintenance of chronic skin inflammation (41). In the current study, ISTP
significantly inhibited the production of the pro-inflammatory
cytokines MCP-1/CCL2, IL-1β, IL-6 and sICAM-1. IL-1 promotes the
expression of adhesion molecules on keratinocytes and endothelial
cells, allowing the infiltration of inflammatory factors (42). IL-1 and IL-33 may function as
pro-inflammatory cytokines and intracellular nuclear factors
involved in transcriptional regulation. IL-33 mRNA expression
levels are increased almost 10-fold in the skin of AD patients
compared with healthy controls (20). TNF-α and IFN-γ serve a key role in
type 1 immune responses and induce the expression of IL-33, which
may promote type 2 immune responses in keratinocytes (43). Additionally, IL-33 levels were
relatively greater in the presence of TNF-α and IFN-γ. Pretreatment
with ISTP or APE inhibited TNF-α/IFN-γ-induced IL-33 production in
a dose-dependent manner. However, the current study was limited to
HaCaT keratinocytes and further studies are required to confirm the
effects of ISTP on other cell types, including human primary
keratinocytes from AD patients, or in an animal model of AD. The
results of the present study indicated that ISTP is an active
component in APE, Although APE consists of many components, ISTP is
an active component isolated from Artermisia princeps Pampanini
that may regulate the recruitment of Th2-type cells into AD lesions
by suppressing the expression of inflammatory chemokines associated
with AD (44,45).
In conclusion, the results of the present study
demonstrated that ISTP isolated from APE suppressed TARC and IL-33
production in HaCaT human keratinocytes. Additionally, ISTP
inhibited the activation of ICAM-1/STAT1 induced by TNF-α/IFN-γ.
These results provide novel evidence regarding the
anti-inflammatory functions of ISTP. Furthermore, the results
indicated that ISTP is a potential therapeutic agent for the
treatment of AD and other inflammatory skin diseases.
Acknowledgements
The authors thank SK Bioland Corporation (Cheongju,
Korea) for providing the active compounds.
References
|
1
|
Homey B, Steinhoff M, Ruzicka T and Leung
DY: Cytokines and chemokines orchestrate atopic skin inflammation.
J Allergy Clin Immunol. 118:178–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albanesi C and Pastore S: Pathobiology of
chronic inflammatory skin diseases: Interplay between keratinocytes
and immune cells as a target for anti-inflammatory drugs. Curr Drug
Metab. 11:210–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gouwy M, Struyf S, Proost P and Van Damme
J: Synergy in cytokine and chemokine networks amplifies the
inflammatory response. Cytokine Growth Factor Rev. 16:561–580.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koide M, Tokura Y, Furukawa F and Takigawa
M: Soluble intercellular adhesion molecule-1 (sICAM-1) in atopic
dermatitis. J Dermatol Sci. 8:151–156. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gniadecki R, Zachariae C and Calverley M:
Trends and developments in the pharmacological treatment of
psoriasis. Acta Derm Venereol. 82:401–410. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pastore S, Mascia F and Girolomoni G: The
contribution of keratinocytes to the pathogenesis of atopic
dermatitis. Eur J Dermatol. 16:125–131. 2006.PubMed/NCBI
|
|
7
|
Saeki H and Tamaki K: Thymus and
activation regulated chemokine (TARC)/CCL17 and skin diseases. J
Dermatol Sci. 43:75–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vestergaard C, Bang K, Gesser B, Yoneyama
H, Matsushima K and Larsen CG: A Th2 chemokine, TARC, produced by
keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional
atopic dermatitis skin. J Invest Dermatol. 115:640–646. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toda S: Inhibitory effects of polyphenols
in leaves of Artemisia princeps PAMP on protein fragmentation by
Cu(II)-H2O2 in vitro. J Med Food. 7:52–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim TH, Lee SJ, Rim HK, Shin JS, Jung JY,
Heo JS, Kim JB, Lee MS and Lee KT: In vitro and in vivo
immunostimulatory effects of hot water extracts from the leaves of
Artemisia princeps Pampanini cv. Sajabal. J Ethnopharmacol.
