Introduction
Arsenic (As) is a naturally occurring toxic metal
which was classified as potentially poisonous substance (1). Excessive exposure to arsenic damages
multiple organs (2). Presently, a
mounting number of studies preferably examined the molecular
mechanisms of apoptosis induced by arsenic (3,4). It
was believed that the mitogen-activated protein kinases (MAPK)
signaling pathway was implicated in cell injury, proliferation, and
apoptosis (5). The extracellular
signal-regulated MAP kinases (ERK), an important member of the MAPK
families, became phosphorylated and activated in response to
diverse environmental stimuli (6).
It had been postulated that ERK was consequently a
participant in arsenic-induced apoptosis (7,8). In
all these studies, however, not all scholars were in agreement on
the issue of arsenic mediating ERK signaling. Escudero-Lourdes
et al (9) found that
exposure of urothelial cells to 0.05 µmol/l of arsenic for 12
months significantly increased protein expression of p-ERK1 and
p-ERK2, which indicated that the ERK signaling pathway was
activated by arsenic. Conversely, Wang et al (10) drew a different conclusion stating
that arsenic restrained the ERK signaling pathway due to the fact
that inhibition and lowering of p-ERK1 and p-ERK2 levels were
observed in human leukemia cell lines after being exposed to 2.5
µmol/l of arsenic for 24 h. Evidently, the effects of arsenic on
ERK signaling pathway remained a debatable issue.
To probe the role of ERK signaling pathway in
arsenic-induced apoptosis, a meta-analysis of experimental studies
published in domestic and foreign literature was performed in our
paper. The present study may be helpful in providing a theoretical
basis for the diverging result of arsenic adverse effects on one
hand and therapeutic mechanisms on the other concerning
arsenic-induced apoptosis.
Materials and methods
Inclusion criteria
Inclusion Criteria and literature search terms were
identified according to the PICO principle.
Study design
Experimental studies published in Chinese and
English.
Participants (P)
All cell lines and animals, disregarding age, gender
and weight.
Intervention (I)
All experimental groups treated with any kind of
arsenic or its compounds. Arsenic model groups might show the
change in indicators associated with ERK signaling pathway and
apoptosis. If variable dosages of arsenic or exposure times were
used in a study, the highest or longest one was chosen for this
analysis.
Comparison (C)
The control group without any intervention (blank
control group).
Outcome (O)
Following indicators in mediating of ERK signaling
were used, Ras (or p21) protein, Raf protein, Mitogen-induced
extracellular kinase (MEK), Total extracellular signal-regulated
MAP kinases (ERK), Extracellular signal-regulated kinase 1 (ERK1),
Extracellular signal-regulated kinase 2 (ERK2), Total
phosphorylated extracellular signal-regulated kinase (p-ERK),
Phosphorylated extracellular signal-regulated kinase 1 (p-ERK1),
Phosphorylated extracellular signal-regulated kinase 2 (p-ERK2),
Cysteinyl aspartate-specific protease-3 (caspase-3), Apoptotic
cells (%), Pro-apoptotic protein-Bcl-associated X protein (Bax),
Anti-apoptotic protein-B-cell lymphoma/leukemia-2 protein
(Bcl-2).
Exclusion criteria
We excluded the studies upon following criteria: i)
the papers focused only on ERK but not arsenic; ii) the papers
focused on arsenic without investigating ERK; iii) no outcome
indicators (as stated in ‘2.1.5 Outcome’); iv) duplicate
publications; v) review articles; vi) inadequate information; and
vii) no available data.
Search strategy
A systematic search was conducted using Cochrane
Library, PubMed, Excerpta Medica database (EMBASE), Springer, Web
of Science, Chinese Biomedical Literature Database (CBM), China
National Knowledge Infrastructure (CNKI) and Wan Fang Data
databases (last search conducted on January 24th, 2017). The key
search string was ‘arsenic AND (Ras OR Raf OR MEK OR ERK)’.
Quality assessment
The Cochrane collaboration's tool for bias risk
assessment was used to evaluate the quality of 42 articles
identified in the present study. The evaluation system consisted of
seven aspects, viz. i) Random sequence generation (selection bias);
ii) allocation concealment (selection bias); iii) blinding of
participants and personnel (performance bias); iv) blinding of
outcome assessment (detection bias); v) incomplete outcome data
(attrition bias); vi) Selective reporting (reporting bias); and
vii) other bias. The rating criteria were as follows, low risk of
bias, unclear risk of bias and high risk of bias.
Data collection
Two reviewers (Dongjie Li and Yutao Wei)
independently extracted data which was then cross-checked before
putting the results into a collective spreadsheet. If the results
seem to be inconsistent, Dr. Shugang Li and Mingxia Jing were asked
to verify before final confirmation. The following information was
documented meticulously out of completed manuscripts from each
qualified study: i) information about the paper including title,
first author, publication date and the name of the journal where
published; ii) characteristics of the research object including the
type and source of cell lines and breed of animals; iii) type,
dosage and exposure time of arsenic; iv) outcome indicators; and v)
baseline data for experimental and control groups, viz. number of
groups (n), mean and standard deviation (SD).
Data analysis
Forty-two articles were analyzed using Review
Manager Version 5.2 (The Nordic Cochrane Centre, The Cochrane
Collaboration 2012, Portland, OR, USA) and Stata 12.0 (StataCorp
LP, College Station, TX, USA). Standardized mean difference (SMD)
was chosen for consolidating statistical data. Heterogeneity was
detected by calculating the I2 index. I2 ≤50%
and >50% represented low and high levels of heterogeneity,
respectively. Random effects model was chosen when P<0.05 and
I2 >50%, and fixed effects model was used when
P>0.05 and I2 ≤50%. Subgroup analyses and
meta-regression analyses (including univariate and multivariate
meta-regression analyses) were conducted to examine sources of
heterogeneity among 42 studies. Subgroup analyses were performed on
the basis of the source (normal and cancer cells), exposure time
(>24 h and ≤24 h) and dosage of arsenic (≥2 and <2 µmol/l).
The combined effect was estimated as SMD with 95% confidence
interval (95% CI) between arsenic model and control group. All
reported P-values were two-sided and P<0.05 was considered to
indicate a statistically significant difference. Small-study
effects were assessed by using funnel plots. Egger's tests and
sensitivity analyses were conducted using Stata 12.0.
Results
Search results
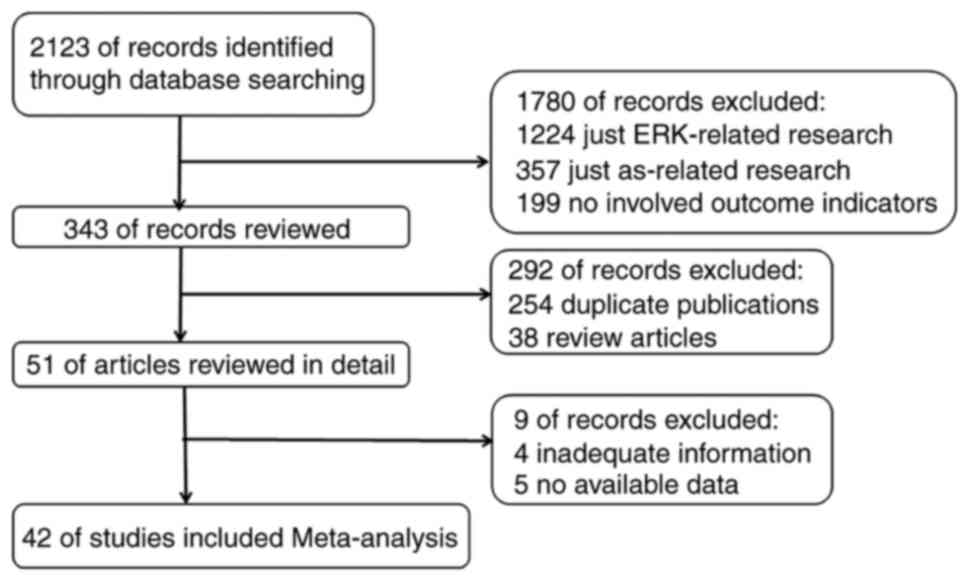
A total of 2,123 articles were initially identified
by search criteria. Utilizing our inclusion and exclusion criteria,
42 of those articles were qualified for meta-analysis (Fig. 1).
