Introduction
Galectins, whose name was proposed in 1994 from
their description as β-galactoside-binding lectins, are a family of
animal carbohydrate-binding proteins that agglutinate cells
(1–3).
Since their initial isolation from animals, including electric
eels, chicks and calves in the 1970s, at least 15 members of this
family have been identified. Galectins were systematically renamed
in 1994, primarily based on the order in which they were identified
(4).
Galectins perform their biological functions
principally through interactions with specific glycoconjugates.
Galectins possess high affinity for N-acetyllactosamine sequences
through their highly conserved carbohydrate recognition domains
(CRDs) (5). All galectins possess at
least one CRD.
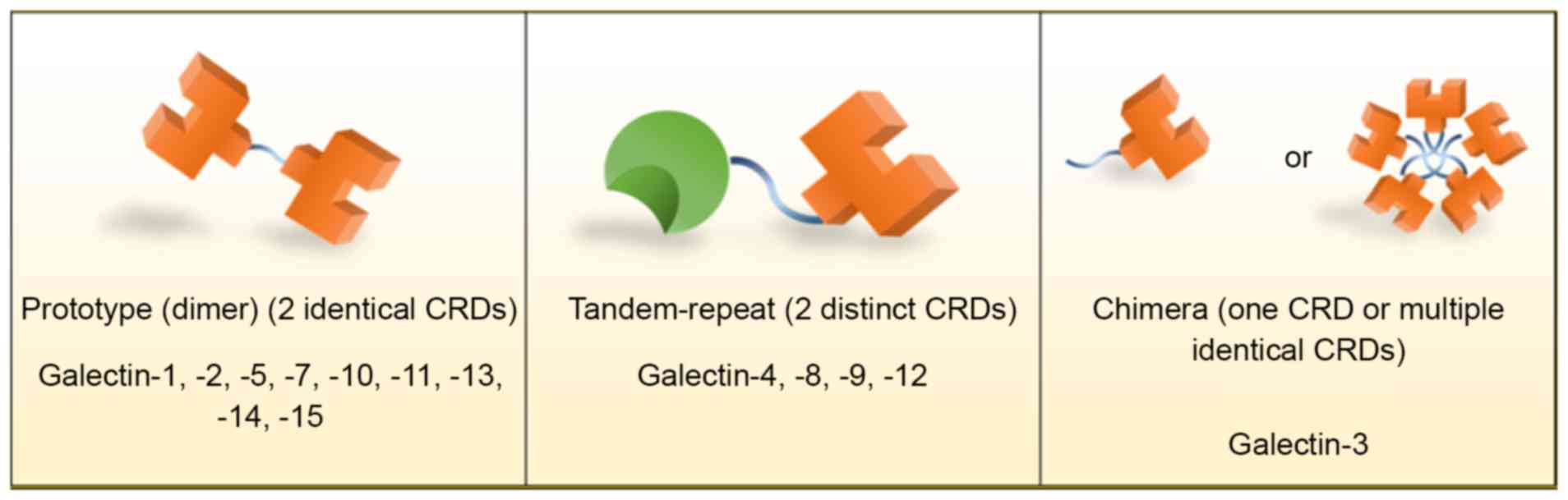
Galectins are classified by the number and structure
of CRDs into three major groups: Prototypical galectins,
tandem-repeat galectins and chimeric galectins (Fig. 1) (1,4,6,7). The
prototypical galectins, including galectins-1, −2, −5, −7, −10,
−11, −13, −14 and −15, contain two identical CRDs to form
homodimers and are therefore occasionally known as dimeric
galectins. Tandem-repeat galectins, including galectins-4, −6, −8,
−9 and −12, are characterized by containing at least two distinct
CRDs, connected by linker domains, within a single polypeptide.
Galectin-3 is the only chimeric galectin identified in vertebrates;
it contains a single CRD and a large amino-terminal domain, which
contributes to self-aggregation, resulting in its common appearance
of multiple identical subunits (6).
Not all galectins are present in humans: Only
galectin-1, −2, −3, −4, −7, −8, −9, −10, −12, and −13 (6). Galectin-5 and galectin-6 are present in
rodents, and galectin-11, −14, and −15 are observed in sheep and
goats (4,6). Notably, a number of galectin transcripts
may be differentially spliced in certain types of tissue to
generate a variety of isoforms. For example, at least seven
different mRNAs encoding human galectin-8 have been identified,
some in prototypical isoforms and others in tandem-repeat isoforms;
these isoforms may be expressed differentially in various tissues
(4). Three isoforms of galectin-9
with variations in their linker domain length have been identified
(4).
