Pancreatic ductal adenocarcinoma (PDAC), globally
recognized as the ‘king of cancers’, is projected to become the
second deadliest cancer in the United States by 2026, but its cause
remains elusive (1,2). This lethal disease is typically
diagnosed at an advanced metastatic stage owing to a lack of early
clinical manifestations (3). The
mortality rate of patients with PDAC closely mirrors the morbidity
rate, with a brief disease course and a survival period typically
<1 year (4). Early screening
techniques such as endoscopic ultrasound, magnetic
resonance/magnetic resonance pancreaticobiliary imaging and PDAC
detection with artificial intelligence can accurately detect and
classify pancreatic lesions using non-contrast CT (5,6).
However, early diagnosis and screening for pancreatic cancer remain
extremely limited in most countries (7).
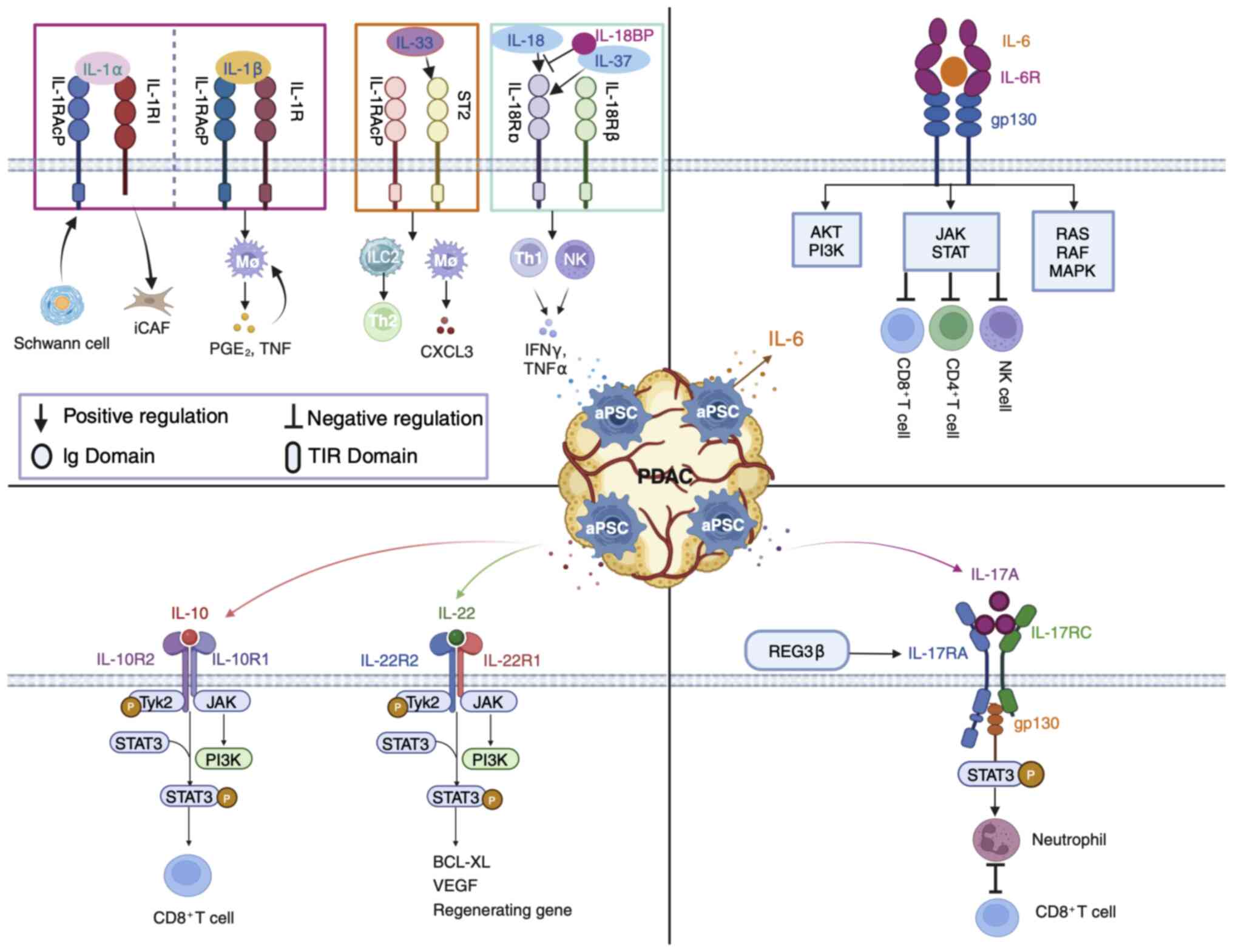
The present review focuses on the effects of aPSCs
in the interleukin-1 (IL-1) family, interleukin-6 (IL-6) family,
interleukin-10 (IL-10) family and interleukin-17A (IL-17A)
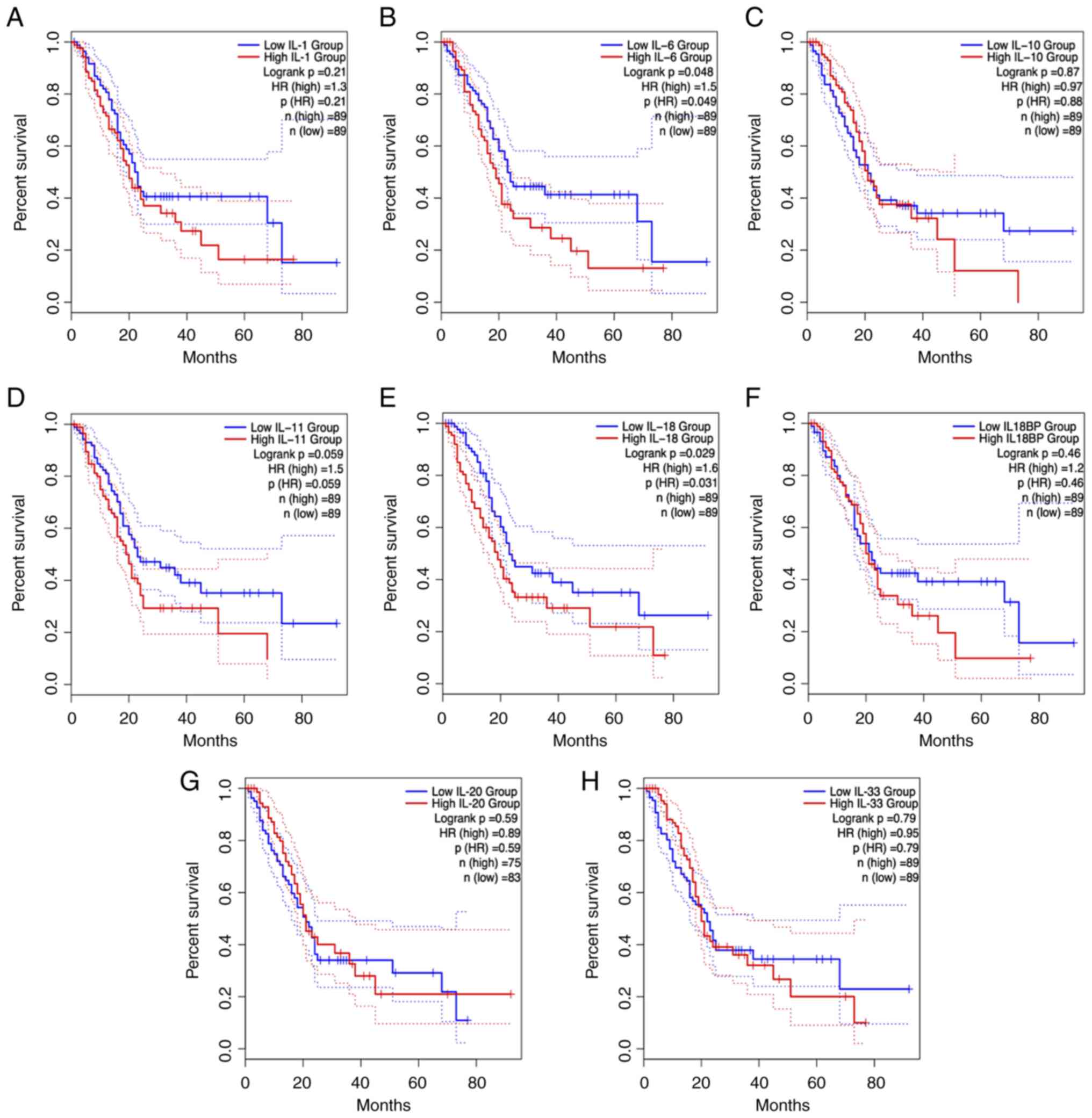
cytokines on pancreatic cancer development (Fig. 2). In addition to the IL-1 family,
the IL-6 family and IL-10 family of cytokines are involved in the
survival of patients with PDAC (Fig.
3) highlighting their potential as critical targets for early
cancer screening and diagnosis. These interleukin family cytokines
are prospective candidates in the field of fibrosis as well as
immune regulation and have considerable potential to improve the
early developmental profile of PDAC (15–17).
However, most other interleukin family members have only been
evaluated in preclinical or clinical trials for immune regulatory
signatures (Table I).