149:254–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarath VJ, So CS, Won YD and Gollapudi S:
Artemisia princeps var orientalis induces apoptosis in human breast
cancer MCF-7 cells. Anticancer Res. 27:3891–3898. 2007.PubMed/NCBI
|
|
12
|
Kim MJ, Han JM, Jin YY, Baek NI, Bang MH,
Chung HG, Choi MS, Lee KT, Sok DE and Jeong TS: In vitro
antioxidant and anti-inflammatory activities of Jaceosidin from
Artemisia princeps Pampanini cv. Sajabal. Arch Pharm Res.
31:429–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trinh HT, Lee IA, Hyun YJ and Kim DH:
Artemisia princeps Pamp. Essential oil and its constituents
eucalyptol and α-terpineol ameliorate bacterial vaginosis and
vulvovaginal candidiasis in mice by inhibiting bacterial growth and
NF-κB activation. Planta Med. 77:1996–2002. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ryu SY, Kim JO and Choi SU: Cytotoxic
components of Artemisia princeps. Planta Med. 63:384–385. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ryu SH, Jo H, Kim JW, Youn HJ and Kim KB:
Four-Week repeated oral toxicity study of Aip1, a water-soluble
carbohydrate fraction from artemisia iwayomogi in mice. Toxicol
Res. 27:261–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn H, Kim JY, Lee HJ, Kim YK and Ryu JH:
Inhibitors of inducible nitric oxide synthase expression from
Artemisia iwayomogi. Arch Pharm Res. 26:301–305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sismanopoulos N, Delivanis DA,
Alysandratos KD, Angelidou A, Therianou A, Kalogeromitros D and
Theoharides TC: Mast cells in allergic and inflammatory diseases.
Curr Pharm Des. 18:2261–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voehringer D: Protective and pathological
roles of mast cells and basophils. Nat Rev Immunol. 13:362–375.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cevikbas F and Steinhoff M: IL-33: A novel
danger signal system in atopic dermatitis. J Invest Dermatol.
132:1326–1329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huneck S, Zdero C and Bohlmann F:
Seco-guaianolides and other constituents from Artemisia species.
Phytochemistry. 25:883–889. 1986. View Article : Google Scholar
|
|
22
|
Kwon TR, Oh CT, Choi EJ, Kim SR, Jang YJ,
Ko EJ, Suh D, Yoo KH and Kim BJ: Ultraviolet light-emitting-diode
irradiation inhibits TNF-α and IFN-γ-induced expression of ICAM-1
and STAT1 phosphorylation in human keratinocytes. Lasers Surg Med.
47:824–832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwon TR, Mun SK, Oh CT, Hong H, Choi YS
and Kim BJ and Kim BJ: Therapeutic effects of full spectrum light
on the development of atopic dermatitis-like lesions in NC/Nga
mice. Photochem Photobiol. 90:1160–1169. 2014.PubMed/NCBI
|
|
24
|
Chung KS, Choi JH, Back NI, Choi MS, Kang
EK, Chung HG, Jeong TS and Lee KT: Eupafolin, a flavonoid isolated
from Artemisia princeps, induced apoptosis in human cervical
adenocarcinoma HeLa cells. Mol Nutr Food Res. 54:1318–1328. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee TH, Jung H, Park KH, Bang MH, Baek NI
and Kim J: Jaceosidin, a natural flavone, promotes angiogenesis via
activation of VEGFR2/FAK/PI3K/AKT/NF-κB signaling pathways in
endothelial cells. Exp Biol Med (Maywood). 239:1325–1334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ju SM, Song HY, Lee SJ, Seo WY, Sin DH,
Goh AR, Kang YH, Kang IJ, Won MH, Yi JS, et al: Suppression of
thymus- and activation-regulated chemokine (TARC/CCL17) production
by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose via blockade of
NF-kappaB and STAT1 activation in the HaCaT cells. Biochem Biophys
Res Commun. 387:115–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho JW, Lee KS and Kim CW: Curcumin
attenuates the expression of IL-1beta, IL-6,and TNF-alpha as well
as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs
as potential upstream targets. Int J Mol Med. 19:469–474.
