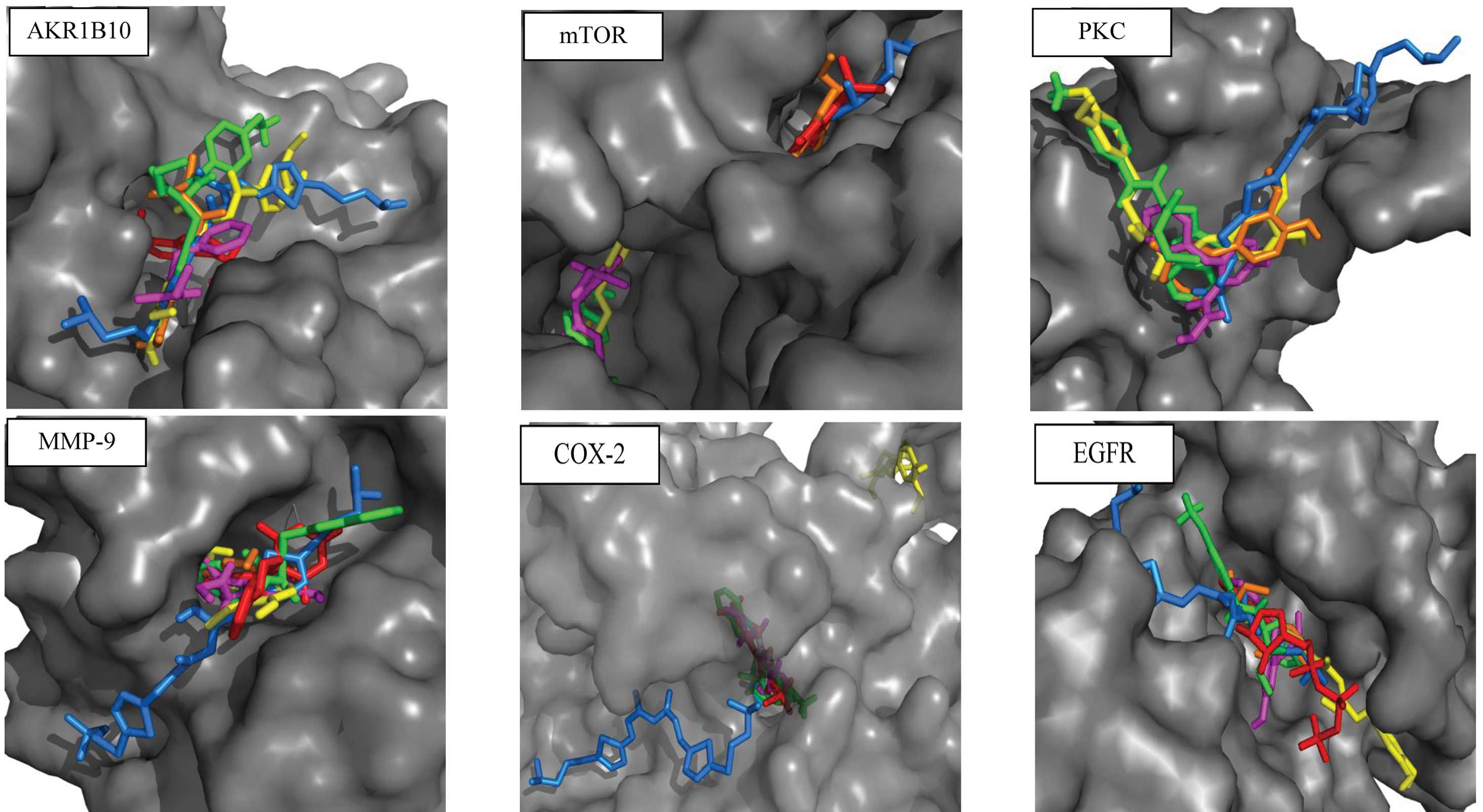
|
1
|
American Cancer Society. Cancer Facts
& Figures. 2013, http://www.cancer.org/research/cancerfactsstatistics/index.