Basic characteristics of included
studies
Characteristics of the studies included in this
meta-analysis were listed in Table
I. In the present study, the effects of arsenic on ERK
signaling pathway was assessed. Arsenic model groups encompasses
those cell lines which were treated with various forms of arsenic
including sodium arsenite (NaAsO2), arsenic trioxide
(As2O3), monomethyl arsenous acid (MMA) and
arsenious acid (AA). The control models were blank controls without
any exposure to arsenic. In subgroup analyses, arsenic exposure
time varied among the studies and hence was stratified into ≤24 h
(n=30) and >24 h (n=12). The dosage of arsenic was also variable
and thus was differentiated into ≥2 µmol/l (n=32) and <2 µmol/l
(n=10) groups. Likewise, different cell lines were separated into
normal cells (n=19) and cancer cells (n=23). In this review, cancer
cells included the following ones, U937 cells (human leukemia cell
line), A431 cells (human epidermoid carcinoma cells), CL3 cell line
(non-small-cell lung carcinoma cell line), Flt3-ITD cells (acute
myeloid leukemia cells), JB6 Cl 41 mass cells, NCI-H2052 cells
(human mesothelioma cells), HL-60 cells (human leukemia cell line),
SGC7901/ADM (human gastric cancer cell line), MDA-MB-468 (breast
cancer cells), SH-SY5Y (human neuroblastoma cells), Neuro-2a cells
(murine neuroblastoma cell line), CLL cells (chronic lymphocytic
leukemia cell line, but not the WSU-CLL cell line), SGC7901/S
(human gastric cancer cell line), NCI-H1793 (non-small-cell lung
carcinoma cell line), U-251 MG cells (human glioma cells), NCI-H157
(non-small-cell lung carcinoma cell line), BEL-7402 cells (human
hepatocarcinoma cells), FRO (anaplastic thyroid cancer cell line)
and Hela cells (cervical cancer cells). NCI-H157 was a
misidentified cell line according to http://iclac.org/wp-content/uploads/Cross-Contaminations-v8_0.pdf.
Taking all these factors into consideration, we have arrived at the
conclusion that time (P=0.012) and dosage (P=0.037) were
statistically significant in the univariate meta-regression
analysis. Outcome variables were assessed for any association with
apoptosis (including apoptotic cells, caspase-3, Bax, and Bcl-2)
and ERK signaling pathway (i.e., Ras, Raf, MEK, ERK, ERK1, ERK2,
p-ERK, p-ERK1, and p-ERK2).
 | Table I.Characteristics of the studies
included in the meta-analysis. |
Table I.
Characteristics of the studies
included in the meta-analysis.
| Author (Refs.) | Year | Language | n | Type of arsenical
compounds | Dosage of arsenic,
µmol/l | Time of exposure,
h | Type of cells | Outcome
indicators |
|---|
| Yen et al
(2) | 2011 | English | 12 | As2O3 | <2 | >24 | Normal cells | 5, 6, 8, 9, 11,
12 |
| Eguchi et al
(3) | 2011 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 5, 6, 8, 9, 11 |
| Ray et al
(4) | 2013 | English | 3 | As2O3 | ≥2 | ≤24 | Normal cells | 4, 7, 11 |
| Lau et al
(5) | 2004 | English | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 5, 6, 8, 9 |
| Li et al
(6) | 2006 | English | 3 | NaAsO2 | ≥2 | ≤24 | Cancer cells | 6, 8, 9 |
| Lozano-Santos et
al (7) | 2015 | English | 3 | As2O3 | <2 | >24 | Cancer cells | 7, 12, 13 |
| Ge et al
(8) | 2005 | Chinese | 3 | AA | ≥2 | >24 | Cancer cells | 10 |
| Escudero-Lourdes
et al (9) | 2010 | English | 3 | MMA | <2 | >24 | Normal cells | 4, 5, 6, 8, 9 |
| Wang et al
(10) | 2012 | Chinese | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 8, 9, 11 |
| Daum et al
(11) | 2001 | English | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 8, 9 |
| Benbrahim-Tallaa
et al (12) | 2005 | English | 3 | NaAsO2 | ≥2 | >24 | Normal cells | 1 |
| Chowdhury et
al (13) | 2010 | English | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 5, 6, 8, 9 |
| Li et al
(14) | 2010 | Chinese | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 7 |
| Suzuki et al
(15) | 2011 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 5, 6, 8, 9 |
| Guilbert et
al (16) | 2013 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 4, 8, 9 |
| Huff et al
(17) | 2016 | English | 3 | NaAsO2 | <2 | ≤24 | Cancer cells | 5, 6, 8, 9 |
| Wang et al
(18) | 2012 | English | 3 | As2O3 | ≥2 | ≤24 | Normal cells | 5, 6, 8, 9(18) |
| Aodengqimuge et
al (19) | 2014 | English | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 4, 7, 10 |
| Gong et al
(20) | 2016 | English | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 5, 6, 8, 9, 10,
12 |
| Person et al
(21) | 2015 | English | 3 | NaAsO2 | ≥2 | >24 | Normal cells | 1, 4, 7 |
| Huang et al
(22) | 1999 | English | 3 | NaAsO2 | ≥2 | ≤24 | Cancer cells | 5, 6, 8, 9 |
| Martinez-Finley
et al (23) | 2011 | English | 4 | NaAsO2 | <2 | >24 | Normal cells | 1, 2, 5, 6, 8,
9 |
| Estañ et al
(24) | 2012 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 5, 6, 8, 9, 10,
11,12 |
| Zheng et al
(25) | 2006 | Chinese | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 4, 10, 11 |
| Zhang et al
(26) | 2015 | Chinese | 3 | NaAsO2 | ≥2 | >24 | Normal cells | 1, 2, 3, 5, 6,
7 |
| Luo et al
(27) | 2012 | Chinese | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 5, 6, 8, 9, 10, 11,
12, 13 |
| Banerjee et
al (28) | 2011 | English | 3 | As2O3 | <2 | ≤24 | Normal cells | 4, 7, 11 |
| Wu et al
(29) | 2008 | Chinese | 3 | As2O3 | ≥2 | >24 | Cancer cells | 8, 9, 10, 13 |
| Li et al
(30) | 2016 | Chinese | 8 | As2O3 | <2 | ≤24 | Cancer cells | 1, 2, 3, 4, 11, 12,
13 |
| Ye (31) | 2006 | Chinese | 3 | As2O3 | <2 | ≤24 | Cancer cells | 5, 6, 10 |
| Iwama et al
(32) | 2001 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 3, 5, 6, 8, 9, 10,
11, 13 |
| Calviño et
al (33) | 2011 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 4, 7, 10, 11,
12 |
| Liu et al
(34) | 2006 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 1, 6, 8, 9 |
| Huang et al
(35) | 2006 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 1, 4, 5, 6, 7 |
| Liao et al
(36) | 2015 | English | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 5, 6,7 |
| Wang (37) | 2012 | Chinese | 3 | NaAsO2 | ≥2 | ≤24 | Normal cells | 8, 9 |
| Ngalame et
al (38) | 2014 | English | 3 | NaAsO2 | ≥2 | >24 | Normal cells | 1, 7 |
| Ju (39) | 2007 | Chinese | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 8, 9, 10, 11 |
| Petit et al
(40) | 2013 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 5, 6, 8, 9 |
| Lu et al
(41) | 2014 | English | 3 | As2O3 | ≥2 | ≤24 | Cancer cells | 5, 6, 8, 9, 11, 12,
13 |
| Liu et al
(42) | 2013 | Chinese | 3 | As2O3 | <2 | >24 | Cancer cells | 1, 7 |
|
| Zhao et al
(43) | 2015 | English | 3 | As2O3 | <2 | >24 | Cancer cells | 1 |
Quality assessment of included
studies
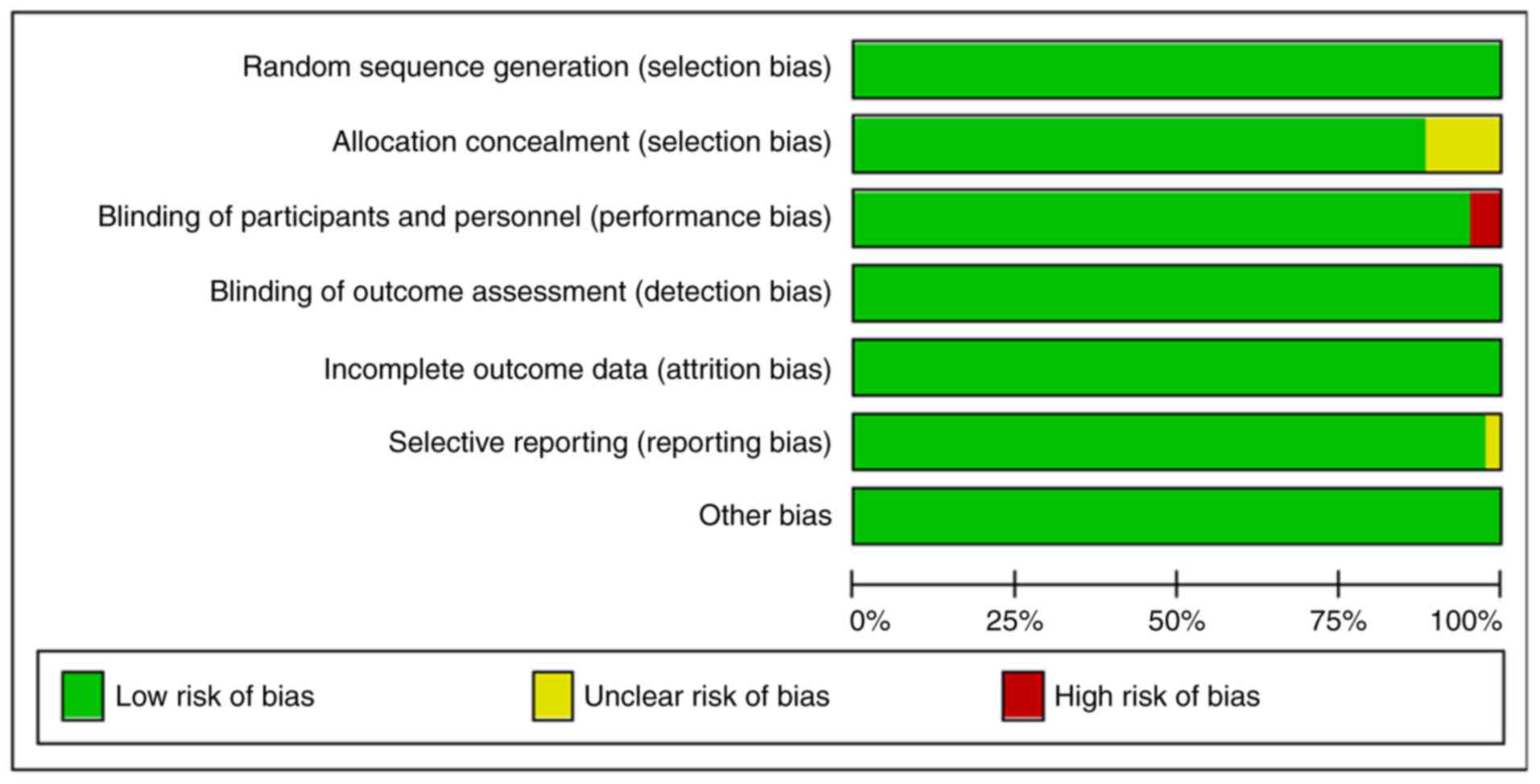
The quality of the 42 articles identified in the
study was evaluated (Table II)
and the proportion of low risk was accounted for more than 75%
(Fig. 2).