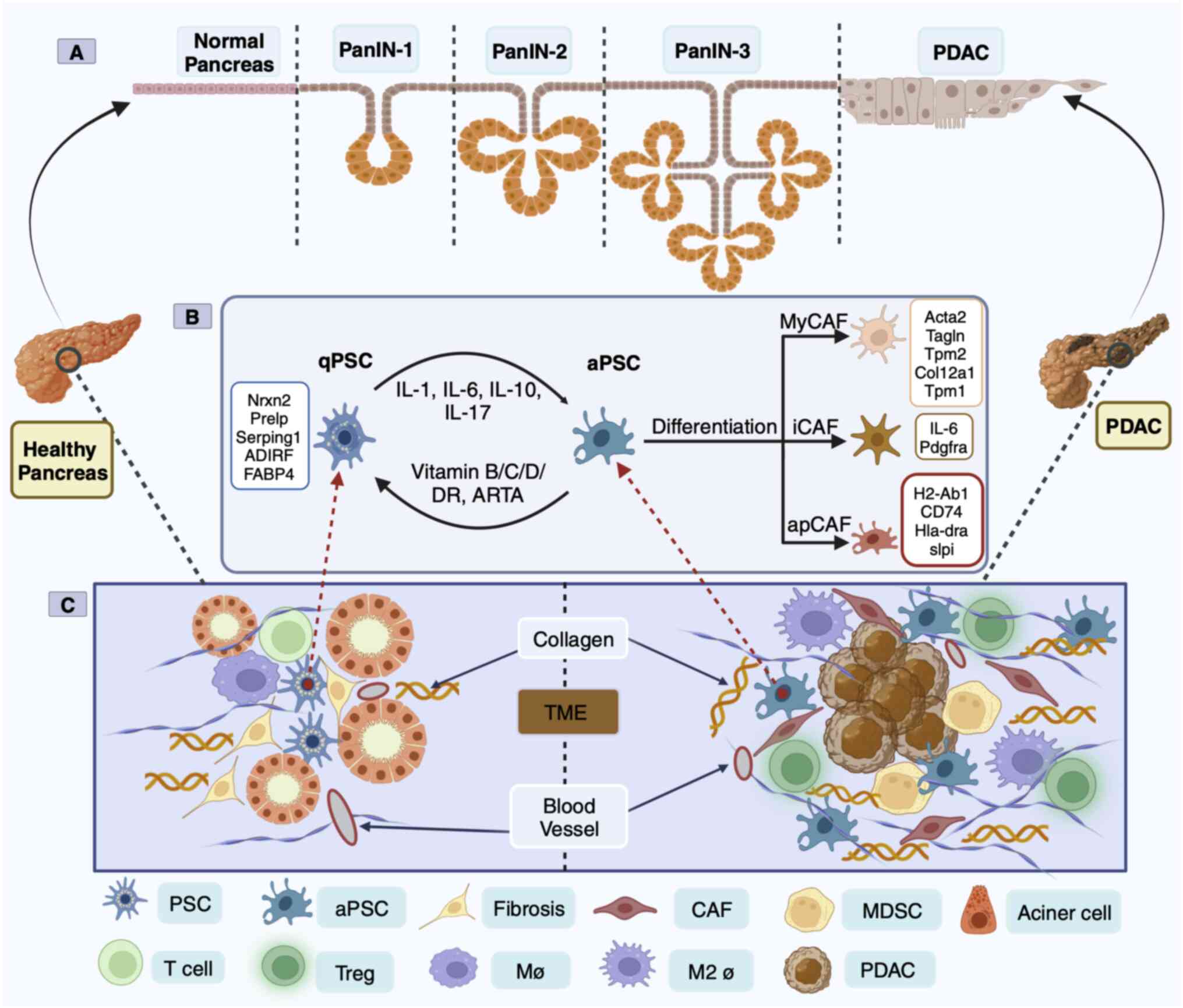
In 1998, PSCs from healthy human and rat pancreases
were successfully isolated and cultured for the first time
(18). Most of the initial
understanding of PSCs was based on their similarity with hepatic
stellate cells, but in-depth exploration has led to a more
comprehensive understanding of the morphology and location of PSCs,
as well as their unique characteristics of being able to store
lipid droplets and vitamin A (19). Transcriptome analysis of human and
mouse PSCs revealed two distinct clusters of PSCs including
‘activate’ [enrichment of ECM genes, collagen type 1 α1 (COL1A1)
and fibronectin 1] and ‘quiescent’ (enrichment of adipose genes,
adipogenesis regulatory factor and fatty acid binding protein 4)
(20). Under physiological
conditions, PSCs are in a quiescent state and are the only
pancreatic cells that store vitamin A (21). PSC activation is characterized by a
spindle-like shape in vitro and the disappearance of vitamin
A lipid droplets, although the mechanism of the disappearance or
absence of vitamin A in PDAC progression has yet to be elucidated
(22). In the aPSC state, there
are α-smooth mucle actin (SMA) and collagen fibers, ECM deposition,
and the release of epithelial-mesenchymal transition
(EMT)-associated soluble inflammatory factors (23). Simultaneously, aPSCs begin to
proliferate and manifest the proinflammatory phenotype, releasing
chemokines, cytokines and growth factors that recruit other
inflammatory factors into the pancreas, perpetuating the
inflammatory response (24). aPSCs
can also promote an immunosuppressive microenvironment in mouse
models of pancreatic cancer (25).
Sustained aPSCs can create a TME conducive to cancer cell growth
(Fig. 1C).
PSC-derived CAFs in human and mouse PDAC were
analyzed by scRNA-seq technology and classified into myofibroblast
CAFs (myCAFs), inflammatory CAFs (iCAFs) and antigen-presenting
CAFs (apCAFs) based on positional and functional characteristics
(26,27). These three heterogeneous CAF
subsets function in mutual conversion (26,27).
However, apCAFs are rarely found in patients with PDAC (9). MyCAFs dominate connective tissue
proliferation and form a physical protective barrier outside the
cells of pancreatic cancers, protecting them from drug intervention
and immune recognition (28). ECM
depletion has been proposed to remove the fibrous barrier, but it
has not been successful in the treatment of pancreatic cancer
(29). The depletion of
SMA+ myCAFs led to a reduction in the tumor stroma in
mice (30). This accelerates PanIN
and PDAC formation and development and reduces survival (30). The second CAF subgroup includes
iCAFs. In a broad sense, myCAFs appear to be involved in EMT and
ECM remodeling, whereas iCAFs appear to be associated with
inflammation and ECM deposition (31). Mouse pancreatic tumor scRNA-seq
revealed that the IL-1/JAK/STAT3 and TGFβ/Smad3 signaling are key
pathways that regulate iCAF and MyCAF heterogeneity and function
(32). Furthermore, myCAFs and
iCAFs are considered to play opposing roles in cancer (33). The third subpopulation of CAFs is
apCAFs, which express a wide range of fibroblast markers including
COL1A1, COL1A2, decorin and podoplanin as well as major
histocompatibility complex class II (MHC II)-related genes that are
limited to antigen-presenting cells (APC) of the immune system and
present model antigens to CD4+ T cells in an
antigen-dependent manner (26).
The source and nature of the antigen presented by apCAFs are not
known, which is an important mystery in the study of the
interaction between CAFs and tumor-infiltrating T cells.
PSC-derived CAFs are classified as cancer-promoting
CAFs (pCAFs) or cancer-restraining CAFs (rCAFs) based on their role
in fighting cancer (26,34). Fibroblasts that maintain
homeostasis and innately suppress tumorigenesis are collectively
referred to as rCAFs (35).
However, the nature and characteristic markers of rCAFs are
unknown. Meflin is highly expressed in quiescent PSCs, oncogenic
meflin-tagged rCAFs appear around cells in PanIN, meflin expression
decreases during PDAC progression, and α-SMA expression increases,
leading to phenomena such as pCAFs (34). pCAFs secrete matrix components such
as collagen, fibronectin and proteoglycans that are involved in
EMT, invasion, metastasis and tumor angiogenesis and have been
extensively studied (35).
Currently, using pharmacological methods to synthesize the
unnatural retinoid Am80, clinical trials on the conversion of pCAFs
to rCAFs for the treatment of pancreatic cancer have been initiated
(clinical trial NCT05064618). Recently, several vitamin analogs
that downregulate α-SMA, reduce the movement and activation of
PSCs, restore PSCs to quiescence, and lead to increased apoptosis
in neighboring cancer cells in combination with chemotherapy drugs
have entered clinical trials in patients with PDAC (https://clinicaltrials.gov/; Table II). However, PSC-derived CAF
subpopulations are diverse (26,27,34).
Thus, interfering with PSC subpopulations is one of the more
challenging therapeutic strategies for pancreatic cancer.
