Introduction
Immunoglobulin A vasculitis (IgAV), formerly known
as Henoch-Schönlein purpura, is a systemic vasculitis caused by the
deposition of immune complexes and is common in children. Its main
feature is to cause skin purpura, joint pain, abdominal pain,
hematuria and proteinuria (1).
Worldwide, the incidence of IgAV in children is 10–20 per 100,000
per year, of which 90% of affected children are 2–10 years old,
with a peak of 4–7 years old (2).
Kidney involvement is an essential factor affecting long-term
prognosis. IgAV with nephritis (IgAVN) accounts for 1–2% of
children with end-stage kidney disease. Although the incidence of
IgAVN varies with different research samples and diagnostic
criteria, it can reach 20–54% (3,4). For
most children with IgAV, abdominal pain or joint pain is only acute
damage. Once the kidney is involved (when IgAV progresses to
IgAVN), IgAVN will affect the long-term quality of life of
children. Therefore, early identification of IgAVN and assessment
of the level of risk of renal involvement are clinically
critical.
Currently, there is a lack of specific biomarkers
for renal damage in IgAV, so it is necessary to use proteomics to
perform such research. There have been proteomic studies based on
serum and renal tissues in children with IgAV (5,6).
However, there are few experiments on proteomics using urine and
there is a lack of large-sample experiments (7). The present study chose urine as the
sample. Urine has some advantages and it is relatively easy to
obtain samples. The urine test is non-invasive and renal function
problems can be determined without pain or discomfort (8). In addition to routine renal biopsy,
the biomarkers found in urine are the most reliable for renal
disease assessment (9).
Liquid chromatography-tandem mass spectrometry
(LC-MS/MS)-based proteomics technology provides a high-throughput
method for protein quantification, which is significantly higher
than that of western blotting and ELISA (10). Compared with data-dependent
acquisition, data-independent acquisition (DIA) technology used in
large samples is superior, but due to the complexity of proteomics
samples and the limitations of collection speed and sensitivity of
mass spectrometry, the unbiased measurement of proteomes remains
difficult (11,12). It has been shown that the addition
of the separation of ion mobility can further improve the
sensitivity of protein identification and reduce spectral
complexity based on retention time, m/z and intensity (11). Parallel accumulation-serial
fragmentation (PASEF), which is a method for synchronizing mobility
separation with quadrupole quality selection, is joined to DIA and
termed diaPASEF (11,12). As a new generation of proteomics
technology, diaPASEF can comprehensively improve the ability of
protein identification, detection sensitivity and data
integrity.
Therefore, the present study aimed to determine the
difference in urinary protein content between children with IgAVN
and normal children based on the technical platform of diaPASEF and
bioinformatics analysis. Subsequently, the changes of identified
protein content were verified by ELISA. It is suggested that the
present study can provide valuable potential for the early
identification and treatment of IgAVN.
Materials and methods
Specimens and clinical data
The present study involved children hospitalized in
the First Affiliated Hospital of Anhui Medical University between
2020 and 2022. The diagnostic criteria of children with IgAVN were
based on the criteria of The European League Against
Rheumatism/Pediatric Rheumatology International Trials
Organisation/Paediatric Rheumatology European Society
(EULAR/PRINTO/PRES) (13): First,
there is non-thrombocytopenic purpura that mainly occurs in the
lower extremities and second, there is one of the following four
criteria: i) Abdominal pain, ii) histologically positive IgA, iii)
arthritis or joint pain and iv) renal damage (13). In addition, the criteria of IgAVN
included in the present study met one of the following: i)
Pathologically confirmed by renal biopsy and ii) 24-h urinary
protein ≥150 mg/d. The healthy children in the experiment,
according to the medical history, urine routine and renal function
tests, were free of leukuria, proteinuria, hematuria and renal
damage and children with kidney-related diseases were excluded.
The present study was divided into two parts. One
was to analyze urinary proteomics between the IgAVN group and the
healthy group and the other is to perform ELISA verification of
specific proteins, including the IgAVN group, IgAV group and the
healthy group. All the urine samples in the experiment were from
mid-morning urine. The present study was approved by the Medical
Ethics Review Committee of Anhui Medical University and written
agreement was obtained from the guardians of all the children
recruited.
Preparation, separation and digestion
of proteins
Following centrifugation at 3,500 × g and 4°C for 10
min, the samples were stored at −80°C with low speed refrigerated
centrifuge (cat. no. JW-1044R; Anhui Jiawen Instrument Equipment
Co., Ltd.). After removing impurities, the samples were frozen at
−80°C for storage. SDT buffer (4% SDS, 100 mM DTT and 150 mM
Tris-HCl; pH 8.0) was incorporated into all samples. The detergent,
DTT and other low-molecular-weight components were removed using UA
buffer (8 M Urea and 150 mM Tris-HCl; pH 8.0) by repeated
ultrafiltration (Microcon-10 kDa; MilliporeSigma). Then 100 µl
iodoacetamide (100 mM IAM in UA buffer) was added to block reduced
cysteine residues and the samples were incubated for 30 min in the
dark. The filters were washed with 100 µl UA buffer three times and
then 100 µl 25 mM NH4HCO3 buffer twice.
Finally, the protein suspensions were digested with 4 µg trypsin
(Promega Corporation) in 40 µl 25 mM NH4HCO3
buffer overnight at 37°C and the resulting peptides were collected
as a filtrate. The peptides of each sample were desalted on C18
Cartridges [Empore SPE Cartridges C18 (standard density), bed I.D.
7 mm, volume 3 ml, MilliporeSigma], concentrated by vacuum
centrifugation (cat. no. FD-80CE; Shanghai Bilon Instrument Co.,
Ltd.) at 25°C for 18 h and reconstituted in 40 µl of 0.1% (v/v)
formic acid. The peptide content was estimated by UV light spectral
density at 280 nm with NanoDrop 2000c (Thermo Fisher Scientific,
Inc.). For DIA experiments, iRT (indexed retention time)
calibration peptides were spiked into the sample.
A new generation of mass
spectrometry-diaPASEF
The peptides from each sample were analyzed by
nanpLC-MS/MS operating in the DIA mode. Nano-liter flow rate Evosep
one system (Evosep Biosystems) was used for chromatographic
separation. Buffer solutions: Solution A was 0.1% formic acid
aqueous solution and solution B was 0.1% formic acid acetonitrile
aqueous solution. The samples separated by nano-upgraded
high-performance liquid chromatography were analyzed by trapped Ion
Mobility Spectrometry (TIMS) time-of-flight mass spectrometer
(Bruker Daltonics). The mass spectrometer collected ion mobility MS
spectra over a mass range of m/z 100–1,700 and according to the
m/z-ion mobility plane, up to eight windows for a single 100 ms
TIMS scan were defined. During PASEF MS/MS scanning, the collision
energy was ramped linearly as a function of the mobility from 20eV
to 59 eV (14,15).
MS data analysis
The DIA data were processed by Spectronaut software
(Spectronaut 14.4.200727.47784; Biognosys AG). The database was the
UniProt_Human database (https://www.uniprot.org/; 2022_01 release), adding the
indexed retention time (IRT) polypeptide sequence (Biognosys
|iRTKit|). The search parameters were as follows: The enzyme was
trypsin, the maximum number of miscleavage was 1, the fixed
modification was carbamidomethyl and the dynamic modification is
oxidation and acetyl (Protein N-term). The protein identified by
database search had to pass the set filter parameter false
discovery rate (FDR) <1%. Based on the spectrum library obtained
by the above retrieval, the parameters of the Spectronaut software
were set as follows: The retention time prediction type was dynamic
IRT, interference on MS2 level correction is enabled and cross-run
normalization is enabled. The results of all samples were filtered
based on Q value cutoff of 0.01 (equivalent to FDR <1%).