Accessed March 24, 2013
|
|
2
|
Arcangeli S, Pinzi V and Arcangeli G:
Epidemiology of prostate cancer and treatment remarks. World J
Radiol. 4:241–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cimino S, Sortino G, Favilla V, et al:
Polyphenols: key issues involved in chemoprevention of prostate
cancer. Oxid Med Cell Longev. 2012:6329592012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fleshner N and Zlotta AR: Prostate cancer
prevention: past, present, and future. Cancer. 110:1889–1899. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agrawal DK and Mishra PK: Curcumin and its
analogues: potential anticancer agents. Med Res Rev. 30:818–860.
2010.PubMed/NCBI
|
|
6
|
Da-Lozzo EJ, Moledo RC, Faraco CD,
Ortolani-Machado CF, Bresolin TM and Silveira JL:
Curcumin/xanthan-galactomannan hydrogels: rheological analysis and
biocompatibility. Carbohydr Polym. 93:279–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu CH and Cheng AL: Clinical studies with
curcumin. Adv Exp Med Biol. 595:471–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park W, Amin AR, Chen ZG and Shin DM: New
perspectives of curcumin in cancer prevention. Cancer Prev Res
(Phila). 6:387–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strimpakos AS and Sharma RA: Curcumin:
preventive and therapeutic properties in laboratory studies and
clinical trials. Antioxid Redox Signal. 10:511–545. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin L, Shi Q, Nyarko AK, et al: Antitumor
agents. 250. Design and synthesis of new curcumin analogues as
potential anti-prostate cancer agents. J Med Chem. 49:3963–3972.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mimeault M and Batra SK: Potential
applications of curcumin and its novel synthetic analogs and
nanotechnology-based formulations in cancer prevention and therapy.
Chin Med. 6:312011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dorai T, Gehani N and Katz A: Therapeutic
potential of curcumin in human prostate cancer-I. curcumin induces
apoptosis in both androgen-dependent and androgen-independent
prostate cancer cells. Prostate Cancer Prostatic Dis. 3:84–93.
2000. View Article : Google Scholar
|
|
13
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: from ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shehzad A, Wahid F and Lee YS: Curcumin in
cancer chemoprevention: molecular targets, pharmacokinetics,
bioavailability, and clinical trials. Arch Pharm (Weinheim).
343:489–499. 2010. View Article : Google Scholar
|
|
15
|
Beevers CS, Chen L, Liu L, Luo Y, Webster
NJ and Huang S: Curcumin disrupts the Mammalian target of
rapamycin-raptor complex. Cancer Res. 69:1000–1008. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuendet M and Pezzuto JM: The role of
cyclooxygenase and lipoxygenase in cancer chemoprevention. Drug
Metabol Drug Interact. 17:109–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rao CV: Regulation of COX and LOX by
curcumin. Adv Exp Med Biol. 595:213–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verderio P, Bonetti P, Colombo M, Pandolfi
L and Prosperi D: Intracellular drug release from curcumin-loaded
PLGA nanoparticlesinduces G2/M block in breast cancer cells.
Biomacromolecules. 14:672–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zlotogorski A, Dayan A, Dayan D, Chaushu
G, Salo T and Vered M: Nutraceuticals as new treatment approaches
for oral cancer - I: Curcumin. Oral Oncol. 49:187–191. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trott O and Olson AJ: AutoDock Vina:
improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.PubMed/NCBI
|
|
21
|
Gallego O, Ruiz FX, Ardèvol A, et al:
Structural basis for the high all-trans-retinaldehyde reductase
activity of the tumor marker AKR1B10. Proc Natl Acad Sci USA.
104:20764–20769. 2007. View Article : Google Scholar
|
|
22
|
Liu KK, Bagrodia S, Bailey S, et al:
4-methylpteridinones as orally active and selective PI3K/mTOR dual
inhibitors. Bioorg Med Chem Lett. 20:6096–6099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benes CH, Wu N, Elia AE, Dharia T, Cantley
LC and Soltoff SP: The C2 domain of PKCdelta is a phosphotyrosine
binding domain. Cell. 121:271–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tochowicz A, Maskos K, Huber R, et al:
Crystal structures of MMP-9 complexes with five inhibitors:
contribution of the flexible Arg424 side-chain to selectivity. J
Mol Biol. 371:989–1006. 2007. View Article : Google Scholar
|
|
25
|
Wang JL, Limburg D, Graneto MJ, et al: The
novel benzopyran class of selective cyclooxygenase-2 inhibitors.