Spatial transcriptomics and scRNA-seq have revealed
distinct transcriptomic features of PanIN fibroblasts and
macrophages in healthy adult organ donors and patients with PDAC
(36). Furthermore, macrophages
are observed in close proximity to PSCs, and aPSCs drive
anti-inflammatory M2 type macrophages to produce an
immunosuppressive environment for pancreatic cancer, which may
explain the aberrant interactions between PSCs and immune cells
(37,38). scRNA-seq analysis conducted on PDAC
samples before and after chemotherapy revealed that chemotherapy
might enhance resistance to immunotherapy (9). However, T cells and macrophages are
the primary populations shaping the immune landscape in the TME
(39). Baron et al
(20) conducted scRNA-seq on four
cases of PDAC and found that PSC, in addition to expressing genes
associated with the ECM, were also closely associated with high
interleukin expression. Interleukins are cytokines with
immunomodulatory functions that serve as a means of communication
between cells and tissues (20).
Interleukins cultivate an environment conducive to cancer growth
and are critical for tumor immunotherapy and targeting (15).
The IL-1 family was originally used to generalize
the products of macrophage-induced inflammation. A total of seven
agonist cytokines (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β and
IL-36γ), three receptor antagonists (IL-1Ra, IL-36Ra and IL-38) and
one anti-inflammatory cytokine (IL-37) currently belong to the IL-1
family (40,41). With the notable exception of
IL-1Ra, each family member is produced as a precursor (40). Most of these precursors, except for
IL-1α and IL-33, which are biologically inactive until they are
cleaved and mature, do not activate their receptors (40). The antagonistic function of the
IL-1 family and its own proinflammatory effects confer a wide range
of biological activities, including proinflammatory and
anti-inflammatory activities (42). The receptor structure contains one
or three extracellular immunoglobulin structural domains and a
transmembrane structural domain (42). Apart from type 2 IL-1 receptor
(IL-1R2), other receptors share a conserved intracellular Toll/IL-1
receptor signaling domain, which is the structural basis for the
IL-1 response to immunology (43).
Therefore, the regulation of these signals is critical for the
regulation of the immune system. In summary, the IL-1 family is a
powerful toolbox.
IL-18 is mainly secreted by macrophages, dendritic
cells and epithelial cells, and can stimulate a variety of cell
types and has numerous biological functions (52). IL-18 exists in an inactive form
within cells (53). Structurally,
IL-18-like IL-1β is an important effector molecule downstream of
the NLRP3 and NLRP1 inflammasomes (53). IL-18 mediates the MyD88-NFκΒ
signaling pathway in combination with its heterodimeric receptor
(IL-18Rα/βR), is activated by the rapid release of caspase-1
excised precursor peptides from the inflammasome during
inflammation and is considered a proinflammatory cytokine (53,54).
Preliminary studies have revealed that IL-18 plays an instrumental
role in the development of the pancreas from the acute to the
chronic disease stage, while activating the PSC to promote fibrosis
in the pancreas (55,56). Concurrently, elevated levels of
IL-1β and IL-18 proteins in chronic pancreatitis (CP) have been
attributed to the direct involvement of the NLRP3 inflammasome in
PSC activation both in vivo and in vitro (57,58).
The subsequent promotion of pancreatic fibrosis is mediated by
pathogen-associated molecular patterns (57,58).
In the search for specific cytokines in the TME, the
application of scRNA-seq technology to tumor-infiltrating
lymphocytes revealed specifically high expression of IL-18 and its
receptor (59). High expression of
IL-18 in the stroma is associated with poor prognosis in patients
(60). One function of IL-18 is to
stimulate the production of IFNγ by natural killer (NK) cells and
Th1 cells and synergize with IL-12 to enhance cytotoxicity against
tumor cells (53,54). Therefore, IL-18 has been used in
tumor immunotherapy, however, this approach has failed in phase II
clinical trials (61). This raises
the question of why IL-18 is ineffective against solid tumors.
First, the Cancer Genome Atlas (TCGA) database and tissue
microarrays revealed that IL-18 binding protein (IL-18BP) is more
widely distributed than IL-18R in various solid tumor tissues and
sera. Second, IL-18BP binds IL-18 with high affinity (1.1 pM),
prevents it from binding to the receptor and reduces the
IFNγ-secreting activity of IL-18 (62,63).
Thus, IL-18BP is a major barrier to IL-18 immunotherapy (62). The next question was whether
bypassing IL-18BP would exert an antitumor effect. A mutant
decoy-resistant IL-18 (DR-18) variant, which combines with IL-18Rα
but not with IL-18BP, has been screened by directed evolutionary
means for its full mobilization of a variety of immune cells,
including CD8+ T cells, NK cells and intratumor
cell-like T cells (60). DR-18
exhibits good antitumor activity, and its efficacy alone is
superior to that of anti-PD-1 monotherapy (60). In 2022, Simcha Therapeutics
(60) commenced a phase I clinical
trial on the safety and bioactivity of ST-067 (DR-18) in multiple
solid tumor types based on this basic study. In a second study,
IL-37 was shown to have high homology with IL-18 (61). IL-37 binds to IL-18Rα after
maturation via caspase-1 cleavage but binds less efficiently than
does IL-18 (62). However, IL-37
can act as a binder for IL-18BP and antagonize the binding of
IL-18/IL-18Rα to IL-18Rβ, whereas the low-affinity dimer
IL-18/IL-18Rα needs to bind to IL-18Rβ for cell signaling, thus
inhibiting IL-18 innate immunity and reducing IFNγ expression
(61,64). IL-18 is a promising alternative
therapeutic target for pancreatitis, PanIN and pancreatic
cancer.
IL-33, the most responsive chromatin-activated
tumor-forming effector, cooperates with mutant KRAS to generate a
specific transcriptional program for tumor formation that
contributes to epigenetic remodeling of early neoplasia and tumor
transformation (72). Despite
being expressed in only a fraction of KRAS-mutant pancreatic
epithelial cells, IL-33 resulted in marked changes in the
premalignant pancreatic cellular state and subsequently prevented
the transition of the plasticized progenitor-like state to the
PanIN populations (73). Among the
downstream targets of oncogenic genes, IL-33 is the most altered
cytokine (72,73). In conclusion, IL-33 is a vital
target allele in damaged pancreatic tissue, acting as an
immunotherapeutic enhancer in the PDAC stage while exerting
opposite proinflammatory and fibrotic effects in precancerous
tissue (72). IL-33 directly
facilitates TGFβ-triggered differentiation of immunosuppressive
Treg cells and IFNγ production in other solid tumors, thereby
reducing immunotherapy efficacy (74). IL-33 is specifically elevated in
human PDACs and is positively associated with tumor immunity in
human patients with PDAC (71).
However, the combined clinical effects of IL-33 require further
investigation.
The IL-6 family comprises cytokines with a similar
structure and signaling mechanism as the subunit glycoprotein 130
kDa (GP130) (75). GP130 dimers
are recruited through conjugation to the non-signaling α receptor
(IL-6R) to form an IL-6/IL-6R/GP130 hexamer that initiates the
intracellular signaling chain (75). During PanIN-PDAC progression,
resident PSCs in the ECM secrete large amounts of inflammatory
cytokines IL-6 and IL-11 (76).
Bazedoxifene has been shown to have effective antitumor effects on
pancreatic cancer via inhibition of the IL-6 (GP130/STAT3) pathway
(clinical trial NCT04812808). Specifically, the JAK/STAT pathway
activates JAK proteins in cells and phosphorylates the
transcription factor STAT3, which translocates to the nucleus to
regulate target gene expression (77). Although JAK/STAT signaling is the
primary pathway for downstream activation of the IL-6 family of
cytokines, the mitogen-activated protein kinase (MAPK) pathway can
also undergo activation (78).
IL-6 collaborates with the oncogene KRAS to activate the reactive
oxygen species detoxification program downstream of the MAPK/ERK
signaling pathway (79). For
instance, a synergistic therapeutic combination with the CAF
inhibitor nintedanib enhances PDAC chimeric antigen
receptor-NK-mediated cytotoxicity via a reduction in CAF-released
IL-6 (80). Clinically high levels
of IL-6 in patients with PDAC are typically associated with large
tumor volumes and distant metastases (81). Moreover, patients with unresectable
and systemic metastatic PDAC have high IL-6 production (82). Thus, high levels of IL-6 indicate
an accurate prognosis for adverse outcomes (82). In addition, the serum marker IL-6
is superior to C-reactive protein, carcinoembryonic antigen and
carbohydrate antigen 19-9 for the diagnosis and prognosis of
patients with PDAC (83). These
phenomena clearly demonstrate that IL-6 is the intrinsic mechanism
of PDAC development, recapitulating most of the hallmarks of
cancer. However, it should be noted that IL-6 can be secreted from
other compartments, such as immune cells and can affect PDAC growth
and progression (31). Therefore,
systemic depletion of IL-6 affects CAF-independent pathways in
PDAC.