Differential protein map and
correlation analysis
To analyze the proteins with different expressions
between the experimental groups, the preliminary experimental data
were further screened for differences. In the screening of proteins
with a significant difference, the following two screening
conditions had to be met: The expression multiple (Fold Change, FC)
between the two groups was >1.5 (upregulated by >1.5 or
downregulated by <0.67 times) and two independent samples after
Shapiro-Wilk test for normality were t-test (P<0.05). As a
result, all the upregulated and downregulated proteins were
obtained. Then hierarchical clustering analysis was carried out by
using Cluster 3.0 (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm)
and Java TreeView software (http://jtreeview.sourceforge.net). Blast2Go
(https://www.blast2go.com/) (16) software and the Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway database (http://geneontology.org/) (17) were used to analyze the differential
proteins. To find the more dominant differential proteins from the
significant differential proteins, the protein-protein interaction
network map was constructed by using Cytoscape software (http://www.cytoscape.org; version 3.2.1) based on the
protein-protein interaction relationship in the STRING database
(18).
ELISA identification of related
specific proteins
Following thawing and centrifugation (3,500 × g and
4°C for 10 min), the three groups of urine samples used in the
verification experiment were treated according to the instructions
of the AZGP1 ELISA kit (cat. no. KA6083; Abnova). The optical
density of the sample was measured by an optical densitometer and
the quantitative analysis result of the verified protein was
obtained.
Statistical analysis of data
In the baseline data, numerical variables are
expressed as mean ± standard deviation, and the Student Newman
Keuls test following one-way analysis of variance (ANOVA) was used
for post-hoc analysis. In the bioinformatics analysis, unpaired
t-test was used to screen differential proteins and Fisher's Exact
Test was used to analyze the significance of differences. For AZGP1
protein, G*Power (3.1.9.7) (https://www.gpower.hhu.de/) was used to calculate the
power of the results. Tukey's post-hoc multiple comparisons were
performed following one-way ANOVA to compare differential levels of
AZGP1, and the Spearman's correlation coefficient was used to
evaluate its correlation with clinical data. SPSS (version 16.0;
SPSS, Inc.) was used in data analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical data of experimental
specimens
The clinical data for all specimens used in the
experiment are shown in Table I.
First, eight specimens from the disease group (Group 1) and eight
samples from the healthy group (Group 2) were selected for the
proteomics experiment. Then, 30 samples of IgAVN, IgAV and healthy
children were collected for verification experiments. According to
the results of S-N-K test of analysis of variance, the P-values of
age, body weight, eGFR and serum creatinine were all >0.05. No
statistical difference was observed in these indexes between the
experimental group and the verification group, suggesting that
there was little interference from the baseline data difference in
the follow-up experiment, which further ensured its accuracy.
 | Table I.Basic clinical data of experimental
subjects. |
Table I.
Basic clinical data of experimental
subjects.
|
| Experimental
groups |
|
|
|
|
|
|---|
|
|
| Verification
groups |
|
|
|
|---|
| Characteristic | IgAVN (n=8) | Healthy children
(n=8) |
| Healthy children
(n=10) |
|
|
|---|
| IgAVN (n=10) | IgAV (n=10) | F-value | P-value |
|---|
| Age, years mean
(SD) | 11.5 | 10.4 | 11.3 | 8.9 | 9.1 | 1.615a | 0.224a |
|
| (1.4) | (2.1) | (1.6) | (2.8) | (2.1) | 3.614b | 0.057b |
| Weight, kg mean
(SD) | 41.3 | 39.4 | 42.6 | 37.4 | 30.1 | 0.062a | 0.806a |
|
| (13.8) | (15.2) | (10.1) | (10.3) | (11.0) | 3.623b |
>0.05b |
| Laboratory index,
mean (SD) |
|
|
|
|
|
|
|
| eGFR,
ml/min/1.73 m2 | 169.4 | 164.5 | 177.2 | 176.7 | 174.1 | 0.228a | 0.641a |
|
| (27.0) | (10.4) | (18.6) | (21.5) | (15.2) | 0.080b | 0.927b |
| Serum
creatinine, mmol/l | 45.0 | 48.1 | 40.8 | 41.8 | 42.8 | 0.244a | 0.629a |
|
| (13.4) | (11.7) | (8.5) | (10.3) | (5.9) | 0.134b | 0.864b |
Significant difference in protein
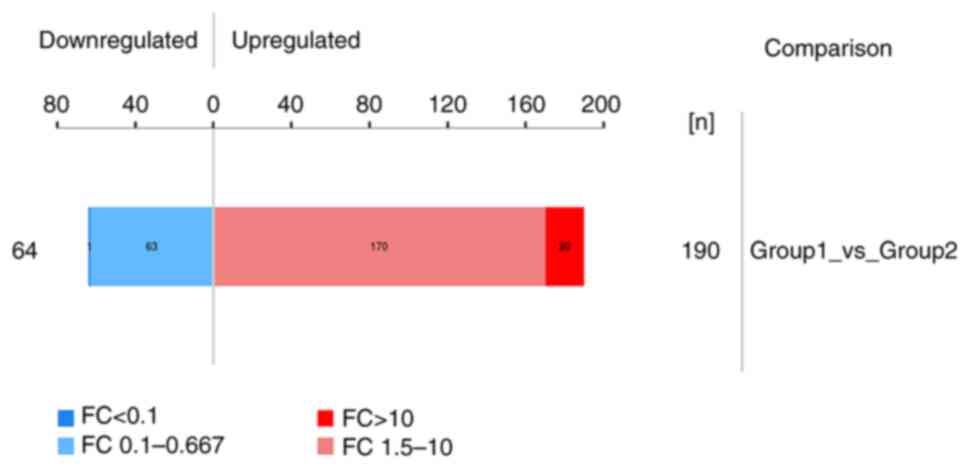
Using the diaPASEF technique, 254 differential
proteins were screened from the experimental group according to the
expression multiple (FC) and P<0.05. Among the differential
proteins, 190 proteins were upregulated (FC >1.5) and 64
proteins were downregulated (FC <0.67; Fig. 1).