Functions of galectins
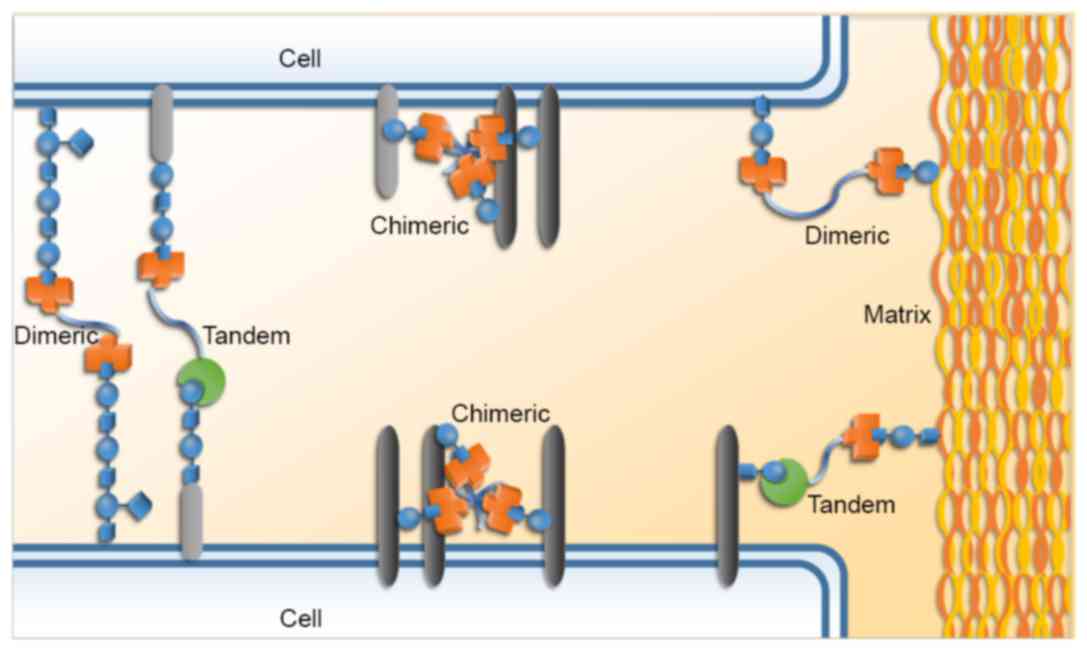
Galectins are able to mediate certain interactions
between cells, including homotypic and heterotypic interactions;
they also facilitate the bindings between cells and extracellular
matrix components. These cell-cell and cell-matrix interactions, as
well as galectin signaling on the cell surface, are able to
modulate signaling pathways and thereby influence cellular
functions and behaviors (Fig. 2)
(7–9).
Roles of galectins in apoptosis
Galectin-1, −2, −3, −7, −8, −9, and −12 are able to
induce apoptosis in certain types of blood cells (10). Galectin-1-induced apoptosis involves
interactions between T cells and surface glycoproteins, associated
with cluster of differentiation (CD)7, CD29 and CD43. The apoptosis
induced by galectin-3 is associated with CD71 and CD45 (11), and the overexpression of intracellular
galectin-3 may exhibit anti-apoptotic activity (12).
Roles of galectins in development
Galectins have also been demonstrated to be involved
in animal development. For example, galectin-3 ablation in mice is
associated with decreased mast cell function, as well as fatty
liver disease, liver fibrosis, age-dependent glomerular lesions and
lung fibrosis (13). Galectin-1 is
involved in pathfinding and axon regeneration, and its ablation in
mice is associated with a decreased sensitivity response to noxious
thermal stimuli. As such, galectin-1 may be associated with
anatomical and functional deficits in the development of neurons
(14). Additionally, galectins are
also involved in post-developmental processes, including immune
regulation (10).
Roles of galectins in inflammation and
immune responses
The regulation of inflammatory and immune responses
is a major function of galectins. Numerous types of blood cells
express galectins, including activated T and B cells, regulatory T
cells, dendritic cells, mast cells, monocytes/macrophages,
eosinophils and neutrophils. Depending on various inflammatory
stimuli, the microenvironment and the target cells, galectins are
associated with pro- or anti-inflammatory responses (10). In a study of autoimmune retinal
disease, galectin-1 was demonstrated to be able to induce
anti-inflammatory cytokines, including interleukin (IL)-5 and
IL-10, and to suppress the expression of interferon γ, ultimately
suppressing the inflammatory response (15). Additionally, galectin-3 serves an
important role in the activation of T cells, potentially associated
with its interaction with poly-N-acetyllactosamine-containing
N-glycans on T-cell receptors (16).