The IL-6 series of cytokines and their downstream
mediators contributes to the initiation of PDAC. IL-6 release by
aPSCs leads to the conversion of immature myeloid cells into
myeloid-derived suppressor cells (MDSCs), which then inhibit the
action of CD4+ T cells, CD8+ T cells and NK
cells, thus forming an immunosuppressive environment for PDAC
(22). Moreover, Nagathihalli
et al (84) demonstrated
that IL-6 secreted by PSCs causes marked fibrosis and
catheterization of pancreatic tissue and that compromising the
IL-6/Stat3 axis inhibits PanIN carcinogenesis. Several clinical
trials targeting the IL-6/JAK/STAT3 pathway have been conducted
(77). The chimeric mouse-human
antibody siltuximab is the most widely developed anti-IL-6 clinical
drug, but the highly heterogeneous nature of KRAS-mutated PDAC
tumors and their autocrine IL-6 status may result in the clinical
ineffectiveness of siltuximab against these tumors (85). Tocilizumab is an antibody against
IL-6R that inhibits IL-6 signaling to significantly reduce the
growth and recurrence of primary cancer (86). An early phase clinical trial on the
safety and efficacy of tocilizumab in patients with PDAC is ongoing
(clinical trial NCT02767557). Ruxolitinib is a clinically useful
oral inhibitor of JAK and has been shown to inhibit tumor growth in
several preclinical studies in mouse models of pancreatic cancer
(87). However, ruxolitinib did
not improve the survival rate of patients with advanced/metastatic
pancreatic disease (87). The use
of STAT3 as a possible inhibitor is challenging owing to its lack
of enzyme activity (88).
Nevertheless, a synthetic STAT3 inhibitor compound, AZD9150, is now
in a phase 2 clinical trial (NCT02983578), but its clinical outcome
is not yet known. Currently, IL-6 is a critical player in all
stages of PDAC and is a potential therapeutic target.
The IL-10 family is classified based on structural
similarity, common receptor use and downstream signaling. This
family consists of nine members including IL-10 and IL-20 subfamily
members IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and
IL-29, which are categorized as type III interferons (IFNs)
(89,90). All IL-10 family members
preferentially bind to Janus kinase 1 and tyrosine kinase 2, and
mediate differentiation through the downstream signaling JAK/STAT
transcription factor pathway (89). IL-10 and IL-22 are the most widely
studied family members in the pancreas (91,92).
IL-10 cytokines are produced primarily by
macrophages and T cell subsets (Th2 and Treg) in immune cells, and
have been linked to the pathogenesis and development of autoimmune
diseases and cancers (93). IL-10
predominates in inflammatory activity and wound recovery, releasing
regenerative anti-inflammatory factors that suppress inflammation
and promote favorable matrix remodeling and repair to alleviate
organ impairment (94). IL-10
[source bone marrow, (BM)] knockout transplanted mice compared with
wild-type mice exhibit substantially more fibrosis, inflammatory
cell infiltration and BM-derived myofibroblasts, which emphasizes
the crucial role of IL-10 in pancreatitis (95). However, whether IL-10 can be
recovered by aPSCs requires further investigation (95).
The effect of IL-10 multipotency as an
immunotherapeutic strategy has been investigated. A cetuximab-based
IL-10 fusion protein exhibits powerful antitumor activity by
blocking dendritic cell-mediated apoptosis of tumor-infiltrating
CD8+ T cells (96).
Conversely, IL-10 levels in the blood of patients with PDAC were
35-fold greater than the systemic concentrations, which may be
associated with NK cells immune escape, a cytotoxic
CD16hiCD57hi NK phenotype and impeded
expression of cytotoxic T lymphocytes and IFNγ (91). PEGylated IL-10 treatment restored
tumor-specific CD8+ T cell reactions and diminished
tumor proliferation (97).
However, a phase 3 trial of single pegilodecakin (PEGylated human
IL-10) tumor immunotherapy revealed no evidence of improved
survival in patients with PDAC (clinical trial NCT02923921). This
may be the reason why IL-10 research in the field of pancreatic
fibrosis has ended abruptly in recent years. However, it has also
been shown that genetically engineered macrophages producing an
IL-10-blocking antibody (αIL-10) can increase cancer cell death in
human gastrointestinal tumors (98). A trial with a combination treatment
is anticipated based on successful preclinical outcomes.
The IL-17 family comprises six subtypes of
multifunctional cytokines (IL-17A to IL-17F) that perform diverse
functions despite their amino acid sequence homology (110). Th17 cells preferentially produce
IL-17A, IL-17F, IL-21 and IL-2 (110). IL-17A is active in epithelial
cells and fibroblasts and is a characteristic hallmark of the
proinflammatory cytokine Th17 cells, which participate in
autoimmune, inflammatory and tumor pathogenesis, whereas IL-17F is
mainly involved in mucosal host defense mechanisms (110,111). IL-17A signal-mediated tissue
remodeling MMP may lead to ECM destruction and tissue lesions and
is also capable of downregulating tissue inhibitors of
metalloproteinases (111). IL-1β,
TNFα, IL-6, IL-10 and IL-2 are typically induced following IL-17A
stimulation of macrophages (112). The inflammatory and cancer
paracrine factor regenerating islet-derived 3β stimulates IL-17RA,
promotes cell proliferation, reduces susceptibility to apoptosis
through coupling of the gp130-JAK2-pSTAT3 signaling pathway and
initiates PanIN onset (113).
IL-17A binds to IL-17RA/RC complexes to secrete cytokines to
recruit neutrophils, which inhibits CD8+ T cells in the
PDAC TME (114). Intestinal
IL-17-IL-17RA signaling regulates microbes to promote barrier
immunity and drive distant pancreatic tumor proliferation (115). Subsequently, IL-17A participates
in the evolution of PanIN and promotes PDAC via the induction of
iCAFs, which results in poor prognosis for patients (116,117). Ablation of IL-17A limits the
immunosuppression of T cells to alter cytokine release by tumor
fibroblasts (118). Thus, the
inhibition of IL-17A may be a novel combination treatment (118). To assess the role of IL-17
therapy in tumor initiation and early tumor progression, an
anti-IL-17 antibody was injected weekly to ensure inhibition of the
IL-17A/IL-17RA axis throughout tumor development (119). However, there was no prolongation
of overall survival in this curative model, which indicates that
IL-17A may have a variable effect on PanIN and PDAC progression
(119). Blocking the IL-17A/RA
axis with antibodies alone is ineffective in preventing the
development and progression of pancreatic cancer (119). Therefore, IL-17A is not suitable
as primary monotherapy for pancreatic cancer in clinical practice.
IL-17A is also a double-edged sword in the immune system (120). During tumorigenesis, IL-17A
recruits MDSCs to suppress antitumor immunity, but it also
activates STAT3 to induce IL-6 to promote tumor growth in
vivo and upregulates oncogenes to promote tumor survival and
angiogenesis (121). Additional
studies are required to clarify the role of IL-17A in the
progression of PanIN and PDAC.
In conclusion, the interaction of PDAC with its TME
components is becoming increasingly important. However, long-term
fibrosis evolution and tumor immune cell imbalance result in an
egregious TME (11). Currently,
depletion of the ECM to remove the fibrous barrier has been
proposed, but this approach has been found to be ineffective for
PDAC treatment (122). Therefore,
ameliorating the accumulation of the ECM in PDAC and targeting the
ECM for immune cell modulation may have unexpected outcomes.
Despite well-developed theories related to fibrosis or immune
modulation in the pancreas, several questions remain to be
addressed to translate mechanistic insights into new and effective
therapeutic interventions. First, even with comprehensive combined
systematic screening of fibrosis and immunomodulation with PSC and
interleukins for PanIN and PDAC, there are limitations in
extracting only gross information from the available study data.
Utilizing scRNA-seq data for in-depth exploration of the mechanisms
of PSC to CAF conversion remains a priority. Second, CAF-forming
fibrosis interacts with immune cells in various ways, with the mode
of action depending in part on the type of CAF under investigation.
As markers for different CAF subgroups are not common to all CAF
regulatory factors, personalized regimens are needed for the
treatment of different stages of PDAC. Moreover, interleukin
families affect the development of PDAC to varying extents, but a
standard for interleukins to cause PDAC fibrosis directly by
inducing PSC activation is lacking. Consequently, IL-1, IL-6, IL-10
and IL-17A are promising targets for the treatment of PDAC.
Targeting the PanIN-PDAC process for immunity and fibrosis
treatment is complex and requires further rigorous testing and
validation of targets. The complex interactions between these
elements and the development of specific therapeutic strategies
require further elucidation.
Future research directions must focus on the stroma
and immune cells in the TME of PDAC, both of which play critical
roles in establishing structural and functional barriers to protect
PDAC from external attack. Most studies investigating the
interactions between PDAC and its ecotope have relied on
traditional two-dimensional cell cultures, which may not accurately
represent the complex three-dimensional microenvironment of PDAC
in vivo and could be utilized in coculture modeling or
organoid culture (123). Organoid
technology has revolutionized in the field of precision medicine
for treating PDAC (124).
Concurrently, the emergence of high-resolution scRNA-seq technology
provides an in-depth characterization of malignant cell types and
increases the understanding of the heterogeneity and plasticity of
PDAC in response to homeostatic and therapeutic perturbations
(125).
In conclusion, pancreatic fibrosis and
immunomodulation have been identified as key drivers in the
development of PDAC and are major impediments to the efficacy of
therapeutics for PDAC. With increasing research on PDAC-related
signaling, it is anticipated that combination therapy regimens will
be more successful, providing a more authoritative basis for drug
development and clinical treatment, and thus improving the survival
of patients with PDAC.