In the common clinical urine protein, consistent
with the baseline data, some of the common urinary proteins were
different, such as serotransferrin (TF; FC18.59; P=0.002) and
albumin (ALB; FC9.256; P=0.002), while no significant differences
were observed in some of them, such as retinol-binding protein
(FC1.438; P=0.2453), α-1-microglobulin (FC1.310; P=0.4438) and β-2
microglobulin (FC1.587; P=0.2839). With cystatin-C (FC0.9379;
P=0.7529), no differential expression was found in the experimental
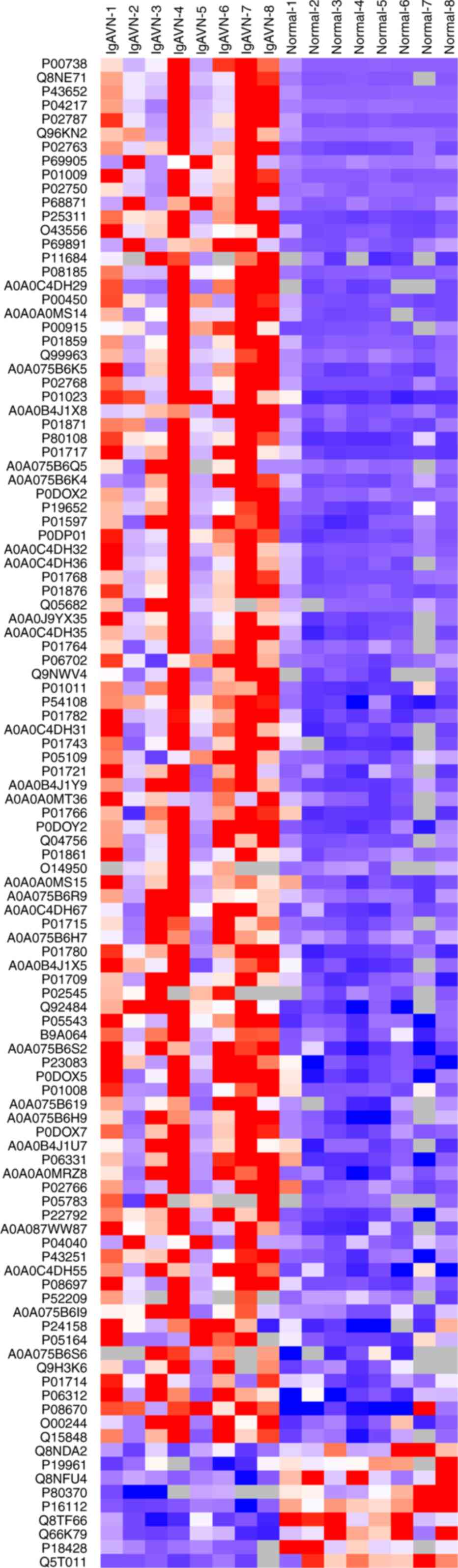
results. Part of the differential proteins (FC >3 or FC
<0.34) were analyzed by hierarchical cluster analysis using
Cluster 3.0 and Java Tree View software and were represented by
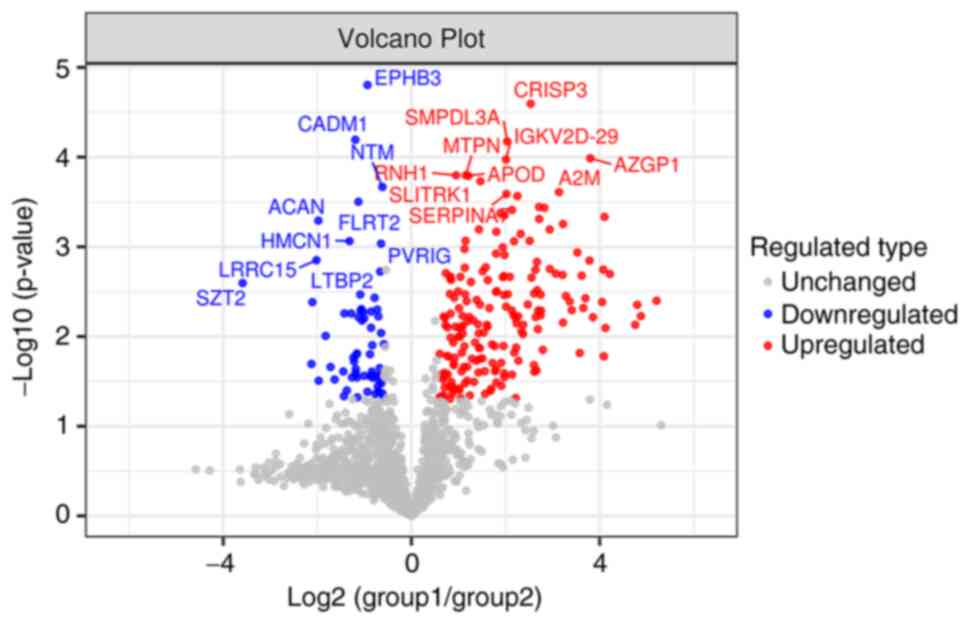
tree heat map (Fig. 2). To better
show the significant difference in proteins between the
experimental groups, a volcano plot was drawn based on the
expression difference multiple and P-value, and the 10 proteins
with the most significant differences between upregulated and
downregulated proteins were labeled (Fig. 3).
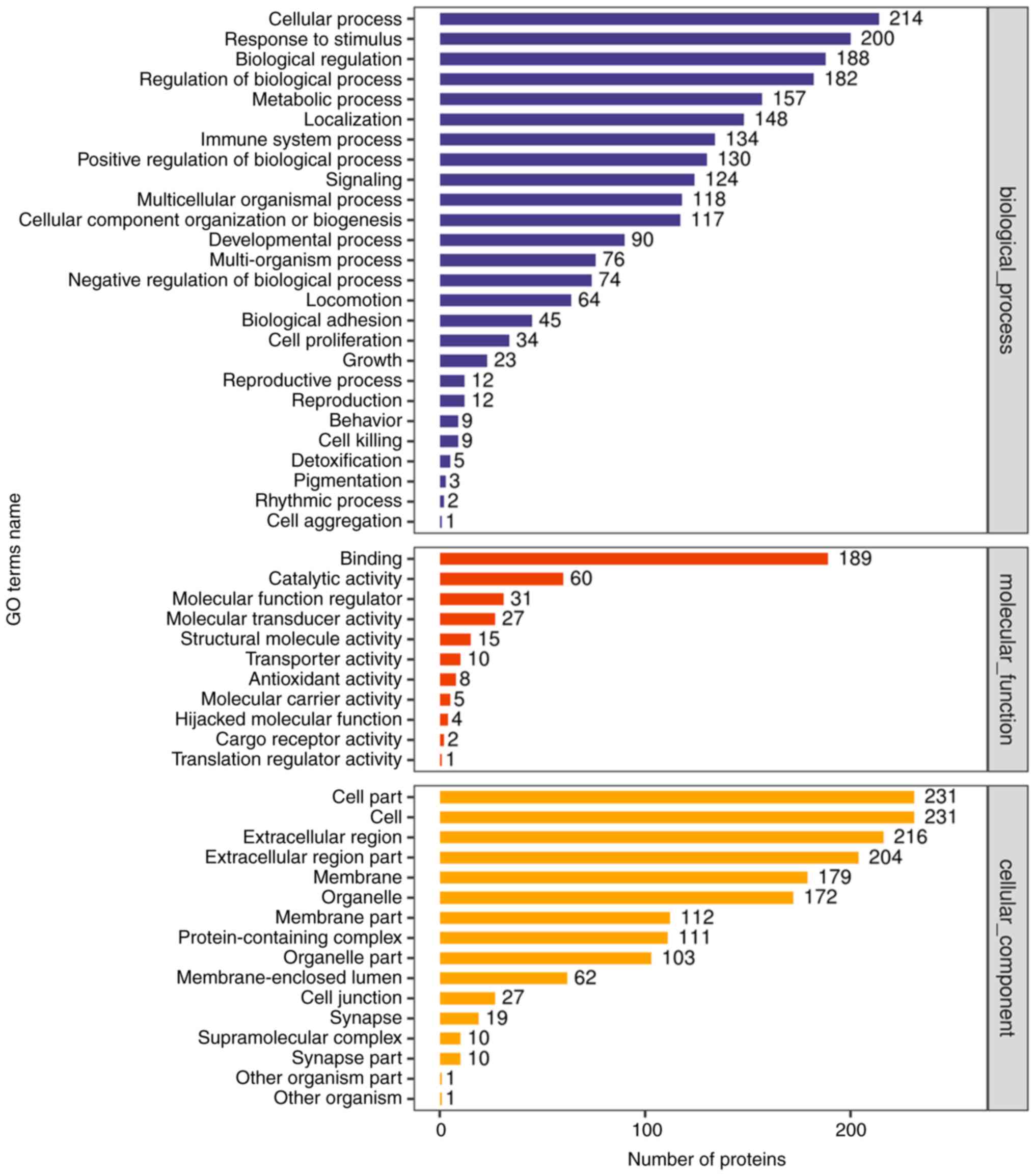
Functional notes and analysis of GO
(Gene ontology)
To fully understand the function, location and
biological pathway of proteins in organisms, proteins were
annotated by GO. GO functional annotations are mainly divided into
three categories: Biological Process (BP), Cellular Component (CC)
and Molecular Function (MF). In the present study, GO functional
annotation was performed on all differentially expressed proteins
by using Blast2Go (https://www.blast2go.com/) software (Fig. 4).