Part 2: the second clinical candidate having a shorter and
favorable human half-life. Bioorg Med Chem Lett. 20:7159–7163.
2010. View Article : Google Scholar
|
|
26
|
Yun CH, Boggon TJ, Li Y, et al: Structures
of lung cancer-derived EGFR mutants and inhibitor complexes:
mechanism of activation and insights into differential inhibitor
sensitivity. Cancer Cell. 11:217–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bikádi Z, Hazai E, Zsila F and Lockwood
SF: Molecular modeling of non-covalent binding of homochiral
(3S,3′S)-astaxanthin to matrix metalloproteinase-13 (MMP-13).
Bioorg Med Chem. 14:5451–5458. 2006.PubMed/NCBI
|
|
28
|
D’hoedt D and Bertrand D: Nicotinic
acetylcholine receptors: an overview on drug discovery. Expert Opin
Ther Targets. 13:395–411. 2009.
|
|
29
|
Hetényi C and van der Spoel D: Blind
docking of drug-sized compounds to proteins with up to a thousand
residues. FEBS Lett. 580:1447–1450. 2006.PubMed/NCBI
|
|
30
|
Iorga B, Herlem D, Barré E and Guillou C:
Acetylcholine nicotinic receptors: finding the putative binding
site of allosteric modulators using the “blind docking” approach. J
Mol Model. 12:366–372. 2006.PubMed/NCBI
|
|
31
|
Seeliger D and de Groot BL: Ligand docking
and binding site analysis with PyMOL and Autodock/Vina. J Computb
Aided Mol Des. 24:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DeLano WL: The case for open-source
software in drug discovery. Drug Discov Today. 10:213–217.
2005.PubMed/NCBI
|
|
33
|
Matsunaga T, Endo S, Soda M, et al: Potent
and selective inhibition of the tumor marker AKR1B10 by
bisdemethoxycurcumin: probing the active site of the enzyme with
molecular modeling and site-directed mutagenesis. Biochem Biophys
Res Commun. 389:128–132. 2009. View Article : Google Scholar
|
|
34
|
Balendiran GK: Fibrates in the chemical
action of daunorubicin. Curr Cancer Drug Targets. 9:366–369. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xuyu Z, Ruilan Y, Jun M, Duan-Fang L and
Deliang C: AKR1B10: A potential target for cancer therapy.
Bioscience Hypotheses. 2:31–33. 2009. View Article : Google Scholar
|
|
36
|
Tokunaga C, Yoshino K and Yonezawa K: mTOR
integrates amino acid- and energy-sensing pathways. Biochem Biophys
Res Commun. 313:443–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beevers CS, Zhou H and Huang S: Hitting
the golden TORget: curcumin’s effects on mTOR signaling. Anticancer
Agents Med Chem. Dec;2012.(epub ahead of print).
|
|
38
|
Deters M, Hütten H and Kaever V:
Synergistic immunosuppressive effects of the mTOR inhibitor
sirolimus and the phytochemical curcumin. Phytomedicine.
20:120–123. 2013. View Article : Google Scholar
|
|
39
|
Lin JK: Molecular targets of curcumin. Adv
Exp Med Biol. 595:227–243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lou JR, Zhang XX, Zheng J and Ding WQ:
Transient metals enhance cytotoxicity of curcumin: potential
involvement of the NF-kappaB and mTOR signaling pathways.
Anticancer Res. 30:3249–3255. 2010.
|
|
41
|
Conboy L, Foley AG, O’Boyle NM, et al:
Curcumin-induced degradation of PKC delta is associated with
enhanced dentate NCAM PSA expression and spatial learning in adult
and aged Wistar rats. Biochem Pharmacol. 77:1254–1265. 2009.
View Article : Google Scholar
|
|
42
|
Tartour E, Pere H, Maillere B, et al:
Angiogenesis and immunity: a bidirectional link potentially
relevant for the monitoring of antiangiogenic therapy and the
development of novel therapeutic combination with immunotherapy.
Cancer Metastasis Rev. 30:83–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
van Kempen LC and Coussens LM: MMP9
potentiates pulmonary metastasis formation. Cancer Cell. 2:251–252.