Not applicable.
The present study was supported by The National Natural Science
Foundation of China (grant no. 82372686) and The Guangzhou Medical
University Research Capacity Enhancement Program (grant no.
2024SRP192).
The survival data of patients with pancreatic cancer
generated in the present study may be found in the gene expression
profiling interactive analysis 2 (GEPIA 2) using data from The
Cancer Genome Atlas (http://gepia2.cancer-pku.cn/#survival).
HL and DL wrote the manuscript; KL acquired the
data, YW and GZ analyzed and interpreted the data included in the
review. KL, YW and GZ also contributed to the study design. LQ and
KX conceived the original idea, corrected and finalized the
manuscript and contributed to critical discussion. All authors read
and approved the final version of the manuscript and checked and
confirmed the authenticity of all the raw data.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Rahib L, Wehner MR, Matrisian LM and Nead
KT: Estimated projection of US cancer incidence and death to 2040.
JAMA Netw Open. 4:e2147082021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang J, Lok V, Ngai CH, Zhang L, Yuan J,
Lao XQ, Ng K, Chong C, Zheng ZJ and Wong MCS: Worldwide burden of,
risk factors for, and trends in pancreatic cancer.
Gastroenterology. 160:744–754. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Infante-Cossio P, Duran-Romero AJ,
Castaño-Seiquer A, Martinez-De-Fuentes R and Pereyra-Rodriguez JJ:
Estimated projection of oral cavity and oropharyngeal cancer deaths
in Spain to 2044. BMC Oral Health. 22:4442022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Viale PH: The American cancer society's
facts & figures: 2020 Edition. J Adv Pract Oncol. 11:135–136.
2020.PubMed/NCBI
|
|
5
|
Blackford AL, Canto MI, Klein AP, Hruban
RH and Goggins M: Recent trends in the incidence and survival of
stage 1A pancreatic cancer: A surveillance, epidemiology, and end
results analysis. J Natl Cancer Inst. 112:1162–1169. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao K, Xia Y, Yao J, Han X, Lambert L,
Zhang T, Tang W, Jin G, Jiang H, Fang X, et al: Large-scale
pancreatic cancer detection via non-contrast CT and deep learning.
Nat Med. 29:3033–3043. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng R, Zhang S, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2016. J Nat Cancer Cent. 2:1–9. 2022. View Article : Google Scholar
|
|
8
|
Yachida S, Jones S, Bozic I, Antal T,
Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Werba G, Weissinger D, Kawaler EA, Zhao E,
Kalfakakou D, Dhara S, Wang L, Lim HB, Oh G, Jing X, et al:
Single-cell RNA sequencing reveals the effects of chemotherapy on
human pancreatic adenocarcinoma and its tumor microenvironment. Nat
Commun. 14:7972023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Du W, Xia X, Hu F and Yu J: Extracellular
matrix remodeling in the tumor immunity. Front Immunol.
14:13406342024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen K, Wang Q, Li M, Guo H, Liu W, Wang
F, Tian X and Yang Y: Single-cell RNA-seq reveals dynamic change in
tumor microenvironment during pancreatic ductal adenocarcinoma
malignant progression. EBioMedicine. 66:1033152021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Storz P and Crawford H: Carcinogenesis of
pancreatic ductal adenocarcinoma. Gastroenterology. 158:2072–2081.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Li Y, Xing C, Ding C, Zhang H,
Chen L, You L, Dai M and Zhao Y: Tumor microenvironment in
chemoresistance, metastasis and immunotherapy of pancreatic cancer.
Am J Cancer Res. 10:1937–1953. 2020.PubMed/NCBI
|
|
14
|
Shi C, Washington MK, Chaturvedi R, Drosos
Y, Revetta FL, Weaver CJ, Buzhardt E, Yull FE, Blackwell TS,
Sosa-Pineda B, et al: Fibrogenesis in pancreatic cancer is a
dynamic process regulated by macrophage-stellate cell interaction.
Lab Invest. 94:409–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Briukhovetska D, Dörr J, Endres S, Libby
P, Dinarello CA and Kobold S: Interleukins in cancer: From biology
to therapy. Nature Rev Cancer. 21:481–499. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kartsonaki C, Pang Y, Millwood I, Yang L,
Guo Y, Walters R, Lv J, Hill M, Yu C, Chen Y, et al: Circulating
proteins and risk of pancreatic cancer: A case-subcohort study
among Chinese adults. Int J Epidemiol. 51:817–829. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nie YJ, Wu SH, Xuan YH and Yan G: Role of
IL-17 family cytokines in the progression of IPF from inflammation
to fibrosis. Mil Med Res. 9:212022.PubMed/NCBI
|
|
18
|
Apte MV, Haber PS, Applegate TL, Norton
ID, McCaughan GW, Korsten MA, Pirola RC and Wilson JS: Periacinar
stellate shaped cells in rat pancreas: identification, isolation,
and culture. Gut. 43:128–133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Wang H, Zhou J, Qiu S, Cai T, Li
H, Shen Z, Hu Y, Ding B, Luo M, et al: Vitamin A and its
multi-effects on pancreas: Recent advances and prospects. Front
Endocrinol (Lausanne). 12:6209412021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baron M, Veres A, Wolock SL, Faust AL,
Gaujoux R, Vetere A, Ryu JH, Wagner BK, Shen-Orr SS, Klein AM, et
al: A single-cell transcriptomic map of the human and mouse
pancreas reveals inter- and intra-cell population structure. Cell
Syst. 3:346–360.e4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikejiri N: The vitamin A-storing cells in
the human and rat pancreas. Kurume Med J. 37:67–81. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmad RS, Eubank TD, Lukomski S and Boone
BA: Immune cell modulation of the extracellular matrix contributes
to the pathogenesis of pancreatic cancer. Biomolecules. 11:9012021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bazzichetto C, Conciatori F, Luchini C,
Simionato F, Santoro R, Vaccaro V, Corbo V, Falcone I, Ferretti G,
Cognetti F, et al: From genetic alterations to tumor
microenvironment: The Ariadne's String in pancreatic cancer. Cells.
9:3092020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mews P, Phillips P, Fahmy R, Korsten M,
Pirola R, Wilson J and Apte M: Pancreatic stellate cells respond to
inflammatory cytokines: Potential role in chronic pancreatitis.
Gut. 50:535–541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang X, Chen J, Wang J, Ma S, Feng W, Wu
Z, Guo Y, Zhou H, Mi W, Chen W, et al: Very-low-density lipoprotein
receptor-enhanced lipid metabolism in pancreatic stellate cells
promotes pancreatic fibrosis. Immunity. 55:1185–1199.e8. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elyada E, Bolisetty M, Laise P, Flynn WF,
Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS,
et al: Cross-species single-cell analysis of pancreatic ductal
adenocarcinoma reveals antigen-presenting cancer-associated
fibroblasts. Cancer Discov. 9:1102–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Öhlund D, Handly-Santana A, Biffi G,
Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA,
Lee EJ, et al: Distinct populations of inflammatory fibroblasts and
myofibroblasts in pancreatic cancer. J Exp Med. 214:579–596. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schnittert J, Bansal R and Prakash J:
Targeting pancreatic stellate cells in cancer. Trends Cancer.
5:128–142. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, McAndrews KM and Kalluri R:
Clinical and therapeutic relevance of cancer-associated
fibroblasts. Nat Rev Clin Oncol. 18:792–804. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Y, Kim J, Yang S, Wang H, Wu CJ,
Sugimoto H, LeBleu VS and Kalluri R: Type I collagen deletion in
αSMA+ myofibroblasts augments immune suppression and
accelerates progression of pancreatic cancer. Cancer Cell.
39:548–565.e6. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Duijneveldt G, Griffin MDW and
Putoczki TL: Emerging roles for the IL-6 family of cytokines in
pancreatic cancer. Clin Sci (Lond). 134:2091–2115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Biffi G, Oni TE, Spielman B, Hao Y, Elyada
E, Park Y, Preall J and Tuveson DA: IL1-induced JAK/STAT signaling
is antagonized by TGFβ to shape CAF heterogeneity in pancreatic
ductal adenocarcinoma. Cancer Discov. 9:282–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huber M, Brehm CU, Gress TM, Buchholz M,
Alashkar Alhamwe B, von Strandmann EP, Slater EP, Bartsch JW, Bauer
C and Lauth M: The immune microenvironment in pancreatic cancer.
Int J Mol Sci. 21:73072020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyai Y, Esaki N, Takahashi M and Enomoto
A: Cancer-associated fibroblasts that restrain cancer progression:
Hypotheses and perspectives. Cancer Sci. 111:1047–1057. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Opitz F, Haeberle L, Daum A and Esposito
I: Tumor microenvironment in pancreatic intraepithelial neoplasia.