All differentially expressed proteins and all
identified proteins were compared with the annotation results of GO
function and the functional categories of all differentially
expressed proteins were found by Fisher's Exact Test (P<0.05). A
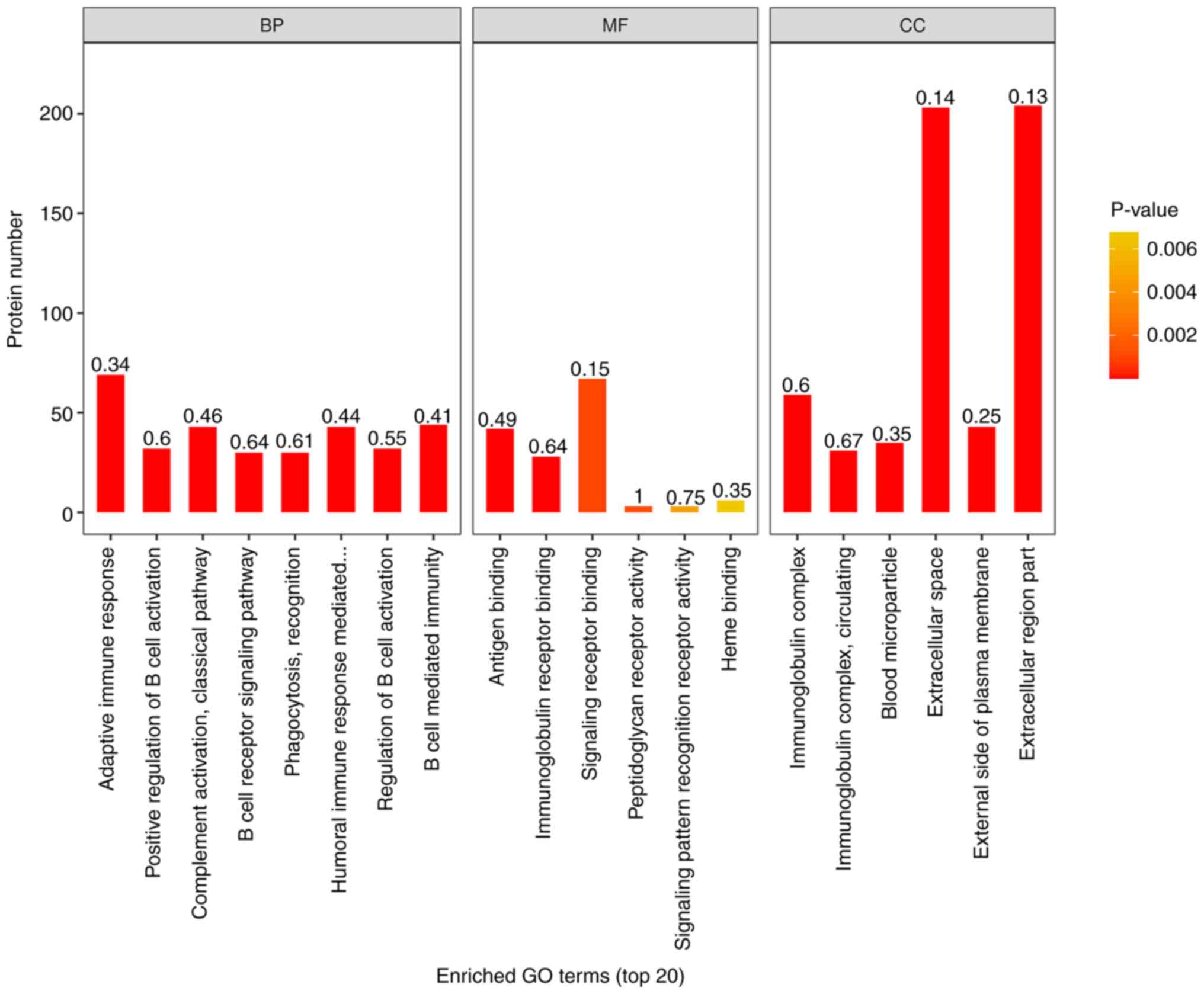
histogram was used to show the enrichment of top20 under the three
classifications of GO function (Fig.
5).
The results of GO enrichment analysis showed that,
among the proteins involved in BP, ‘adaptive immune response’,
‘positive regulation of B cell activation’, ‘classical complement
activation pathway’, ‘B cell receptor signaling pathway’,
‘phagocytosis, recognition’, ‘humoral immune response mediated by
circulating immunoglobulin’, ‘regulation of B cell activation’, ‘B
cell-mediated immunity’ and ‘immunoglobulin mediated immune
response’ were significantly enriched. Among CC, the expression of
‘immunoglobulin complex’ and ‘circulating immunoglobulin complex’
was the most evident. In MF, significant enrichment was also
associated with ‘antigen binding’ and ‘immunoglobulin receptor
binding’.
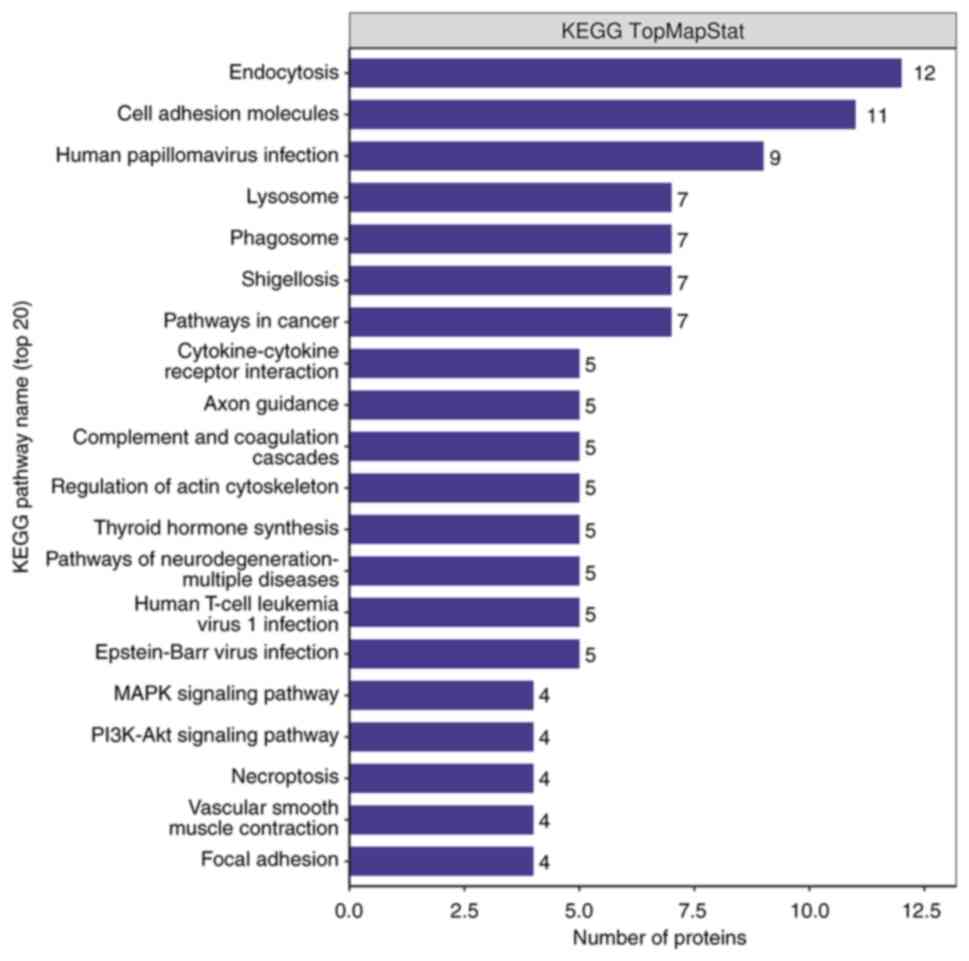
KEGG pathway analysis
In order to further study the biological function of
differential proteins, all differential proteins were subjected to
KEGG pathway analysis. As shown in the Fig. 6, there were 20 major pathways,
namely ‘Endocytosis’, ‘Cell adhesion molecules’, ‘Human
papillomavirus infection’, ‘Lysosome’, ‘Phagosome’, ‘Shigellosis’,
‘Pathways in cancer’, ‘Cytokine-cytokine receptor interaction’,
‘Axon guidance’, ‘Complement and coagulation cascades’, ‘Regulation
of actin cytoskeleton’, ‘Thyroid hormone synthesis’, ‘Pathways of
neurodegeneration-multiple diseases’, ‘Human T-cell leukemia virus
1 infection’, ‘Epstein-Barr virus infection’, ‘MAPK signaling
pathway’, ‘PI3K-Akt signaling pathway’, ‘Necroptosis’, ‘Vascular
smooth muscle contraction’ and ‘Focal adhesion’. Among them, the
leading ones were ‘Endocytosis’ and ‘Cell adhesion molecules’.