 | Table II.Quality assessment of included
studies. |
Table II.
Quality assessment of included
studies.
| Author/(Refs.) | Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| Huang et al
(22) | 1999 | L | L | L | L | L | L | L |
| Iwama et al
(32) | 2001 | L | L | L | L | L | L | L |
| Daum et al
(11) | 2001 | L | U | L | L | L | L | L |
| Benbrahim-Tallaa
et al (12) | 2005 | L | L | L | L | L | L | L |
| Liu et al
(34) | 2006 | L | L | L | L | L | L | L |
| Huang et al
(35) | 2006 | L | L | L | L | L | L | L |
| Li et al
(6) | 2006 | L | L | L | L | L | L | L |
| Chowdhury et
al (13) | 2010 | L | L | H | L | L | U | L |
| Li et al
(14) | 2010 | L | L | L | L | L | L | L |
| Calviño et
al (33) | 2011 | L | L | L | L | L | L | L |
| Suzuki et al
(15) | 2011 | L | L | L | L | L | L | L |
| Banerjee et
al (28) | 2011 | L | L | L | L | L | L | L |
| Eguchi et al
(3) | 2011 | L | L | L | L | L | L | L |
| Martinez-Finley
et al (23) | 2011 | L | L | L | L | L | L | L |
| Estañ et al
(24) | 2012 | L | L | L | L | L | L | L |
| Wang et al
(10) | 2012 | L | U | L | L | L | L | L |
| Liu et al
(42) | 2013 | L | L | L | L | L | L | L |
| Guilbert et
al (16) | 2013 | L | L | L | L | L | L | L |
| Ray et al
(4) | 2013 | L | L | L | L | L | L | L |
| Ngalame et
al (38) | 2014 | L | L | L | L | L | L | L |
| Lu et al
(41) | 2014 | L | L | L | L | L | L | L |
| Lozano-Santos et
al (7) | 2015 | L | L | H | L | L | L | L |
| Zhao et al
(43) | 2015 | L | L | L | L | L | L | L |
| Huff et al
(17) | 2016 | L | L | L | L | L | L | L |
| Zheng et al
(25) | 2006 | L | U | L | L | L | L | L |
| Liao et al
(36) | 2015 | L | L | L | L | L | L | L |
| Wu et al
(29) | 2008 | L | L | L | L | L | L | L |
| Zhang (26) | 2015 | L | L | L | L | L | L | L |
| Escudero-Lourdes
et al (9) | 2010 | L | L | L | L | L | L | L |
| Yen et al
(2) | 2011 | L | L | L | L | L | L | L |
| Wang et al
(18) | 2012 | L | L | L | L | L | L | L |
| Aodengqimuge et
al (19) | 2014 | L | L | L | L | L | L | L |
| Gong et al
(20) | 2016 | L | L | L | L | L | L | L |
| Ju (39) | 2007 | L | L | L | L | L | L | L |
| Ge et al
(8) | 2005 | L | U | L | L | L | L | L |
| Luo (27) | 2012 | L | U | L | L | L | L | L |
| Li et al
(30) | 2016 | L | L | L | L | L | L | L |
| Wang et al
(10) | 2012 | L | L | L | L | L | L | L |
| Ye (31) | 2006 | L | L | L | L | L | L | L |
| Person et al
(21) | 2015 | L | L | L | L | L | L | L |
| Lau et al
(5) | 2004 | L | L | L | L | L | L | L |
Meta-analysis of arsenic-related
apoptosis
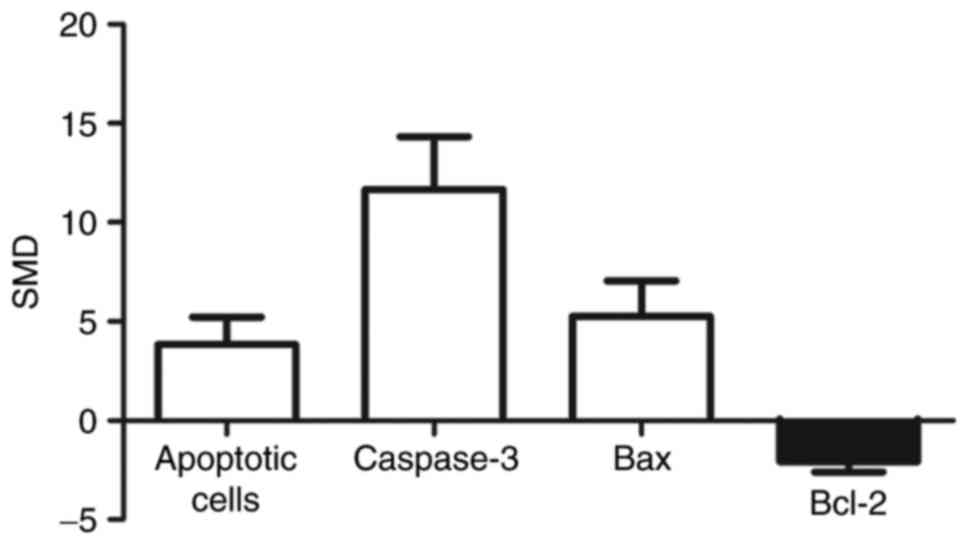
A pooled analysis showed that apoptotic cell levels
were 3.84-fold higher in arsenic exposed group compared to those of
control (95% CI (1.44, 6.24)). Levels of caspase-3 were 11.67 times
higher in the exposed group than in control group (95% CI (7.06,
16.28)). Bax levels were 5.27-fold higher in the exposed group
compared to control group (95% CI (2.18, 8.36)). Levels of Bcl-2
were 2.08 times lower in the exposed group than in control group
(95% CI (−2.96, −1.21)) (Fig.
3).
Meta-analysis regarding arsenic and
level of ERK
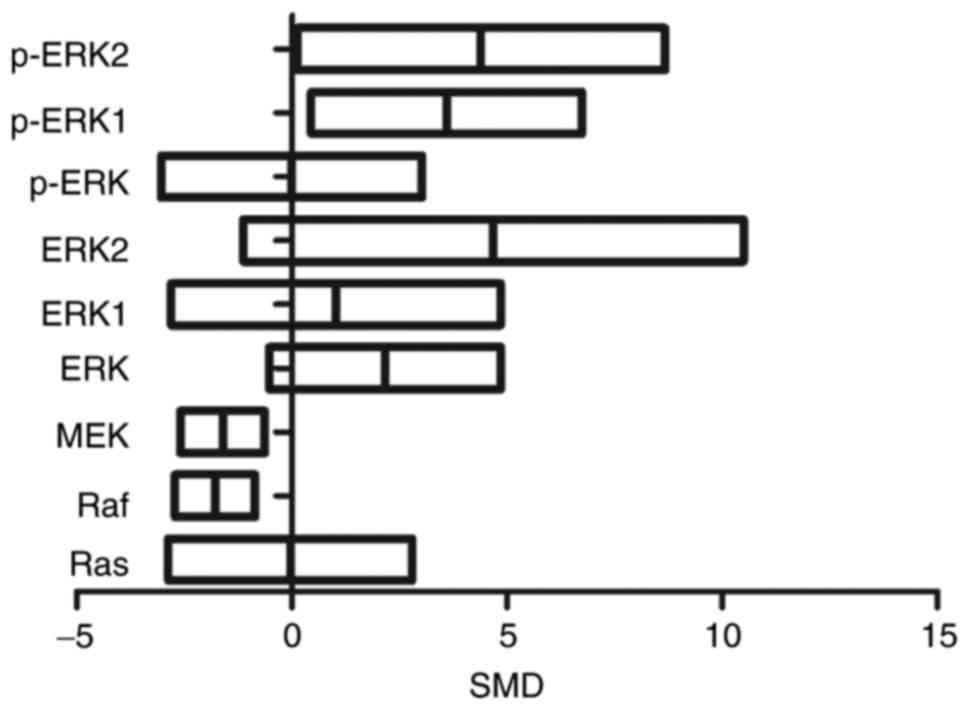
Levels of p-ERK1 were 3.59 times higher in exposed
group as compared to control group (95% CI (0.45, 6.74)). The
levels of p-ERK2 were comparatively 4.39 times higher in exposed
than in control group (95% CI (0.12, 8.67)). Raf levels were
1.78-fold lower in exposed than in control group (95% CI (−2.72,
−0.85)). Similarly, MEK levels were 1.61 times lower in exposed
group as compared to control group (95% CI (−2.59, −0.63)). There
was no statistical difference in Ras, ERK, ERK1, ERK2, and p-ERK
levels (P>0.05) (Fig. 4).