Cancers (Basel). 13:61882021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carpenter ES, Elhossiny AM, Kadiyala P, Li
J, McGue J, Griffith BD, Zhang Y, Edwards J, Nelson S, Lima F, et
al: Analysis of donor pancreata defines the transcriptomic
signature and microenvironment of early neoplastic lesions. Cancer
Discov. 13:1324–1345. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xue J, Sharma V, Hsieh MH, Chawla A,
Murali R, Pandol SJ and Habtezion A: Alternatively activated
macrophages promote pancreatic fibrosis in chronic pancreatitis.
Nat Commun. 6:71582015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu J, Zhang L, Shi J, He R, Yang W,
Habtezion A, Niu N, Lu P and Xue J: Macrophage phenotypic switch
orchestrates the inflammation and repair/regeneration following
acute pancreatitis injury. EBioMedicine. 58:1029202020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hingorani SR: Epithelial and stromal
co-evolution and complicity in pancreatic cancer. Nat Rev Cancer.
23:57–77. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garlanda C and Mantovani A: Interleukin-1
in tumor progression, therapy, and prevention. Cancer Cell.
39:1023–1027. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boersma B, Jiskoot W, Lowe P and Bourquin
C: The interleukin-1 cytokine family members: Role in cancer
pathogenesis and potential therapeutic applications in cancer
immunotherapy. Cytokine Growth Factor Rev. 62:1–14. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dinarello CA, Simon A and van der Meer
JWM: Treating inflammation by blocking interleukin-1 in a broad
spectrum of diseases. Nat Rev Drug Discov. 11:633–652. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dinarello C: Overview of the IL-1 family
in innate inflammation and acquired immunity. Immunol Rev.
281:8–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Narros-Fernández P, Chomanahalli
Basavarajappa S and Walsh PT: Interleukin-1 family cytokines at the
crossroads of microbiome regulation in barrier health and disease.
FEBS J. 291:1849–1869. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tomimatsu S, Ichikura T and Mochizuki H:
Significant correlation between expression of interleukin-1alpha
and liver metastasis in gastric carcinoma. Cancer. 91:1272–1276.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xue M, Zhu Y, Jiang Y, Han L, Shi M, Su R,
Wang L, Xiong C, Wang C, Wang T, et al: Schwann cells regulate
tumor cells and cancer-associated fibroblasts in the pancreatic
ductal adenocarcinoma microenvironment. Nat Commun. 14:46002023.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Das S, Shapiro B, Vucic E, Vogt S and
Bar-Sagi D: Tumor cell-derived IL1β promotes desmoplasia and immune
suppression in pancreatic cancer. Cancer Res. 80:1088–1101. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Caronni N, La Terza F, Vittoria FM,
Barbiera G, Mezzanzanica L, Cuzzola V, Barresi S, Pellegatta M,
Canevazzi P, Dunsmore G, et al: IL-1β+ macrophages fuel
pathogenic inflammation in pancreatic cancer. Nature. 623:415–422.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Herremans KD, Szymkiewicz DD, Riner AN,
Bohan RP, Tushoski GW, Davidson AM, Lou X, Leong MC, Dean BD,
Gerber M, et al: The interleukin-1 axis and the tumor immune
microenvironment in pancreatic ductal adenocarcinoma. Neoplasia.
28:1007892022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen L, Huang H, Zheng X, Li Y, Chen J,
Tan B, Liu Y, Sun R, Xu B, Yang M, et al: IL1R2 increases
regulatory T cell population in the tumor microenvironment by
enhancing MHC-II expression on cancer-associated fibroblasts. J
Immunother Cancer. 10:e0045852022. View Article : Google Scholar
|
|
51
|
Underwood PW, Gerber MN, Nguyen K, Delitto
D, Han S, Thomas RM, Forsmark CE, Trevino JG, Gooding WE and Hughes
SJ: Protein signatures and tissue diagnosis of pancreatic cancer. J
Am Coll Surg. 230:26–36.e1. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Waldmann T: Cytokines in cancer
immunotherapy. Cold Spring Hard Perspect Biol. 10:a0284722018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yasuda K, Nakanishi K and Tsutsui H:
Interleukin-18 in health and disease. Int J Mol Sci. 20:6492019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kaplanski G: Interleukin-18: Biological
properties and role in disease pathogenesis. Immunol Rev.
281:138–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schneider A, Haas SL, Hildenbrand R,
Siegmund S, Reinhard I, Nakovics H, Singer MV and Feick P: Enhanced
expression of interleukin-18 in serum and pancreas of patients with
chronic pancreatitis. World J Gastroentero. 12:6507–6514. 2006.
View Article : Google Scholar
|
|
56
|
Manohar M, Verma AK, Venkateshaiah SU and
Mishra A: Role of eosinophils in the initiation and progression of
pancreatitis pathogenesis. Am J Physiol Gastrointest Liver Physiol.
314:G211–G222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li CX, Cui LH, Zhang LQ, Yang L, Zhuo YZ,
Cui NQ and Zhang SK: Role of NLR family pyrin domain-containing 3
inflammasome in the activation of pancreatic stellate cells. Exp
Cell Res. 404:1126342021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu Y, Xu X, Lei W, Hou Y, Zhang Y, Tang
R, Yang Z, Tian Y, Zhu Y, Wang C, et al: The NLRP3 inflammasome in
fibrosis and aging: The known unknowns. Ageing Res Rev.
79:1016382022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou T, Damsky W, Weizman OE, McGeary MK,
Hartmann KP, Rosen CE, Fischer S, Jackson R, Flavell RA, Wang J, et
al: IL-18BP is a secreted immune checkpoint and barrier to IL-18
immunotherapy. Nature. 583:609–614. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ahmed A, Klotz R, Köhler S, Giese N,
Hackert T, Springfeld C, Jäger D and Halama N: Immune features of
the peritumoral stroma in pancreatic ductal adenocarcinoma. Front
Immunol. 13:9474072022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tarhini AA, Millward M, Mainwaring P,
Kefford R, Logan T, Pavlick A, Kathman SJ, Laubscher KH, Dar MM and
Kirkwood JM: A phase 2, randomized study of SB-485232, rhIL-18, in
patients with previously untreated metastatic melanoma. Cancer.
115:859–868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim SH, Eisenstein M, Reznikov L, Fantuzzi
G, Novick D, Rubinstein M and Dinarello CA: Structural requirements
of six naturally occurring isoforms of the IL-18 binding protein to
inhibit IL-18. Proc Natl Acad Sci USA. 97:1190–1195. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Menachem A, Alteber Z, Cojocaru G, Fridman
Kfir T, Blat D, Leiderman O, Galperin M, Sever L, Cohen N, Cohen K,
et al: Unleashing natural IL18 activity using an anti-IL18BP
blocker induces potent immune stimulation and antitumor effects.
Cancer Immunol Res. 12:687–703. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang Y, Zhang ZX, Lian D, Haig A,
Bhattacharjee R and Jevnikar AM: IL-37 inhibits IL-18-induced
tubular epithelial cell expression of pro-inflammatory cytokines
and renal ischemia-reperfusion injury. Kidney Int. 87:396–408.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liew FY, Girard JP and Turnquist HR:
Interleukin-33 in health and disease. Nat Rev Immunol. 16:676–689.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Larsen KM, Minaya MK, Vaish V and Peña
MMO: The role of IL-33/ST2 pathway in tumorigenesis. Int J Mol Sci.
19:26762018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Park JH, Ameri AH, Dempsey KE, Conrad DN,
Kem M, Mino-Kenudson M and Demehri S: Nuclear IL-33/SMAD signaling
axis promotes cancer development in chronic inflammation. EMBO J.
40:e1061512021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Alam A, Levanduski E, Denz P,
Villavicencio HS, Bhatta M, Alhorebi L, Zhang Y, Gomez EC, Morreale
B, Senchanthisai S, et al: Fungal mycobiome drives IL-33 secretion
and type 2 immunity in pancreatic cancer. Cancer Cell.
40:153–167.e11. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Andersson P, Yang Y, Hosaka K, Zhang Y,
Fischer C, Braun H, Liu S, Yu G, Liu S, Beyaert R, et al: Molecular
mechanisms of IL-33-mediated stromal interactions in cancer
metastasis. JCI insight. 3:e1223752018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Moral JA, Leung J, Rojas LA, Ruan J, Zhao
J, Sethna Z, Ramnarain A, Gasmi B, Gururajan M, Redmond D, et al:
ILC2s amplify PD-1 blockade by activating tissue-specific cancer
immunity. Nature. 579:130–135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun X, He X, Zhang Y, Hosaka K, Andersson
P, Wu J, Wu J, Jing X, Du Q, Hui X, et al: Inflammatory
cell-derived CXCL3 promotes pancreatic cancer metastasis through a
novel myofibroblast-hijacked cancer escape mechanism. Gut.