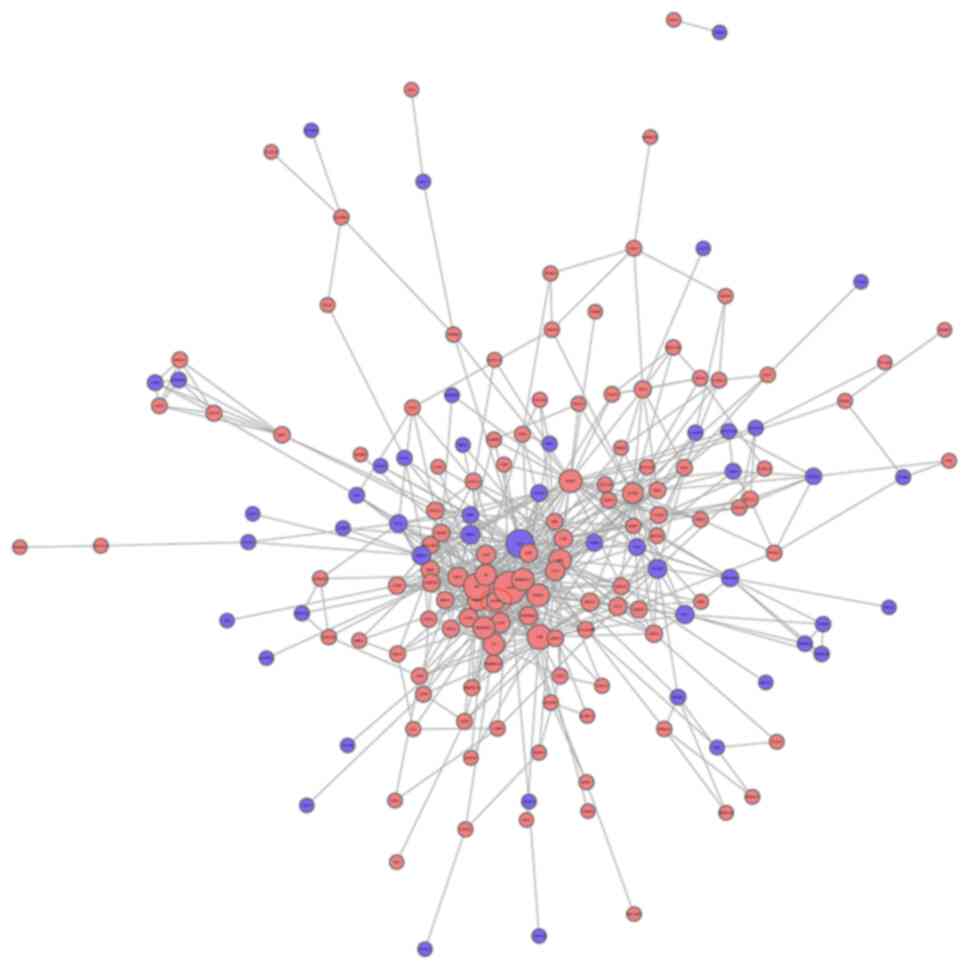
Network analysis of protein-protein
interaction
The interaction network analysis of all differential
proteins is shown in Fig. 7.
According to the two conditions, the specific differential protein
was selected as AZGP1. First, it can be seen from the volcano map
that AZGP1 ranks second in the top 20; in differential expression
AZGP1 participates in the highest degree of connectivity in the
interaction network and has an interaction with 13 differential
proteins. The interacting proteins are transmembrane emp24
domain-containing protein 9, ALB, TF, α-2-macroglobulin,
leucine-rich α-2-glycoprotein, cysteine-rich secretory protein 3,
α-1-antitrypsin, adiponectin, apolipoprotein D, haptoglobin,
ceruloplasmin, α-1-acid glycoprotein 1 and famin. Combined with the
GO function results, it was shown that AZGP1 and interacting
proteins are related to extracellular space, extracellular region
part, extracellular region, establishment of localization,
transport and localization.
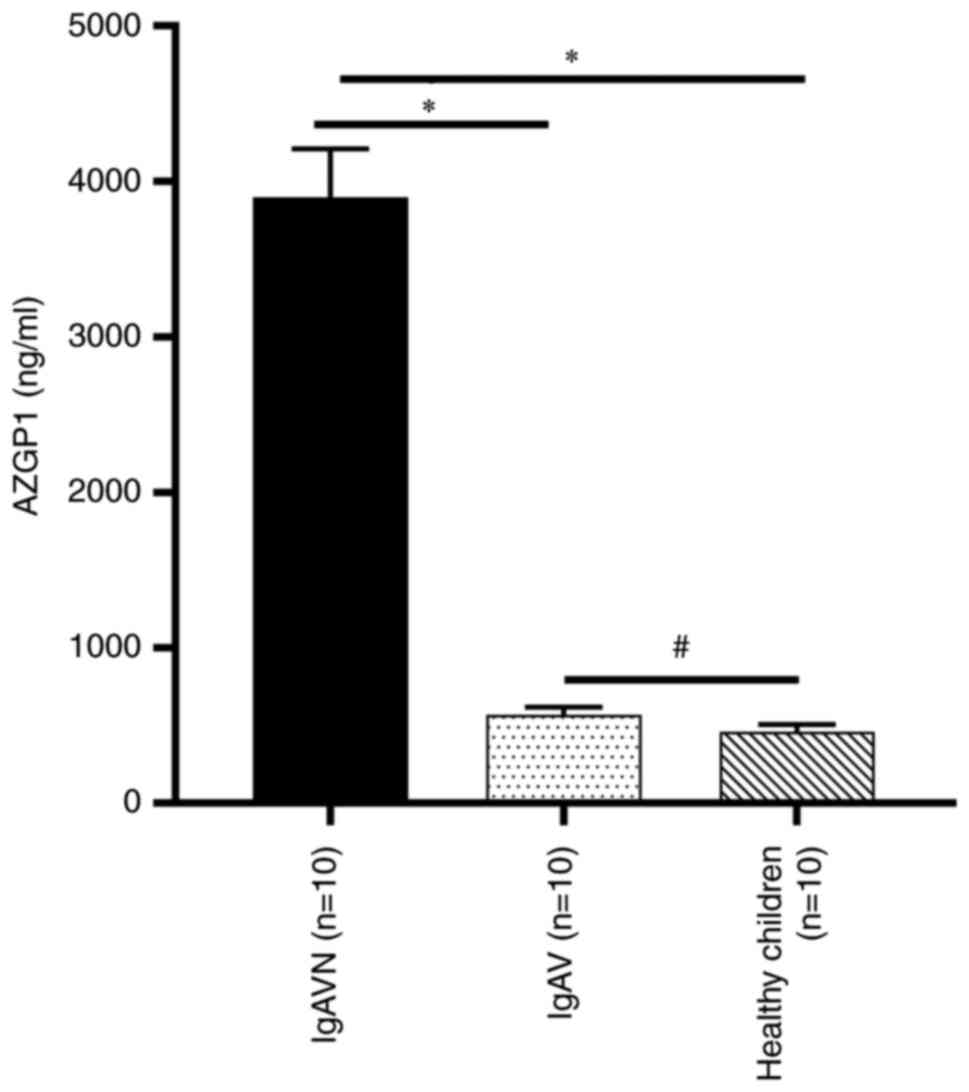
ELISA verification of specific
differential proteins
Through the previous analysis, a more specific
differential protein, AZGP1, was found. Considering that the sample
size was small, a post-hoc analysis of the AZGP1 data by G*Power
(3.1.9.7) was performed and it was found that the power (1-β) was
0.53, where β indicates a class II error when <0.5. To verify
the reliability of this protein, further ELISA experiments were
performed. The ELISA results showed that urinary AZGP1 levels in
IgAVN were significantly higher compared with those in IgAV and
healthy children and that there was no significant difference
between IgAV and healthy children (Fig. 8). The association of urinary levels
of AZGP1 with serum creatinine and eGFR was evaluated and it was
found that urinary AZGP1 levels were no correlation with serum
creatinine (P=0.086) and eGFR (P=0.055).