2007.PubMed/NCBI
|
|
29
|
Sung YY, Kim YS and Kim HK: Illicium verum
extract inhibits TNF-α- and IFN-γ-induced expression of chemokines
and cytokines in human keratinocytes. J Ethnopharmacol.
144:182–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dustin ML, Singer KH, Tuck DT and Springer
TA: Adhesion of T lymphoblasts to epidermal keratinocytes is
regulated by interferon gamma and is mediated by intercellular
adhesion molecule 1 (ICAM-1). J Exp Med. 167:1323–1340. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taniguchi K, Yamamoto S, Hitomi E, Inada
Y, Suyama Y1, Sugioka T and Hamasaki Y: Interleukin 33 is induced
by tumor necrosis factor alpha and interferon gamma in
keratinocytes and contributes to allergic contact dermatitis. J
Investig Allergol Clin Immunol. 23:428–434. 2013.PubMed/NCBI
|
|
32
|
Rainsford KD: Anti-inflammatory drugs in
the 21st century. Subcell Biochem. 42:3–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shu YZ: Recent natural products based drug
development: A pharmaceutical industry perspective. J Nat Prod.
61:1053–1071. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moufid A and Eddouks M: Artemisia herba
alba: A popular plant with potential medicinal properties. Pak J
Biol Sci. 15:1152–1159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park EY, Lee KW, Lee HW, Cho YW, Baek NI,
Chung HG, Jeong TS, Choi MS and Lee KT: The ethanol extract from
Artemisia princeps Pampanini induces p53-mediated G1 phase arrest
in A172 human neuroblastoma cells. J Med Food. 11:237–245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang YJ, Jung UJ, Lee MK, Kim HJ, Jeon SM,
Park YB, Chung HG, Baek NI, Lee KT, Jeong TS and Choi MS:
Eupatilin, isolated from Artemisia princeps Pampanini, enhances
hepatic glucose metabolism and pancreatic beta-cell function in
type 2 diabetic mice. Diabetes Res Clin Pract. 82:25–32. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsuda T, Tohyama M, Yamasaki K, Shirakata
Y, Yahata Y, Tokumaru S, Sayama K and Hashimoto K: Lack of evidence
for TARC/CCL17 production by normal human keratinocytes in
vitro. J Dermatol Sci. 31:37–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boehm U, Klamp T, Groot M and Howard JC:
Cellular responses to interferon-gamma. Annu Rev Immunol.
15:749–795. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park JH, Kim MS, Jeong GS and Yoon J:
Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines
production via blockade of NF-κB, STAT1 and p38-MAPK activation in
human epidermal keratinocytes. J Ethnopharmacol. 171:85–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jung MR, Lee TH, Bang MH, Kim H, Son Y,
Chung DK and Kim J: Suppression of thymus- and activation-regulated
chemokine (TARC/CCL17) production by
3-O-β-D-glucopyanosylspinasterol via blocking NF-κB and STAT1
signaling pathways in TNF-α and IFN-γ-induced HaCaT keratinocytes.
Biochem Biophys Res Commun. 427:236–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carmi-Levy I, Homey B and Soumelis V: A
modular view of cytokine networks in atopic dermatitis. Clin Rev
Allergy Immunol. 41:245–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barker JN, Mitra RS, Griffiths CE, Dixit
VM and Nickoloff BJ: Keratinocytes as initiators of inflammation.
Lancet. 337:211–214. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Meephansan J, Tsuda H, Komine M, Tominaga
S and Ohtsuki M: Regulation of IL-33 expression by IFN-γ and tumor
necrosis factor-α in normal human epidermal keratinocytes. J Invest
Dermatol. 132:2593–2600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yoo JS, Ahn EM, Bang MH, Song MC, Yang HJ,
Kim DH, Lee DY, Chung HG, Jeong TS, Lee KT, et al: Steroids from
the aerial parts of Artemisia princeps Pampanini. Hanguk Yakyong
Changmul Hakhoe Chi. 14:273–277. 2006.
|
|
45
|
Lee YW, Jin Y and Row KH: Extraction and
purification of eupatilin fromArtemisia princeps PAMPAN recycling
preparative HPLC. Korean J Chem Engineering. 23:279–282. 2006.
View Article : Google Scholar
|