71:129–147. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Alonso-Curbelo D, Ho YJ, Burdziak C, Maag
JLV, Morris JP IV, Chandwani R, Chen HA, Tsanov KM, Barriga FM,
Luan W, et al: A gene-environment-induced epigenetic program
initiates tumorigenesis. Nature. 590:642–648. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Burdziak C, Alonso-Curbelo D, Walle T,
Reyes J, Barriga FM, Haviv D, Xie Y, Zhao Z, Zhao CJ, Chen HA, et
al: Epigenetic plasticity cooperates with cell-cell interactions to
direct pancreatic tumorigenesis. Science. 380:eadd53272023.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hatzioannou A, Banos A, Sakelaropoulos T,
Fedonidis C, Vidali MS, Köhne M, Händler K, Boon L, Henriques A,
Koliaraki V, et al: An intrinsic role of IL-33 in Treg
cell-mediated tumor immunoevasion. Nat Immunol. 21:75–85. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Martínez-Pérez C, Kay C, Meehan J, Gray M,
Dixon JM and Turnbull AK: The IL6-like cytokine family: Role and
biomarker potential in breast cancer. J Pers Med. 11:10732021.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shi Y, Gao W, Lytle NK, Huang P, Yuan X,
Dann AM, Ridinger-Saison M, DelGiorno KE, Antal CE, Liang G, et al:
Targeting LIF-mediated paracrine interaction for pancreatic cancer
therapy and monitoring. Nature. 569:131–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang Y, Yan W, Collins MA, Bednar F,
Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD and di Magliano
MP: Interleukin-6 is required for pancreatic cancer progression by
promoting MAPK signaling activation and oxidative stress
resistance. Cancer Res. 73:6359–6374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lee YE, Go GY, Koh EY, Yoon HN, Seo M,
Hong SM, Jeong JH, Kim JC, Cho D, Kim TS, et al: Synergistic
therapeutic combination with a CAF inhibitor enhances
CAR-NK-mediated cytotoxicity via reduction of CAF-released IL-6. J
Immunother Cancer. 11:e0061302023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ramsey ML, Talbert E, Ahn D, Bekaii-Saab
T, Badi N, Bloomston PM, Conwell DL, Cruz-Monserrate Z, Dillhoff M,
Farren MR, et al: Circulating interleukin-6 is associated with
disease progression, but not cachexia in pancreatic cancer.
Pancreatology. 19:80–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ebrahimi B, Tucker SL, Li D, Abbruzzese JL
and Kurzrock R: Cytokines in pancreatic carcinoma: Correlation with
phenotypic characteristics and prognosis. Cancer. 101:2727–2736.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nagathihalli NS, Castellanos JA, VanSaun
MN, Dai X, Ambrose M, Guo Q, Xiong Y and Merchant NB: Pancreatic
stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness
of pancreatic intraepithelial neoplasia and cancer cells.
Oncotarget. 7:65982–65992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Angevin E, Tabernero J, Elez E, Cohen SJ,
Bahleda R, van Laethem JL, Ottensmeier C, Lopez-Martin JA, Clive S,
Joly F, et al: A phase I/II, multiple-dose, dose-escalation study
of siltuximab, an anti-interleukin-6 monoclonal antibody, in
patients with advanced solid tumors. Clin Cancer Res. 20:2192–2204.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Goumas FA, Holmer R, Egberts JH,
Gontarewicz A, Heneweer C, Geisen U, Hauser C, Mende MM, Legler K,
Röcken C, et al: Inhibition of IL-6 signaling significantly reduces
primary tumor growth and recurrencies in orthotopic xenograft
models of pancreatic cancer. Int J Cancer. 137:1035–1046. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Hurwitz H, Van Cutsem E, Bendell J,
Hidalgo M, Li CP, Salvo MG, Macarulla T, Sahai V, Sama A, Greeno E,
et al: Ruxolitinib + capecitabine in advanced/metastatic pancreatic
cancer after disease progression/intolerance to first-line therapy:
JANUS 1 and 2 randomized phase III studies. Invest New Drugs.
36:683–695. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wong ALA, Hirpara JL, Pervaiz S, Eu JQ,
Sethi G and Goh BC: Do STAT3 inhibitors have potential in the
future for cancer therapy? Expert Opin Investig Drugs. 26:883–887.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ouyang W, Rutz S, Crellin NK, Valdez PA
and Hymowitz SG: Regulation and functions of the IL-10 family of
cytokines in inflammation and disease. Annu Rev Immunol. 29:71–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lazear HM, Schoggins JW and Diamond MS:
Shared and distinct functions of type I and type III interferons.
Immunity. 50:907–923. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Marcon F, Zuo J, Pearce H, Nicol S,
Margielewska-Davies S, Farhat M, Mahon B, Middleton G, Brown R,
Roberts KJ and Moss P: NK cells in pancreatic cancer demonstrate
impaired cytotoxicity and a regulatory IL-10 phenotype.
Oncoimmunology. 9:18454242020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xuan X, Tian Z, Zhang M, Zhou J, Gao W,
Zhang Y, Zhang Y, Lei B, Ni B, Wu Y and Fan W: Diverse effects of
interleukin-22 on pancreatic diseases. Pancreatology. 18:231–237.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhao Y, Chen J, Andreatta M, Feng B, Xie
YQ, Wenes M, Wang Y, Gao M, Hu X, Romero P, et al: IL-10-expressing
CAR T cells resist dysfunction and mediate durable clearance of
solid tumors and metastases. Nat Biotechnol. Jan 2–2024.(Epub ahead
of print). View Article : Google Scholar
|
|
94
|
Ip WKE, Hoshi N, Shouval DS, Snapper S and
Medzhitov R: Anti-inflammatory effect of IL-10 mediated by
metabolic reprogramming of macrophages. Science. 356:513–519. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lin WR, Lim SN, Yen TH and Alison MR: The
influence of bone marrow-secreted IL-10 in a mouse model of
cerulein-induced pancreatic fibrosis. Biomed Res Int.
2016:46015322016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Qiao J, Liu Z, Dong C, Luan Y, Zhang A,
Moore C, Fu K, Peng J, Wang Y, Ren Z, et al: Targeting tumors with
IL-10 prevents dendritic cell-mediated CD8+ T cell
apoptosis. Cancer Cell. 35:901–915.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Naing A, Infante JR, Papadopoulos KP, Chan
IH, Shen C, Ratti NP, Rojo B, Autio KA, Wong DJ, Patel MR, et al:
PEGylated IL-10 (pegilodecakin) induces systemic immune activation,
CD8+ T cell invigoration and polyclonal T cell expansion
in cancer patients. Cancer Cell. 34:775–791.e3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Labadie KP, Kreuser SA, Brempelis KJ,
Daniel SK, Jiang X, Sullivan KM, Utria AF, Kenerson HL, Kim TS,
Crane CA and Pillarisetty VG: Production of an interleukin-10
blocking antibody by genetically engineered macrophages increases
cancer cell death in human gastrointestinal tumor slice cultures.
Cancer Gene Ther. 30:1227–1233. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Perusina Lanfranca M, Lin Y, Fang J, Zou W
and Frankel T: Biological and pathological activities of
interleukin-22. J Mol Med (Berl). 94:523–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Perusina Lanfranca M, Zhang Y, Girgis A,
Kasselman S, Lazarus J, Kryczek I, Delrosario L, Rhim A, Koneva L,
Sartor M, et al: Interleukin 22 signaling regulates acinar cell
plasticity to promote pancreatic tumor development in mice.
Gastroenterology. 158:1417–1432.e11. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Curd LM, Favors SE and Gregg RK:
Pro-tumour activity of interleukin-22 in HPAFII human pancreatic
cancer cells. Clin Exp Immunol. 168:192–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Arshad T, Mansur F, Palek R, Manzoor S and
Liska V: A double edged sword role of interleukin-22 in wound
healing and tissue regeneration. Front Immunol. 11:21482020.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Feng D, Park O, Radaeva S, Wang H, Yin S,
Kong X, Zheng M, Zakhari S, Kolls JK and Gao B: Interleukin-22
ameliorates cerulein-induced pancreatitis in mice by inhibiting the
autophagic pathway. Int J Biol Sci. 8:249–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yang H, Cao R, Zhou F, Wang B, Xu Q, Li R,
Zhang C and Xu H: The role of Interleukin-22 in severe acute
pancreatitis. Mol Med. 30:602024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang T, Wahib R, Zazara DE, Lücke J,
Shiri AM, Kempski J, Zhao L, Agalioti T, Machicote AP, Giannou O,
et al: CD4+ T cell-derived IL-22 enhances liver metastasis by
promoting angiogenesis. Oncoimmunology. 12:22696342023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Xue J, Zhao Q, Sharma V, Nguyen LP, Lee
YN, Pham KL, Edderkaoui M, Pandol SJ, Park W and Habtezion A: Aryl
hydrocarbon receptor ligands in cigarette smoke induce production
of interleukin-22 to promote pancreatic fibrosis in models of
chronic pancreatitis. Gastroenterology. 151:1206–1217. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zelante T, Iannitti RG, Cunha C, De Luca
A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C,
Massi-Benedetti C, Fallarino F, et al: Tryptophan catabolites from
microbiota engage aryl hydrocarbon receptor and balance mucosal
reactivity via interleukin-22. Immunity. 39:372–385. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Liu B, Fu T, He P, Du C and Xu K:
Construction of a five-gene prognostic model based on
immune-related genes for the prediction of survival in pancreatic
cancer. Biosci Rep. 41:BSR202043012021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lu SW, Pan HC, Hsu YH, Chang KC, Wu LW,
Chen WY and Chang MS: IL-20 antagonist suppresses PD-L1 expression
and prolongs survival in pancreatic cancer models. Nat Commun.