2002.PubMed/NCBI
|
|
44
|
Bolignano D, Donato V, Lacquaniti A, et
al: Neutrophil gelatinase-associated lipocalin (NGAL) in human
neoplasias: a new protein enters the scene. Cancer Lett. 288:10–16.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Masson V, de la Ballina LR, Munaut C, et
al: Contribution of host MMP-2 and MMP-9 to promote tumor
vascularization and invasion of malignant keratinocytes. FASEB J.
19:234–236. 2005.PubMed/NCBI
|
|
46
|
Jankun J, Aleem AM, Selman SH, Basrur V
and Skrzypczak-Jankun E: VLHL plasminogen activator inhibitor
spontaneously reactivates from the latent to active form. Int J Mol
Med. 23:57–63. 2009.PubMed/NCBI
|
|
47
|
Jankun J, Aleem AM, Specht Z, et al: PAI-1
induces cell detachment, downregulates nucleophosmin (B23) and
fortilin (TCTP) in LnCAP prostate cancer cells. Int J Mol Med.
20:11–20. 2007.
|
|
48
|
Jankun J and Skrzypczak-Jankun E: Yin and
yang of the plasminogen activator inhibitor. Pol Arch Med Wewn.
119:410–417. 2009.PubMed/NCBI
|
|
49
|
Ravindranath MH, Muthugounder S, Presser N
and Viswanathan S: Anticancer therapeutic potential of soy
isoflavone, genistein. Adv Exp Med Biol. 546:121–165. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fujisawa S, Atsumi T, Ishihara M and
Kadoma Y: Cytotoxicity, ROS-generation activity and
radical-scavenging activity of curcumin and related compounds.
Anticancer Res. 24:563–569. 2004.PubMed/NCBI
|
|
51
|
Youssef KM, El-Sherbeny MA, El-Shafie FS,
Farag HA, Al-Deeb OA and Awadalla SA: Synthesis of curcumin
analogues as potential antioxidant, cancer chemopreventive agents.
Arch Pharm (Weinheim). 337:42–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zingg JM, Hasan ST and Meydani M:
Molecular mechanisms of hypolipidemic effects of curcumin.
Biofactors. 39:101–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Menon VP and Sudheer AR: Antioxidant and
anti-inflammatory properties of curcumin. Adv Exp Med Biol.
595:105–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gafner S, Lee SK, Cuendet M, et al:
Biologic evaluation of curcumin and structural derivatives in
cancer chemoprevention model systems. Phytochemistry. 65:2849–2859.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guo LY, Cai XF, Lee JJ, et al: Comparison
of suppressive effects of demethoxycurcumin and
bisdemethoxycurcumin on expressions of inflammatory mediators in
vitro and in vivo. Arch Pharm Res. 31:490–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Handler N, Jaeger W, Puschacher H, Leisser
K and Erker T: Synthesis of novel curcumin analogues and their
evaluation as selective cyclooxygenase-1 (COX-1) inhibitors. Chem
Pharm Bull (Tokyo). 55:64–71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Griesser M, Pistis V, Suzuki T, Tejera N,
Pratt DA and Schneider C: Autoxidative and cyclooxygenase-2
catalyzed transformation of the dietary chemopreventive agent
curcumin. J Biol Chem. 286:1114–1124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dong L, Vecchio AJ, Sharma NP, Jurban BJ,
Malkowski MG and Smith WL: Human cyclooxygenase-2 is a sequence
homodimer that functions as a conformational heterodimer. J Biol
Chem. 286:19035–19046. 2011. View Article : Google Scholar
|
|
59
|
Xu YY, Cao Y, Ma H, Li HQ and Ao GZ:
Design, synthesis and molecular docking of α,β-unsaturated
cyclohexanone analogous of curcumin as potent EGFR inhibitors with
antiproliferative activity. Bioorg Med Chem. 21:388–394. 2013.
|
|
60
|
Kolev TM, Velcheva EA, Stamboliyska BA and
Spiteller M: DFT and experimental studies of the structure and
vibrational spectra of curcumin. Int J Quantum Chem. 102:1069–1079.
2005. View Article : Google Scholar
|