Discussion
Generally speaking, IgAV is a common disease in
childhood and, when the kidney is involved, it will affect the
long-term prognosis of children as with all kinds of nephropathy.
Despite the lack of authoritative evidence-based medicine for the
treatment of IgAVN, the earlier the occurrence of IgAV nephritis is
identified, is still valuable (19). In recent years, there have been a
number of reports on urinary proteomics in other kidney diseases,
including IgAN, lupus nephritis (LN) and nephrotic syndrome
(20–31). The study of IgAVN is more limited
to the study and verification of known markers of renal damage and
less valuable markers for future research are suggested through
proteomics, especially with urine as samples (6,7,32).
The purpose of the present study was to explore the specific
markers in urine, which is of value to the future investigation of
pathological mechanism and more accurate treatment of IgAVN. In
addition, through a new generation of proteomics technology, the
number of proteins identified was significantly greater than in the
past, which provided more basic data for further research.
In present study, there was a specific upregulated
protein, AZGP1. According to the results of expression multiple
(FC) and P-value, it can be stated that the expression difference
of AZGP1 is the most significant. According to PPI, AZGP1 has more
correlation with other proteins, which is valuable for further
research. Therefore, the present study chose AZGP1 as the object of
further study. It was further verified that there was differential
expression between IgAVN and non-IgAVN children.
AZGP1 is a secretory glycoprotein of ~40 kDa and was
first isolated from human plasma 60 years ago. It is mainly
expressed in adipocytes and epithelial cells, including renal
tubular epithelial cells (33).
Earlier studies have shown that blood AZGP1 is significantly
increased in patients with chronic hemodialysis and early acute
kidney injury (34). For the study
of urine AZGP1 10 years ago, it was proposed that urine AZGP1 may
be used as a biomarker of diabetic nephropathy (35). Then, in a biomarker experiment of
active LN, it was found that there was no obvious specificity of
urinary AZGP1 (36). Initially, an
experimental study suggested that when the proximal tubule was
damaged, the reabsorption was insufficient, which led to an
increase in the amount of protein excreted from the urine and, due
to increased filtration of other proteins, especially albumin, the
reabsorption capacity of the renal tubule was saturated, increasing
urinary AZGP1 excretion (37).
Moreover, it is generally hypothesized that the passive
accumulation of blood AZGP1 is caused by a decrease in renal
clearance ability. Notably, when studying the factors related to
metabolic disorders related to chronic kidney disease (CKD), some
studies have suggested that AZGP1 may increase lipolysis and reduce
fat production through overproduction (38). At present, it is unclear whether
the increase of AZGP1 in urine comes from kidney damage or the
increase in production is caused by the disease itself. In
particular, a strong correlation between plasma AZGP1 and GFR has
not been found, only confirmation that there is a significant
increase in blood AZGP1 only in CKD 5 phase (39). Therefore, although the metabolic
mechanism of AZGP1 in kidney disease remains to be elucidated,
urine AZPG1 may show abnormalities earlier in disease progression
than blood AZGP1. The reliability of its relative value needs to be
confirmed by further research.
The HLA-B35 gene is not only associated with the
pathogenesis of IgAN but increases the risk of IgAVN (40). Significantly, HLA-B35 is attributed
to MHC-I molecules, while AZGP1 molecules are highly similar to
MHC-I molecules in structure, which may play a role in the
pathogenesis of IgAVN by binding to T cells and presenting lipid
ligands (41). Initially, AZGP1
was isolated as a factor that can lead to weight loss by inhibiting
animal feeding, indicating that there is a direct relationship
between immune function and nutritional metabolism, but the pathway
of action is not clear (42).
AZGP1 plays an important role as a fat factor in the immune process
of atopic dermatitis, rheumatoid arthritis, AIDS, cachexia and
other diseases (43–46). To study AZGP1 in kidney disease,
Schmitt et al (47),
through the study of AZGP1 deficient mice, concluded that AZGP1
deficient mice showed higher susceptibility to renal fibrosis, so
it was found that AZGP1 seemed to play a stable role in cell
integrity and epithelial differentiation (48). However, Schmitt et al
(47) found that aging and loss of
proliferation of renal epithelial cells were associated with
increased expression of AZGP1 and inhibition of AZGP1 in the aging
kidney could increase the proliferative response after injury
(47). Therefore, under normal
expression, AZGP1 should have a protective effect on the kidney,
but when the kidney is damaged, AZGP1 will be overexpressed,
aggravating the kidney damage. Under renal injury, renal injury and
fibrosis can be alleviated by significantly increasing IL-33R+ and
IL-2Ra+ regulatory T cells in renal tissue (49). Although no direct interaction
between AZGP1 and T cells was found, an amino acid sequence
(Arg-Gly-Asp-Val) that could interact with cell surface integrin
was found in AZGP1 (50,51). AZGP1 may cause renal involvement
through the aforementioned processes in the progression of IgAV
disease.
In addition, according to GO enrichment and KEGG
pathway analysis, most of the differential proteins are closely
related to the immune response in biological processes, cell
composition and molecular function and participate in endocytosis
and cell adhesion. In patients with IgAV, immune complexes formed
during immune response not only activate mesangial cells and induce
mesangial proliferation, but also induce the expression of
pro-inflammatory cytokines and chemokines (IL-6, IL-8, TNF and
MCP-1) and induce apoptosis of podocytes and renal tubular
epithelial cells. The above effects lead to the recruitment of
inflammatory cells and further damage to the kidney (52). In a study of IgAVN mechanism,
complement activation is involved in the damage of glomerular
structure (53). The present study
found that complement C4-B and complement C1R were differentially
expressed (data not shown).