11:46112020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
McGeachy MJ, Cua DJ and Gaffen SL: The
IL-17 family of cytokines in health and disease. Immunity.
50:892–906. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Meehan EV and Wang K: Interleukin-17
family cytokines in metabolic disorders and cancer. Genes (Basel).
13:16432022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jovanovic DV, Di Battista JA,
Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F and
Pelletier JP: IL-17 stimulates the production and expression of
proinflammatory cytokines, IL-beta and TNF-alpha, by human
macrophages. J Immunol. 160:3513–3521. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Loncle C, Bonjoch L, Folch-Puy E,
Lopez-Millan MB, Lac S, Molejon MI, Chuluyan E, Cordelier P, Dubus
P, Lomberk G, et al: IL17 functions through the novel
REG3β-JAK2-STAT3 inflammatory pathway to promote the transition
from chronic pancreatitis to pancreatic cancer. Cancer Res.
75:4852–4862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen Z, Qiao S, Yang L, Sun M, Li B, Lu A
and Li F: Mechanistic insights into the roles of the IL-17/IL-17R
families in pancreatic cancer. Int J Mol Sci. 24:135392023.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chandra V, Li L, Le Roux O, Zhang Y,
Howell RM, Rupani DN, Baydogan S, Miller HD, Riquelme E, Petrosino
J, et al: Gut epithelial Interleukin-17 receptor A signaling can
modulate distant tumors growth through microbial regulation. Cancer
Cell. 42:85–100.e6. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Hu F, Guo F, Zhu Y, Zhou Q, Li T, Xiang H
and Shang D: IL-17 in pancreatic disease: Pathogenesis and
pharmacotherapy. Am J Cancer Res. 10:3551–3564. 2020.PubMed/NCBI
|
|
117
|
Picard FSR, Lutz V, Brichkina A, Neuhaus
F, Ruckenbrod T, Hupfer A, Raifer H, Klein M, Bopp T, Pfefferle PI,
et al: IL-17A-producing CD8+ T cells promote PDAC via
induction of inflammatory cancer-associated fibroblasts. Gut.
72:1510–1522. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Mucciolo G, Curcio C, Roux C, Li WY,
Capello M, Curto R, Chiarle R, Giordano D, Satolli MA, Lawlor R, et
al: IL17A critically shapes the transcriptional program of
fibroblasts in pancreatic cancer and switches on their
protumorigenic functions. Proc Natl Acad Sci USA.
118:e20203951182021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Li J, Betzler C, Lohneis P, Popp MC, Qin
J, Kalinski T, Wartmann T, Bruns CJ, Zhao Y and Popp FC: The
IL-17A/IL-17RA axis is not related to overall survival and cancer
stem cell modulation in pancreatic cancer. Int J Mol Sci.
21:22152020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Qian X, Chen H, Wu X, Hu L, Huang Q and
Jin Y: Interleukin-17 acts as double-edged sword in anti-tumor
immunity and tumorigenesis. Cytokine. 89:34–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang J, Zhang Y, Yin K, Xu P, Tian J, Ma
J, Tian X, Wang Y, Tang X, Xu H and Wang S: IL-17A weakens the
antitumor immuity by inhibiting apoptosis of MDSCs in Lewis lung
carcinoma bearing mice. Oncotarget. 8:4814–4825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
McAndrews KM, Chen Y, Darpolor JK, Zheng
X, Yang S, Carstens JL, Li B, Wang H, Miyake T, Correa de Sampaio
P, et al: Identification of functional heterogeneity of
carcinoma-associated fibroblasts with distinct IL6-mediated therapy
resistance in pancreatic cancer. Cancer Discov. 12:1580–1597. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ware MJ, Keshishian V, Law JJ, Ho JC,
Favela CA, Rees P, Smith B, Mohammad S, Hwang RF, Rajapakshe K, et
al: Generation of an in vitro 3D PDAC stroma rich spheroid model.
Biomaterials. 108:129–142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Jiang L, Qin J, Dai Y, Zhao S, Zhan Q, Cui
P, Ren L, Wang X, Zhang R, Gao C, et al: Prospective observational
study on biomarkers of response in pancreatic ductal
adenocarcinoma. Nat Med. 30:749–761. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Bärthel S, Falcomatà C, Rad R, Theis FJ
and Saur D: Single-cell profiling to explore pancreatic cancer
heterogeneity, plasticity and response to therapy. Nat Cancer.
4:454–467. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ayars M, O'Sullivan E, Macgregor-Das A,
Shindo K, Kim H, Borges M, Yu J, Hruban RH and Goggins M: IL2RG,
identified as overexpressed by RNA-seq profiling of pancreatic
intraepithelial neoplasia, mediates pancreatic cancer growth.
Oncotarget. 8:83370–83383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hulst SPL: Zur kenntnis der Genese des
Adenokarzinoms und Karzinoms des Pankreas. Virchows Arch.
180:288–316. 1905. View Article : Google Scholar
|
|
128
|
Dougan M, Ingram JR, Jeong HJ, Mosaheb MM,
Bruck PT, Ali L, Pishesha N, Blomberg O, Tyler PM, Servos MM, et
al: Targeting cytokine therapy to the pancreatic tumor
microenvironment using PD-L1-specific VHHs. Cancer Immunol Res.
6:389–401. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ahmed A, Köhler S, Klotz R, Giese N,
Lasitschka F, Hackert T, Springfeld C, Zörnig I, Jäger D and Halama
N: Peripheral blood and tissue assessment highlights differential
tumor-circulatory gradients of IL2 and MIF with prognostic
significance in resectable pancreatic ductal adenocarcinoma.
Oncoimmunology. 10:19621352021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mayer P, Linnebacher A, Glennemeier-Marke
H, Marnet N, Bergmann F, Hackert T, Klauss M, Poth T and Gaida MM:
The microarchitecture of pancreatic cancer as measured by
diffusion-weighted magnetic resonance imaging is altered by T cells
with a tumor promoting Th17 phenotype. Int J Mol Sci. 21:3462020.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Linnebacher A, Mayer P, Marnet N, Bergmann
F, Herpel E, Revia S, Yin L, Liu L, Hackert T, Giese T, et al:
Interleukin 21 receptor/ligand interaction is linked to disease
progression in pancreatic cancer. Cells. 8:11042019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zaidi N, Quezada SA, Kuroiwa JMY, Zhang L,
Jaffee EM, Steinman RM and Wang B: Anti-CTLA-4 synergizes with
dendritic cell-targeted vaccine to promote IL-3-dependent
CD4+ effector T cell infiltration into murine pancreatic
tumors. Ann N Y Acad Sci. 1445:62–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Savid-Frontera C, Viano ME, Baez NS, Lidon
NL, Fontaine Q, Young HA, Vimeux L, Donnadieu E and Rodriguez-Galan
MC: Exploring the immunomodulatory role of virtual memory
CD8+ T cells: Role of IFN gamma in tumor growth control.
Front Immunol. 13:9710012022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Hussain SM, Reed LF, Krasnick BA,
Miranda-Carboni G, Fields RC, Bi Y, Elahi A, Ajidahun A, Dickson
PV, Deneve JL, et al: IL23 and TGF-ß diminish macrophage associated
metastasis in pancreatic carcinoma. Sci Rep. 8:58082018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Mirlekar B, Michaud D, Lee SJ, Kren NP,
Harris C, Greene K, Goldman EC, Gupta GP, Fields RC, Hawkins WG, et
al: B cell-derived IL35 drives STAT3-dependent CD8+
T-cell exclusion in pancreatic cancer. Cancer Immunol Res.
8:292–308. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Liou GY, Bastea L, Fleming A, Döppler H,
Edenfield BH, Dawson DW, Zhang L, Bardeesy N and Storz P: The
presence of interleukin-13 at pancreatic ADM/PanIN lesions alters
macrophage populations and mediates pancreatic tumorigenesis. Cell
Rep. 19:1322–1333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Shi J, Shen X, Kang Q, Yang X, Denzinger
M, Kornmann M and Traub B: Loss of interleukin-13-receptor-alpha-1
induces apoptosis and promotes EMT in pancreatic cancer. Int J Mol
Sci. 23:36592022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Fujisawa T, Shimamura T, Goto K, Nakagawa
R, Muroyama R, Ino Y, Horiuchi H, Endo I, Maeda S, Harihara Y, et
al: A novel role of interleukin 13 receptor alpha2 in perineural
invasion and its association with poor prognosis of patients with
pancreatic ductal adenocarcinoma. Cancers (Basel). 12:12942020.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Arnoletti JP, Reza J, Rosales A, Monreal
A, Fanaian N, Whisner S, Srivastava M, Rivera-Otero J, Yu G,
Phanstiel Iv O, et al: Pancreatic ductal adenocarcinoma (PDAC)
circulating tumor cells influence myeloid cell differentiation to
support their survival and immunoresistance in portal vein
circulation. PLoS One. 17:e02657252022. View Article : Google Scholar : PubMed/NCBI
|