Subgroup analyses of arsenic exposure
effects
Subgroup analyses based on sources of
arsenic
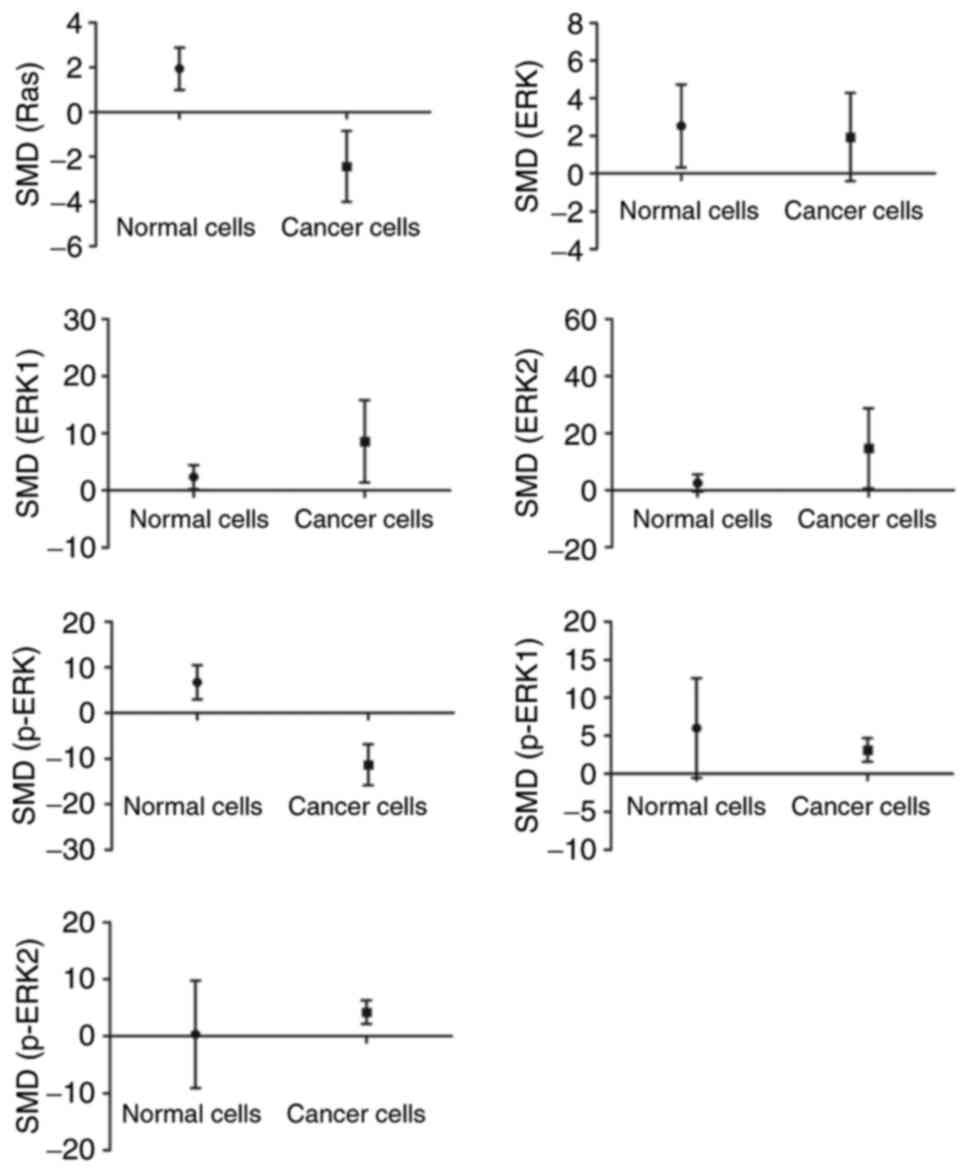
The analysis had demonstrated that arsenic promoted
the expressions of Ras and p-ERK (P<0.05) in normal cells.
Though, in cancer cells, arsenic decreased the expressions of Ras
and p-ERK as well as caused an increase in p-ERK1 and p-ERK2 levels
(P<0.05) (Fig. 5).
Subgroup analyses based on exposure
time of arsenic
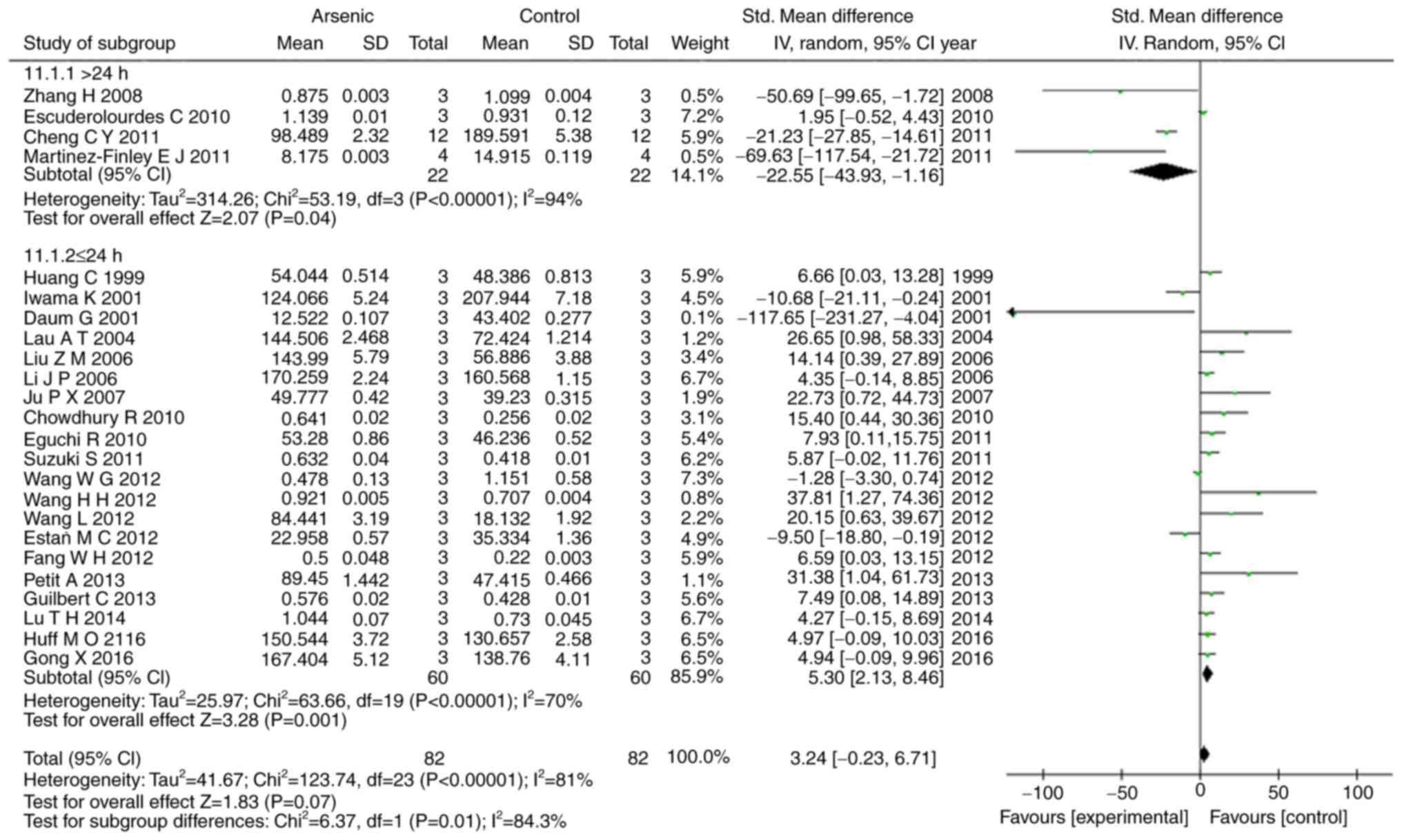
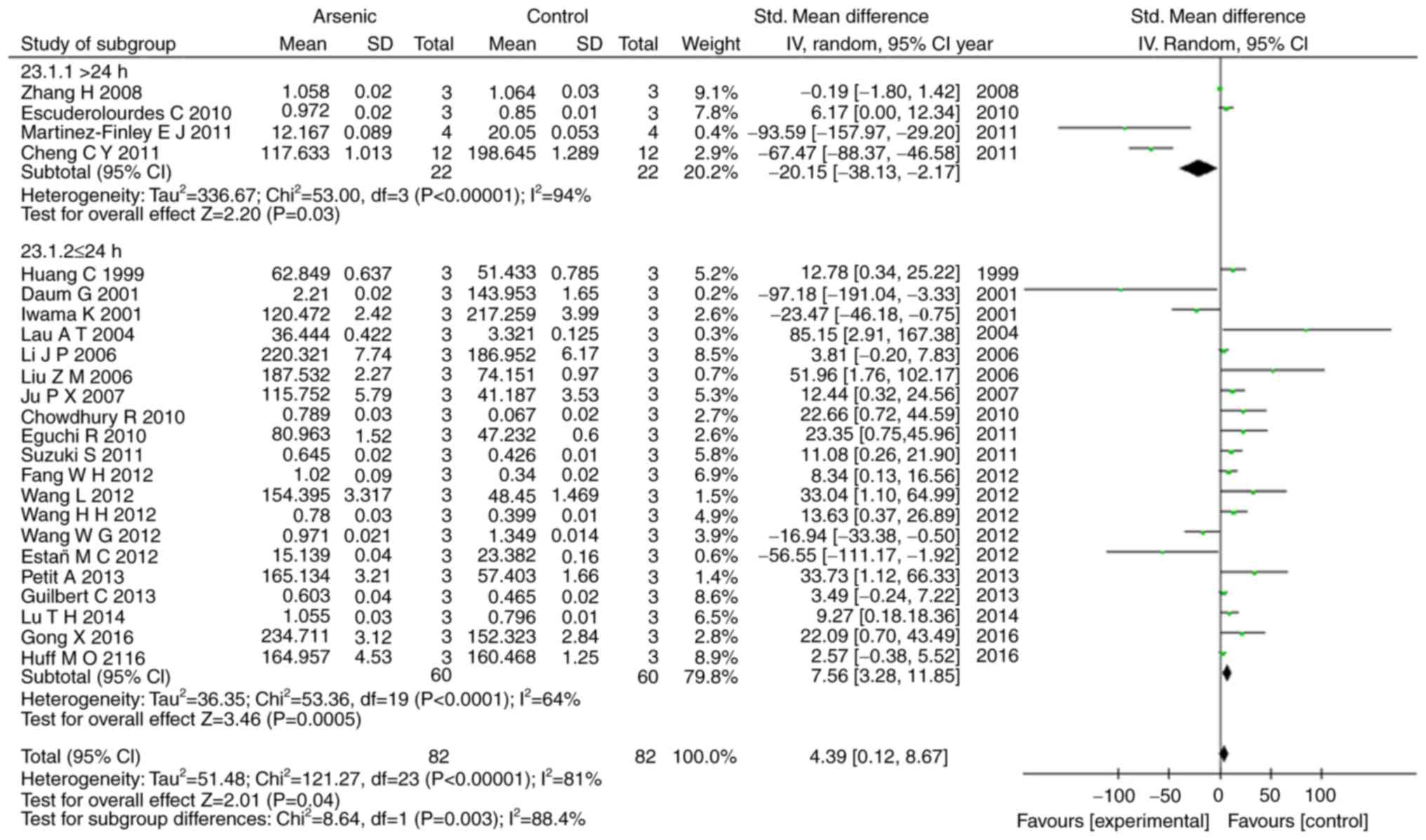
Our results showed that arsenic exposure time of
>24 h had suppressed the levels of ERK1, p-ERK1, and p-ERK2
(P<0.05), conversely arsenic exposure time of ≤24 h promoted the
levels of ERK1, ERK2, p-ERK, p-ERK1, and p-ERK2 (P<0.05)