In summary, 254 differential proteins were screened
by a new generation of proteomic technology. The present study has
some limitations, for example, the sample size was relatively small
and larger samples are needed to verify it so that the protein
differences between different renal pathological types can be
further explored. In addition, the present study failed to further
study the mechanism of AZGP1. However, the differential proteins in
the results of the present study can still provide value for future
research. The present study validated AZGP1 as possessing potential
value as a new biomarker. In the future, the role of AZGP1 in the
pathogenesis of IgAVN and the application of urinary AZGP1 to guide
clinical diagnosis and treatment will be clarified.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Research
Project of Higher Education of Anhui Province of China (grant no.
KJ2019A0245) and the Foundation of Anhui Medical University (grant
no. 2020×kj174). There were no funders involved in the study
design, data analysis, manuscript preparation, or publication of
the study findings.
Availability of data and materials
The mass spectrometry proteomics data have been
deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via
the iProX partner repository with the dataset identifier PXD042355.
The additional datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZQZ, TZ, SC, ZHR, QZ contributed to the study
conception and design. ZQZ, TZ, SC wrote the manuscript and
collected and analyzed data. ZHR and QZ critically revised the
final manuscript. ZQZ and QZ confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The research protocol was approved by the Biomedical
Ethics Committee of Anhui Medical University (Hefei, China;
approval no. 20190812). Written informed consent was obtained from
the guardians of all children prior to the enrollment of this
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AZGP1
|
zinc-alpha-2-glycoprotein
|
|
CKD
|
chronic kidney disease
|
|
DIA
|
data-independent acquisition
|
|
PASEF
|
parallel accumulation-serial
fragmentation
|
|
diaPASEF
|
parallel accumulation-serial
fragmentation combined with data-independent acquisition
|
|
GO
|
Gene Ontology
|
|
IgAV
|
Immunoglobulin A vasculitis
|
|
IgAVN
|
IgAV with nephritis
|
|
KEGG
|
Kyoto Encyclopedia of Gene and
Genome
|
|
LC-MS/MS
|
liquid chromatography-tandem mass
spectrometry
|
References
|
1
|
Chen J, Fang X, Dang X, Wu X and Yi Z:
Association of the paired box 2 gene polymorphism with the
susceptibility and pathogenesis of Henoch-Schönlein Purpura in
children. Mol Med Rep. 11:1997–2003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung AKC, Barankin B and Leong KF:
Henoch-Schönlein Purpura in Children: An updated review. Curr
Pediatric Rev. 16:265–276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pillebout E and Sunderkotter C: IgA
vasculitis. Semin Immunopathol. 43:729–738. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oni L and Sampath S: Childhood IgA
vasculitis (Henoch Schonlein Purpura)-advances and knowledge gaps.
Front Pediatr. 7:2572019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie B, Zhang W, Zhang Q, Zhang Q, Wang Y,
Sun L, Liu M and Zhou P: An integrated transcriptomic and proteomic
analysis identifies significant novel pathways for Henoch-Schönlein
Purpura nephritis progression. Biomed Res Int. 2020:24891752020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao R, Niu X, Zhu L, Qi R and He L: iTRAQ
quantitative proteomic analysis differentially expressed proteins
and signal pathways in Henoch-Schönlein Purpura nephritis. Am J
Transl Res. 12:7908–7922. 2020.PubMed/NCBI
|
|
7
|
Fang X, Wu H, Lu M, Cao Y, Wang R, Wang M,
Gao C and Xia Z: Urinary proteomics of Henoch-Schönlein Purpura
nephritis in children using liquid chromatography-tandem mass
spectrometry. Clin Proteomics. 17:102020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naderi AS and Reilly RF: Primary care
approach to proteinuria. J Am Board Fam Med. 21:569–574. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taherkhani A, Farrokhi Yekta R, Mohseni M,
Saidijam M and Arefi Oskouie A: Chronic kidney disease: A review of
proteomic and metabolomic approaches to membranous
glomerulonephritis, focal segmental glomerulosclerosis, and IgA
nephropathy biomarkers. Proteome Sci. 17:72019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Smith LS and Zhu HJ:
Data-independent acquisition (DIA): An emerging proteomics
technology for analysis of drug-metabolizing enzymes and
transporters. Drug discovery today. Technologies. 39:49–56.
2021.PubMed/NCBI
|
|
11
|
Demichev V, Szyrwiel L, Yu F, Teo GC,
Rosenberger G, Niewienda A, Ludwig D, Decker J, Kaspar-Schoenefeld
S, Lilley KS, et al: dia-PASEF data analysis using FragPipe and
DIA-NN for deep proteomics of low sample amounts. Nat Commun.
13:39442022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mun DG, Vanderboom PM, Madugundu AK,
Garapati K, Chavan S, Peterson JA, Saraswat M and Pandey A:
DIA-based proteome profiling of nasopharyngeal Swabs from COVID-19
patients. J Proteome Res. 20:4165–4175. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozen S, Pistorio A, Iusan SM, Bakkaloglu
A, Herlin T, Brik R, Buoncompagni A, Lazar C, Bilge I, Uziel Y, et
al: EULAR/PRINTO/PRES criteria for Henoch-Schönlein Purpura,
childhood polyarteritis nodosa, childhood Wegener granulomatosis
and childhood Takayasu arteritis: Ankara 2008. Part II: Final
classification criteria. Ann Rheum Dis. 69:798–806. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parker SJ, Rost H, Rosenberger G, Collins
BC, Malmström L, Amodei D, Venkatraman V, Raedschelders K, Van Eyk
JE and Aebersold R: Identification of a set of conserved eukaryotic
internal retention time standards for data-independent acquisition
mass spectrometry. Mol Cell Proteomics. 14:2800–2813. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meier F, Brunner AD, Koch S, Koch H,
Lubeck M, Krause M, Goedecke N, Decker J, Kosinski T, Park MA, et
al: Online parallel accumulation-serial fragmentation (PASEF) with
a novel trapped ion mobility mass spectrometer. Mol Cell
Proteomics. 17:2534–2545. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gotz S, Garcia-Gomez JM, Terol J, Williams
TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J and Conesa A:
High-throughput functional annotation and data mining with the
Blast2GO suite. Nucleic Acids Res. 36:3420–3435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanehisa M, Goto S, Sato Y, Furumichi M
and Tanabe M: KEGG for integration and interpretation of
large-scale molecular data sets. Nucleic Acids Res. 40:D109–D114.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen JY and Mao JH: Henoch-Schönlein
Purpura nephritis in children: Incidence, pathogenesis and
management. World J Pediatr. 11:29–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mucha K, Bakun M, Jazwiec R, Dadlez M,
Florczak M, Bajor M, Gala K and Pączek L: Complement components,
proteolysisrelated, and cell communicationrelated proteins detected
in urine proteomics are associated with IgA nephropathy. Pol Arch
Med Wewn. 124:380–386. 2014.PubMed/NCBI
|
|
21
|
Surin B, Sachon E, Rougier JP, Steverlynck
C, Garreau C, Lelongt B, Ronco P and Piedagnel R: LG3 fragment of
endorepellin is a possible biomarker of severity in IgA
nephropathy. Proteomics. 13:142–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neprasova M, Maixnerova D, Novak J, Reily
C, Julian BA, Boron J, Novotny P, Suchanek M, Tesar V and Kacer P:
Toward noninvasive diagnosis of IgA nephropathy: A Pilot urinary
metabolomic and proteomic study. Dis Markers. 2016:36509092016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo Z, Wang Z, Lu C, Yang S, Sun H, Reziw,
Guo Y, Sun W and Yue H: Analysis of the differential urinary
protein profile in IgA nephropathy patients of Uygur ethnicity. BMC
Nephrol. 19:3582018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rocchetti MT, Papale M, d'Apollo AM,
Suriano IV, Di Palma AM, Vocino G, Montemurno E, Varraso L,
Grandaliano G, Di Paolo S and Gesualdo L: Association of urinary
laminin G-like 3 and free K light chains with disease activity and
histological injury in IgA nephropathy. Clin J Am Soc Nephrol.