(Figs. 6, 7).
Subgroup analyses based on arsenic
dose
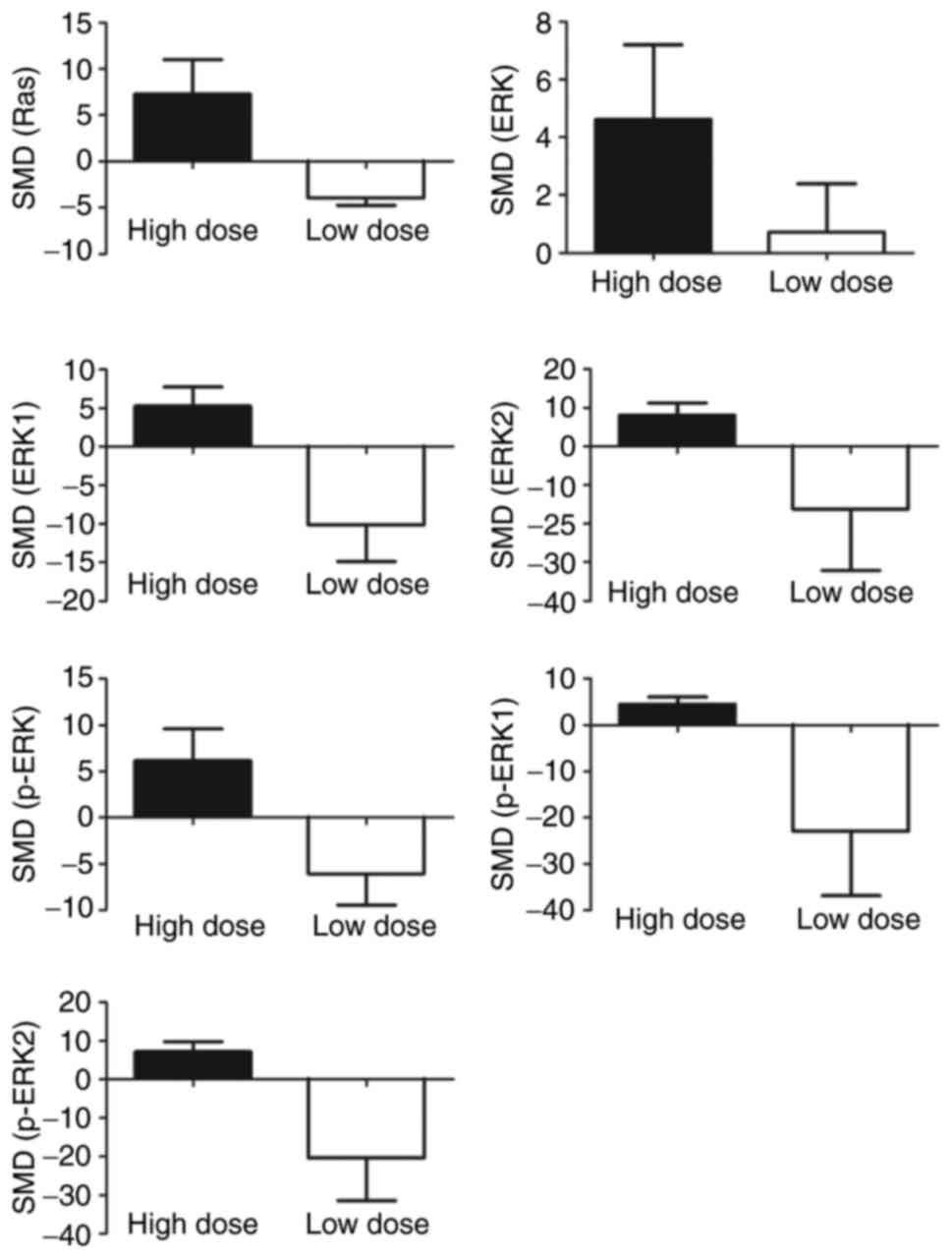
Subgroup analyses exhibited increased expressions of
Ras (SMD=7.29, 95% CI (0.90, 13.68)), ERK (SMD=4.62, 95% CI (0.17,
9.07)), ERK1 (SMD=5.28, 95% CI (1.02, 9.54)), ERK2 (SMD=8.17, 95%
CI (2.73, 13.62)), p-ERK (SMD=6.15, 95% CI (0.20, 12.11)), p-ERK1
(SMD=4.48, 95% CI (1.67, 7.30)), p-ERK2 (SMD=7.28, 95% CI (2.87,
11.70)) with high doses of arsenic (≥2 µmol/l). Conversely
decreased expressions of Ras (SMD=−3.96, 95% CI (−5.36, −2.56)),
ERK1 (SMD=−10.11, 95% CI (−18.40, −1.81)), p-ERK (SMD=−6.07, 95% CI
(−11.89, −0.26)), p-ERK2 (SMD=−20.34, 95% CI (−39.58, −1.11)) were
seen with low doses (<2 µmol/l) (Fig. 8).
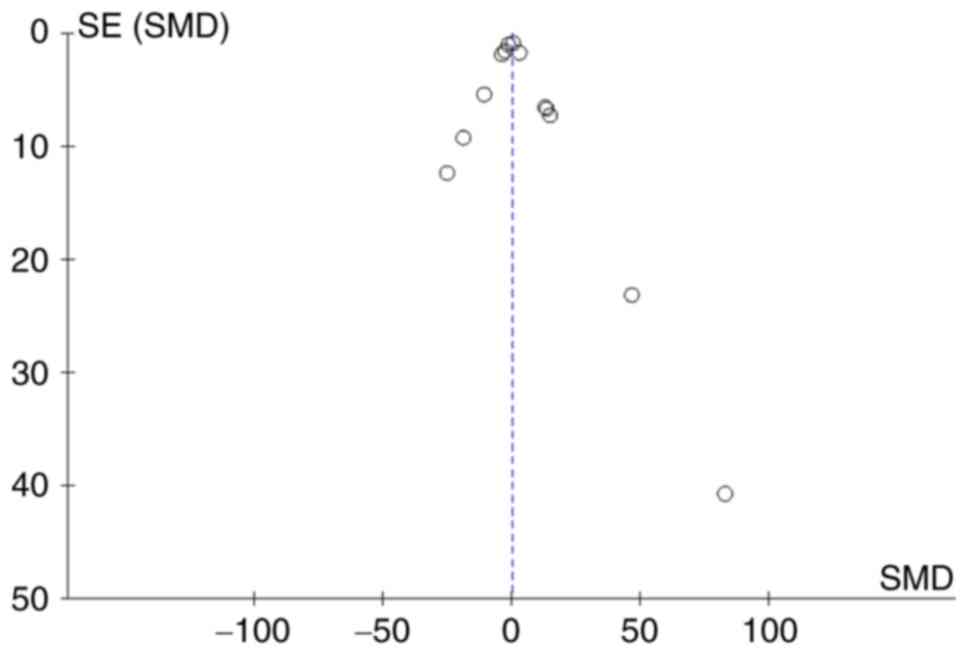
Small-study effect evaluation
The funnel plot (Fig.
9) shows that there was a symmetrical distribution of all the
studies, suggesting no significant small-study effects.
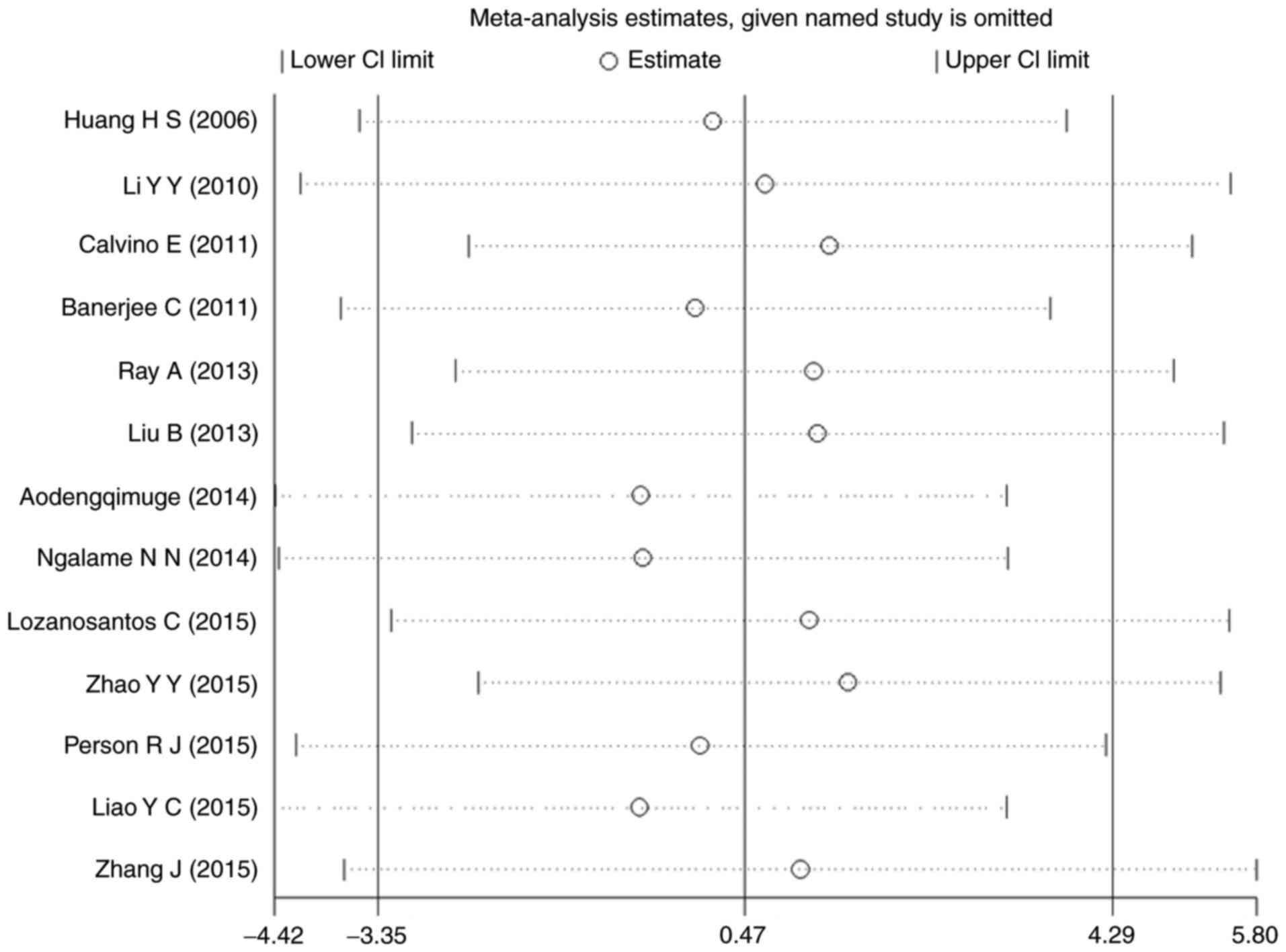
Sensitivity analysis
A sensitivity analysis was performed for p-ERK. The
results of all the studies were distributed evenly from the center
line and no significant deviation was seen. Thus, there seems to be
no individual study affecting the combined results (Fig. 10).
Discussion
Arsenic contributes to cell apoptosis (3) leading to serious damage (23). However, arsenic has recently been
explored for its anti-tumor ability in leukemia and other malignant
tumors using its induction of apoptosis (24). ERK had been reported to participate
in arsenic-induced apoptosis (25), but reports on the interaction
between arsenic and ERK signaling pathway were inconsistent. In our
meta-analysis, we found that arsenic had a bidirectional effect on
ERK signaling pathway. Arsenic could activate it in the normal
cell, but inhibit ERK pathway in cancer cell line, which was also
related to dosage and exposure time. These findings provided a
divergent theoretical basis of injurious as well as beneficial
therapeutic mechanisms of arsenic.
Apoptosis is of great significance in maintaining
normal development and homeostasis (26). As shown in Fig. 3, apoptotic cells, pro-apoptotic
protein (Bax) and activity of caspase-3 had increased while
anti-apoptotic protein (Bcl-2) had decreased suggesting an
undoubted proof of arsenic-induced apoptosis.
Present results suggested that ERK plays an opposing
role in normal and cancer cells. Luo (27) and Banerjee et al (28) had reported that in normal cells,
arsenic-induced apoptosis was brought about by activation of ERK
signaling pathway. As shown in Fig.
5, it can be seen that arsenic increased levels of Ras and
p-ERK in normal cells indicating that arsenic led to ERK signaling
pathway activation. As for cancer cells, induction of apoptosis is
one of the most efficient approaches for the clinical treatment of
cancer. It had been reported that arsenic-induced apoptosis of
cancer cells was correlated with inhibition of ERK (29–32).
Furthermore, arsenic inhibition of ERK signaling pathway in human
leukemia cells was also verified as a fact (7,10,24,33).
Likewise, decreased levels of both Ras and p-ERK were shown in
cancer cells (Fig. 5) along with
restraint of ERK signaling. Obviously, the mechanism of
arsenic-induced apoptosis is different between normal cells and
cancer cells.
ERK was also considered an important mechanism of
arsenic causing toxic injury (34,35).
Our results showed that arsenic increased the levels of Ras and
p-ERK in normal cells (Fig. 5),
suggesting that arsenic may activate ERK signaling pathway via
Ras/Raf/MEK/ERK pathways. Some studies stated that the activation
of ERK signaling pathway leads to DNA damage steering genetic
toxicity (36,37). These results also demonstrated that
arsenic, through activation of ERK signaling pathway in normal
cells, causes toxicity. Activated ERK had been reported to be
involved in pathogenesis and development of tumor and cancer
(30,38). In this study, cancer cells exposed
to arsenic had decreased levels of Ras and p-ERK (Fig. 5) and thus induced suppression of
ERK signaling pathway. Therefore, arsenic could inhibit the
development of tumor by restraining ERK signaling.
A difference in arsenic doses and exposure time
could account for opposite effects caused by arsenic on ERK
activity. Ju (39) found out that
high doses and short exposure time of arsenic led to activation of
ERK. This point was also testified by other studies (40,41).
However, some studies (42,43)
showed that low doses and long exposure time of arsenic contributed
to inactivation of ERK signaling pathway. These discoveries were
consistent with the results of this meta-analysis, suggesting that
high dose and short exposure time may lead to ERK signaling pathway
activation, while low dose and long exposure time depress the
activation of ERK signaling.
From what has been discussed above, we may
reasonably conclude that ERK signaling pathway was activated when
normal cells were exposed to high doses of arsenic for a short
period of time, contributing to cell apoptosis, explaining the
toxic injury caused by arsenic (Fig.
11A). As for cancer cells, low dose arsenic intervention for
long period of time may play a role in promoting cell apoptosis by
inhibiting ERK signaling pathway thus suppressing the growth of the
tumor (Fig. 11B). These findings
not only contributed to a potential approach for seeking ERK
inhibitors which work against toxic injury but also provided a
reference for a long-term treatment of cancer with low doses of
arsenic.