8:1115–1125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He Q, Shao L, Yu J, Ji S, Wang H, Mao Y
and Chen J: Urinary proteome analysis by matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry with
magnetic beads for identifying the pathologic presentation of
clinical early IgA nephropathy. J Biomed Nanotechnol. 8:133–139.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taylor S, Pieri K, Nanni P, Tica J,
Barratt J and Didangelos A: Phosphatidylethanolamine binding
protein-4 (PEBP4) is increased in IgA nephropathy and is associated
with IgA-positive B-cells in affected kidneys. J Autoimmun.
105:1023092019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rudnicki M, Siwy J, Wendt R, Lipphardt M,
Koziolek MJ, Maixnerova D, Peters B, Kerschbaum J, Leierer J,
Neprasova M, et al: Urine proteomics for prediction of disease
progression in patients with IgA nephropathy. Nephrol Dial
Transplant. 37:42–52. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ning X, Yin Z, Li Z, Xu J, Wang L, Shen W,
Lu Y, Cai G, Zhang X and Chen X: Comparative proteomic analysis of
urine and laser microdissected glomeruli in IgA nephropathy. Clin
Exp Pharmacol Physiol. 44:576–585. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Turnier JL, Brunner HI, Bennett M, Aleed
A, Gulati G, Haffey WD, Thornton S, Wagner M, Devarajan P, Witte D,
et al: Discovery of SERPINA3 as a candidate urinary biomarker of
lupus nephritis activity. Rheumatology (Oxford). 58:321–330. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai Z, Zhang S, Wu P, Ren Q, Wei P, Hong
M, Feng Y, Wong CK, Tang H and Zeng H: A novel potential target of
IL-35-regulated JAK/STAT signaling pathway in lupus nephritis. Clin
Transl Med. 11:e3092021. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varghese SA, Powell TB, Budisavljevic MN,
Oates JC, Raymond JR, Almeida JS and Arthur JM: Urine biomarkers
predict the cause of glomerular disease. J Am Soc Nephrol.
18:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He X, Yin W, Ding Y, Cui SJ, Luan J, Zhao
P, Yue X, Yu C, Laing X and Zhao Y: Higher serum angiotensinogen is
an indicator of IgA vasculitis with nephritis revealed by
comparative proteomes analysis. PLoS One. 10:e01305362015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmitt R: ZAG-a novel biomarker for
cardiovascular risk in ESRD patients? Kidney Int. 94:858–860. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sörensen-Zender I, Beneke J, Schmidt BM,
Menne J, Haller H and Schmitt R: Zinc-alpha2-glycoprotein in
patients with acute and chronic kidney disease. BMC Nephrol.
14:1452013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soggiu A, Piras C, Bonizzi L, Hussein HA,
Pisanu S and Roncada P: A discovery-phase urine proteomics
investigation in type 1 diabetes. Acta Diabetol. 49:453–464. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Somparn P, Hirankarn N, Leelahavanichkul
A, Khovidhunkit W, Thongboonkerd V and Avihingsanon Y: Urinary
proteomics revealed prostaglandin H(2)D-isomerase, not
Zn-alpha2-glycoprotein, as a biomarker for active lupus nephritis.
J Proteomics. 75:3240–3247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ekman R, Johansson BG and Ravnskov U:
Renal handling of Zn-alpha2-glycoprotein as compared with that of
albumin and the retinol-binding protein. J Clin Invest. 57:945–954.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pelletier CC, Koppe L, Croze ML, Kalbacher
E, Vella RE, Guebre-Egziabher F, Géloën A, Badet L, Fouque D and
Soulage CO: White adipose tissue overproduces the lipid-mobilizing
factor zinc alpha2-glycoprotein in chronic kidney disease. Kidney
Int. 83:878–886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pelletier CC, Koppe L, Alix PM, Kalbacher
E, Croze ML, Hadj-Aissa A, Fouque D, Guebre-Egziabher F and Soulage
CO: The relationship between renal function and plasma
concentration of the cachectic factor zinc-alpha2-glycoprotein
(ZAG) in adult patients with chronic kidney disease. PLoS One.
9:e1034752014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Robson KJ, Ooi JD, Holdsworth SR, Rossjohn
J and Kitching AR: HLA and kidney disease: From associations to
mechanisms. Nat Rev Nephrol. 14:636–655. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bouchara A, Yi D, Pastural M, Granjon S,
Selag JC, Laville M, Arkouche W, Pelletier S, Fouque D, Soulage CO
and Koppe L: Serum levels of the adipokine zinc-alpha2-glycoprotein
(ZAG) predict mortality in hemodialysis patients. Kidney Int.
94:983–992. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matarese G and La Cava A: The intricate
interface between immune system and metabolism. Trends Immunol.
25:193–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Noh JY, Shin JU, Kim JH, Kim SH, Kim BM,
Kim YH, Park S, Kim TG, Shin KO, Park K and Lee KH: ZAG regulates
the skin barrier and immunity in atopic dermatitis. J Invest
Dermatol. 139:1648–1657. e16472019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Na HS, Kwon JE, Lee SH, Jhun J, Kim SM,
Kim SY, Kim EK, Jung K, Park SH and Cho ML: Th17 and IL-17 cause
acceleration of inflammation and fat loss by inducing
alpha2-glycoprotein 1 (AZGP1) in rheumatoid arthritis with high-fat
diet. Am J Pathol. 187:1049–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yeregui E, Masip J, Viladés C, Domingo P,
Pacheco YM, Blanco J, Mallolas J, Alba V, Vargas M, García-Pardo G,
et al: Adipokines as new biomarkers of immune recovery: Apelin
receptor, RBP4 and ZAG are related to CD4(+) T-cell reconstitution
in PLHIV on suppressive antiretroviral therapy. Int J Mol Sci.
23:22022022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Romauch M: Zinc-alpha2-glycoprotein as an
inhibitor of amine oxidase copper-containing 3. Open Biol.
10:1900352020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schmitt R, Marlier A and Cantley LG: Zag
expression during aging suppresses proliferation after kidney
injury. J Am Soc Nephrol. 19:2375–2383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sörensen-Zender I, Bhayana S, Susnik N,
Rolli V, Batkai S, Baisantry A, Bahram S, Sen P, Teng B, Lindner R,
et al: Zinc-alpha2-Glycoprotein exerts antifibrotic effects in
kidney and heart. J Am Soc Nephrol. 26:2659–2668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
do Valle Duraes F, Lafont A, Beibel M,
Martin K, Darribat K, Cuttat R, Waldt A, Naumann U, Wieczorek G,
Gaulis S, et al: Immune cell landscaping reveals a protective role
for regulatory T cells during kidney injury and fibrosis. JCI
Insight. 5:e1306512020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Adams EJ and Luoma AM: The adaptable major
histocompatibility complex (MHC) fold: Structure and function of
nonclassical and MHC class I-like molecules. Annu Rev Immunol.
31:529–561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takagaki M, Honke K, Tsukamoto T,
Higashiyama S, Taniguchi N, Makita A and Ohkubo I: Zn-alpha
2-glycoprotein is a novel adhesive protein. Biochem Biophys Res
Commun. 201:1339–1347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Song Y, Huang X, Yu G, Qiao J, Cheng J, Wu
J and Chen J: Pathogenesis of IgA Vasculitis: An Up-to-date review.
Front Immunol. 12:7716192021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chimenti MS, Ballanti E, Triggianese P and
Perricone R: Vasculitides and the complement system: A
comprehensive review. Clin Rev Allergy Immunol. 49:333–346. 2015.
View Article : Google Scholar : PubMed/NCBI
|