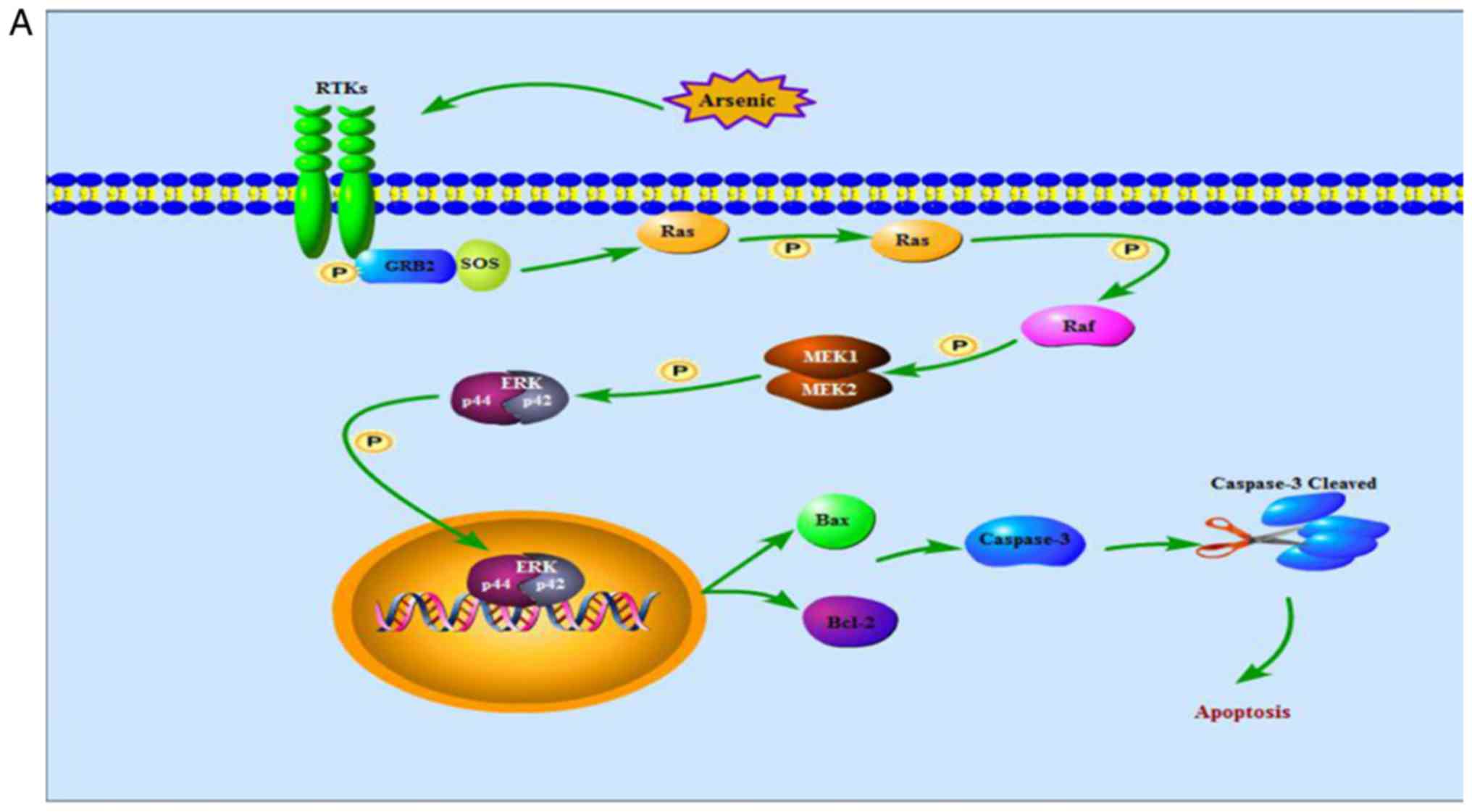 | Figure 11.The ERK signaling pathway. (A) shows
that high doses of arsenic for a short period of time enhances Ras,
Raf, MEK and ERK phosphorylation in normal cells, thereby the
activated ERK translocates from cytoplasm into nucleus, increases
levels of Bax protein, decreases levels of Bcl-2 protein and
cleaves caspase-3, contributing to cell apoptosis. (B) indicates
that, in cancer cells, low dose arsenic intervention for long
period of time suppresses phosphorylation of Ras, Raf, MEK and ERK,
blocking ERK translocation from cytoplasm into nucleus, thereby
increases levels of Bax protein, decreases levels of Bcl-2 protein
and cleaves caspase-3, contributing to cell apoptosis. ERK,
extracellular signal-regulated MAP kinases; MEK, mitogen-induced
extracellular kinase; p-ERK, phosphorylated extracellular
signal-regulated kinase; Raf, serine/threonine-specific protein
kinases; Bcl-2, B-cell lymphoma/leukemia-2 protein; Bax,
Bcl-associated X protein; caspase-3, cysteinyl aspartate-specific
protease-3; RTKs, receptor tyrosine kinases. |
The literature incorporated in this study exhibit
heterogeneity. It may also be related to certain factors such as
strains of objects, the method of arsenic exposure and possibly
others in addition to the factors shown in subgroup analysis. The
existing literature did not provide a detailed description of the
said factors. Moreover, the data in the selected papers do not
support our comparison of time and dose together. Arsenic also
affects JNK, p38 and other MAPK signaling pathways and whether ERK
interacts with all these may be regarded as a new direction for
future research.
Acknowledgements
The authors would like to thank the Department of
Public Health, Shihezi University School of Medicine for assistance
with this work, as well as funding from the National Natural
Science Foundation of China (no. 81560517 and 81760584), the Key
Areas of Science and Technology Research Project of Xinjiang
Production and Construction Corps (nos. 2014BA039 and 2015AG014)
and the International Cooperative Project of Shihezi University
(no. GJHZ201602).
Glossary
Abbreviations
Abbreviations:
|
MAPK
|
mitogen-activated protein kinases
|
|
ERK
|
extracellular signal-regulated MAP
kinases
|
|
MEK
|
mitogen-induced extracellular
kinase
|
|
p-ERK
|
phosphorylated extracellular
signal-regulated kinase
|
|
caspase-3
|
cysteinyl aspartate-specific
protease-3
|
|
Bcl-2
|
B-cell lymphoma/leukemia-2 protein
|
|
Bax
|
Bcl-associated X protein
|
References
|
1
|
Singh N, Kumar D, Lal K, Raisuddin S and
Sahu AP: Adverse health effects due to arsenic exposure:
Modification by dietary supplementation of jaggery in mice. Toxicol
Appl Pharmacol. 242:247–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yen CC, Ho TJ, Wu CC, Chang CF, Su CC,
Chen YW, Jinn TR, Lu TH, Cheng PW, Su YC and Liu SH: Inorganic
arsenic causes cell apoptosis in mouse cerebrum through an
oxidative stress-regulated signaling pathway. Arch Toxicol.
85:565–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eguchi R, Fujimori Y, Takeda H, Tabata C,
Ohta T, Kuribayashi K, Fukuoka K and Nakano T: Arsenic trioxide
induces apoptosis through JNK and ERK in human mesothelioma cells.
J Cell Physiol. 226:762–768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ray A, Chatterjee S, Mukherjee S and
Bhattacharya S: Interplay of loss of ERK dependence and
amplification of apoptotic signals in arsenic treated rat
hepatocytes. Natl Acad Sci Lett. 36:599–602. 2013. View Article : Google Scholar
|
|
5
|
Lau AT, Li M, Xie R, He QY and Chiu JF:
Opposed arsenite-induced signaling pathways promote cell
proliferation or apoptosis in cultured lung cells. Carcinogenesis.
25:21–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li JP, Lin JC and Yang JL: ERK activation
in arsenite-treated G1-enriched CL3 cells contributes to survival,
DNA repair inhibition, and micronucleus formation. Toxicol Sci.
89:164–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lozano-Santos C, Amigo-Jiménez I,
Nova-Gurumeta S, Pérez-Sanz N, García-Pardo A and García-Marco JA:
Arsenic trioxide synergistically potentiates the cytotoxic effect
of fludarabine in chronic lymphocytic leukemia cells by further
inactivating the AKT and ERK signaling pathways. Biochem Biophys
Res Commun. 461:243–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ge Y, Xu G and Zhang C: The effects of
arsenious acid on the apoptosis and the expression of ERK-1 protein
of human hepatocarcinoma cells. J Anhui Med Univ. 40:412–414.
2005.
|
|
9
|
Escudero-Lourdes C, Medeiros MK,
Cárdenas-González MC, Wnek SM and Gandolfi JA: Low level exposure
to monomethyl arsenous acid-induced the over-production of
inflammation-related cytokines and the activation of cell signals
associated with tumor progression in a urothelial cell model.
Toxicol Appl Pharmacol. 244:162–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang WG, Ma LL and Sun BL: Arsenic
trioxide induces apoptosis and inhibit activity of ERK in HL-60
cells. J Mod Oncol. 20:39–42. 2012.
|
|
11
|
Daum G, Pham J and D eou J: Arsenite
inhibits Ras-dependent activation of ERK but activates ERK in the
presence of oncogenic Ras in baboon vascular smooth muscle cells.
Mol Cell Biochem. 217:131–136. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benbrahim-Tallaa L, Waterland RA, Styblo
M, Achanzar WE, Webber MM and Waalkes MP: Molecular events
associated with arsenic-induced malignant transformation of human
prostatic epithelial cells: Aberrant genomic DNA methylation and
K-ras oncogene activation. Toxicol Appl Pharmacol. 206:288–298.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chowdhury R, Chatterjee R, Giri AK, Mandal
C and Chaudhuri K: Arsenic-induced cell proliferation is associated
with enhanced ROS generation, ERK signaling and CyclinA expression.
Toxicol Lett. 198:263–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li YY, Jiang YF, Yang YY, Wang R, Zhang
BY, Li L and Mu XL: Effects of JNK ERK signaling pathway in
proliferation of bone marrow mesenchymal stem cells induced by
NaAsO2. Prog Mod Biomed. 8:1464–1466. 2010.
|
|
15
|
Suzuki S, Inaba H, Satoh T, Okazaki T and
Takahashi S: Activation of ERK and p38 by the addition of arsenic
trioxide in Flt3-ITD cells. Open J Blood Dis. 1:9–11. 2011.
View Article : Google Scholar
|
|
16
|
Guilbert C, Annis MG, Dong Z, Siegel PM,
Miller WH Jr and Mann KK: Arsenic trioxide overcomes
rapamycin-induced feedback activation of AKT and ERK signaling to
enhance the anti-tumor effects in breast cancer. PLoS One.
8:e859952013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huff MO, Todd SL, Smith AL, Elpers JT,
Smith AP, Murphy RD, Bleser-Shartzer AS, Hoerter JE, Radde BN and
Klinge CM: Arsenite and cadmium activate MAPK/ERK via membrane
estrogen receptors and G-protein coupled estrogen receptor
signaling in human lung adenocarcinoma cells. Toxicol Sci.
152:62–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Kou MC, Weng CY, Hu LW, Wang YJ
and Wu MJ: Arsenic modulates heme oxygenase-1, interleukin-6, and
vascular endothelial growth factor expression in endothelial cells:
Roles of ROS, NF-κB, and MAPK pathways. Arch Toxicol. 86:879–896.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aodengqimuge, Liu S, Mai S, Li X, Li Y, Hu
M, Yuan S and Song L: AP-1 activation attenuates the
arsenite-induced apoptotic response in human bronchial epithelial
cells by up-regulating HO-1 expression. Biotechnol Lett.
36:1927–1936. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong X, Ivanov VN and Hei TK:
2,3,5,6-Tetramethylpyrazine (TMP) down-regulated arsenic-induced
heme oxygenase-1 and ARS2 expression by inhibiting Nrf2, NF-κB,
AP-1 and MAPK pathways in human proximal tubular cells. Arch
Toxicol. 90:2187–2200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Person RJ, Ngalame NN, Makia NL, Bell MW,
Waalkes MP and Tokar EJ: Chronic inorganic arsenic exposure in
vitro induces a cancer cell phenotype in human peripheral lung
epithelial cells. Toxicol Appl Pharmacol. 286:36–43. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang C, Ma WY, Li J, Goranson A and Dong
Z: Requirement of ERK, but not JNK, for arsenite-induced cell
transformation. J Biol Chem. 274:14595–14601. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martinez-Finley EJ, Goggin SL, Labrecque
MT and Allan AM: Reduced expression of MAPK/ERK genes in perinatal
arsenic-exposed offspring induced by glucocorticoid receptor
deficits. Neurotoxicol Teratol. 33:530–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Estañ MC, Calviño E, de Blas E,
Boyano-Adánez Mdel C, Mena ML, Gómez-Gómez M, Rial E and Aller P:
2-Deoxy-D-glucose cooperates with arsenic trioxide to induce
apoptosis in leukemia cells: Involvement of IGF-1R-regulated
Akt/mTOR, MEK/ERK and LKB-1/AMPK signaling pathways. Biochem
Pharmacol. 84:1604–1616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng TH, Guo QJ, Wang FM and Liu XH:
Extracellular signal-regulated kinase 1/2 (ERK1/2) participates in
the glioma apoptosis induced by As2O3. J Mod Oncol. 14:1341–1344.
2006.
|
|
26
|
Zhang J: Effects of Fluoride, Arsenic and
co-exposure on learning and memory and Ras/ERK pathway in rats
(dissertation). Med Univ Xinjiang. 2015.
|
|
27
|
Luo P, Zhang AH, Zhang KJ, Zeng XP, Fang
WH, Ye JF, Xiao JY, Zhang Y and Wu XY: The change of ERKs
expression in L-02 cell damage process cause by NaAsO2. Chin Soc
Toxicol. 27:387–388. 2013.(In Chinese).
|
|
28
|
Banerjee C, Goswami R, Datta S, Rajagopal
R and Mazumder S: Arsenic-induced alteration in intracellular
calcium homeostasis induces head kidney macrophage apoptosis
involving the activation of calpain-2 and ERK in Clarias batrachus.
Toxicol Appl Pharmacol. 256:44–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Luo RC, Zhang H and Cui YZ:
Inhibitory effect of sorafenib combined with arsenic trioxide on
hepatocellular carcinoma cells. Nan Fang Yi Ke Da Xue Xue Bao.
28:639–641. 2008.(In Chinese). PubMed/NCBI
|
|
30
|
Li P, Gong XJ and Cao W: Effect of ERK
activation and As2O3 in the apoptosis of anaplastic thyroid cancer
cell line FRO. Oncol Prog. 14:879–881. 2016.
|
|
31
|
Ye J: Involvement of JWA and MAPK in
apoptosis induced by Arsenic trioxide in MCF-7 and Hela cells and
cell differentiation induced by TPA in MCF-7 cells (unpublished PhD
thesis). Med Univ Nanjing. 2006.
|
|
32
|
Iwama K, Nakajo S, Aiuchi T and Nakaya K:
Apoptosis induced by arsenic trioxide in leukemia U937 cells is
dependent on activation of p38, inactivation of ERK and the
Ca2+-dependent production of superoxide. Int J Cancer. 92:518–526.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Calviño E, Estañ MC, Simón GP, Sancho P,
Boyano-Adánez Mdel C, de Blas E, Bréard J and Aller P: Increased
apoptotic efficacy of lonidamine plus arsenic trioxide combination
in human leukemia cells. Reactive oxygen species generation and
defensive protein kinase (MEK/ERK, Akt/mTOR) modulation. Biochem
Pharmacol. 82:1619–1629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu ZM and Huang HS: As2O3-induced
c-Src/EGFR/ERK signaling is via Sp1 binding sites to stimulate
p21WAF1/CIP1 expression in human epidermoid carcinoma A431 cells.
Cell Signal. 18:244–255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang HS, Liu ZM, Ding L, Chang WC, Hsu
PY, Wang SH, Chi CC and Chuang CH: Opposite effect of ERK1/2 and
JNK on p53-independent p21WAF1/CIP1 activation involved in the
arsenic trioxide-induced human epidermoid carcinoma A431 cellular
cytotoxicity. J Biomed Sci. 13:113–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao YC, Chen YF and Lee TC: Increased
susceptibility of H-Ras(G12V)-transformed human urothelial cells to
the genotoxic effects of sodium arsenite. Arch Toxicol.
89:1971–1979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang HH: The effect and mechanism study of
sodium arsenic induces cyclooxygenase-2 expression in Human
urothelial cells. unpublished PhD thesisMed Univ China 2012
|
|
38
|
Ngalame NN, Tokar EJ, Person RJ, Xu Y and
Waalkes MP: Aberrant microRNA expression likely controls RAS
oncogene activation during malignant transformation of human
prostate epithelial and stem cells by arsenic. Toxicol Sci.
138:268–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ju PX: Signal transduction mechanism for
As2O3-induced non-small cell lung cancer cell apoptosis.
unpublished PhD thesisMed Univ China 2007
|
|
40
|
Petit A, Delaune A, Falluel-Morel A,
Goullé JP, Vannier JP, Dubus I and Vasse M: Importance of ERK
activation in As2O3-induced differentiation and promyelocytic
leukemia nuclear bodies formation in neuroblastoma cells. Pharmacol
Res. 77:11–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu TH, Tseng TJ, Su CC, Tang FC, Yen CC,
Liu YY, Yang CY, Wu CC, Chen KL, Hung DZ and Chen YW: Arsenic
induces reactive oxygen species-caused neuronal cell apoptosis
through JNK/ERK-mediated mitochondria-dependent and GRP
78/CHOP-regulated pathways. Toxicol Lett. 224:130–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu B, Zhao Y, Yu L, He X and Zhang B:
Study on the role of Ras/p-ERK signaling pathway in the reversing
effect of As2O3 on multi-drug-resistance. Chin J Clin Oncol.
40:505–512. 2013.
|
|
43
|
Zhao YY, Yu L, Liu BL, He XJ and Zhang BY:
Downregulation of P-gp, Ras and p-ERK1/2 contributes to the arsenic
trioxide-induced reduction in drug resistance towards doxorubicin
in gastric cancer cell lines. Mol Med Rep. 12:7335–7343. 2015.
View Article : Google Scholar : PubMed/NCBI
|