In certain immune cells, particularly eosinophils, the addition of
galectin-3 leads to the suppressed production of IL-5 by
downregulation of the expression of the IL-5 gene (17). Other blood cells, including mast
cells, neutrophils, lymphocytes and monocytes, are activated by
galectin-3 (18). In the function of
mast cells, endogenous galectin-3 is involved in the phagocytosis
of macrophages and the promotion of inflammation in the airway
(19).
Roles of galectins in cancer
Galectins contribute to carcinogenesis
and cancer progression
Protein-glycan interactions are important in the
progression of cancer, including being involved in carcinogenesis,
proliferation, metastasis, angiogenesis and the immune response
(20). Galectins are modulated during
the differentiation of cells and the development of organs; errors
in this modulation occur in a number of physiological and
pathological conditions (21).
Additionally, galectins serve a role in tumor transformation and
survival, having been demonstrated to be involved in tumorigenesis,
the regulation of apoptosis, tumor invasion and angiogenesis, the
adhesion of cells during metastasis and the tumor immune response
(21). Each galectin exhibits
distinct effects in different cancer types. Previous studies have
demonstrated that galectin-1 and −3 promote pro-tumorigenic
activity by binding to the ligands of cancer cells (22), potentially promoting the progression
of distinct types of cancer (6).
These pro-tumorigenic activities may include affecting tumor
growth, metastasis and angiogenesis, and the promotion of radio-
and chemo-resistance, while galactin-1 and −3 also suppress T cell
function to affect the tumor microenvironment (6). Other galectins also contribute to the
progression of various types of cancer, for example galectin-7
promotes metastatic potential and apoptosis-resistance in breast
cancer (23), and is overexpressed in
esophageal squamous cell carcinoma (24). Notably, increased galectin-7
expression is associated with improved outcome following
radiotherapy for cervical cancer (25) and serves a tumor-suppressive role in
gastric cancer (26). In addition,
galectin-4 is important in the metastatic potential of lung
adenocarcinoma (27) and may also
prevent detachment of pancreatic cancer cells through its adhesion
molecule-like function (11).
Galectin-9 is a good prognostic factor in breast cancer due to its
anti-metastatic potential (12).
Galectin-2, −4 and −8 may increase adhesion of colon cancer cells
to endothelial cells, enhancing the metastasis of colon cancer
(28).
Galectin expression in cancerous
tissues
Expression of galectins is observed in numerous
types of cancer tissue, including cancer of the digestive,
reproductive, respiratory, neural, urinary and hematological
systems (7). The galectins most
extensively studied in oncology are galectin-1, −3, −4, −7, −8 and
−9, with numerous studies comparing the expression levels of
galectins between normal and malignant tissues. Notably, each
galectin may exhibit increased expression in distinct types of
cancer, but decreased expression in others; similarly, a specific
cancer may have increased expression levels of certain galectins,
but decreased expression levels of others (7). For example, galectin-1 expression is
increased in the majority of cancer types (7,29),
although certain studies have revealed decreased galectin-1
expression in malignant tissues of bladder cancer (30), uterine cancer (31) and head and neck squamous cell
carcinoma (13), whereas other
studies have observed increased galectin-1 expression in these same
types of cancer (14–16). The expression of galectin-3 is also
heterogeneous, with increased expression levels identified in
thyroid cancer, lung cancer, and the majority of digestive tract
and urinary system tumors, whereas decreased expression levels have
been observed in reproductive system tumors (7). Studying the expression of galectin-8 and
−9 in cancer is complicated due to extensive splicing of their mRNA
transcripts, generating various isoforms (7). As a result, they may exhibit decreased
mRNA levels (17), but increased
protein levels (18).
Galectin expression and cancer
prognosis
Much effort has been made to determine the
association between galectin expression level and cancer prognosis.
Irrespective of the cancer type, increased expression of galectin-1
is generally associated with poor overall and disease-free survival
rates (7). By contrast, studies of
galectin-3 for the prediction of cancer prognosis revealed various
results, mostly depending on the cancer types. Increased expression
of galectin-3 in brain cancer (32),
acute myeloid leukemia (33) and
colorectal cancer (34) is associated
with poorer overall survival times and rates, while increased
expression of galectin-3 is associated with improved survival rate
in gastric cancer (19). In addition
to predicting cancer prognosis, the levels of galectins are also
associated with the stages and grades in different cancer types. A
positive correlation between the expression level of galectin-1 and
cancer stage has been observed in gastric cancer (35), ovarian cancer (36) and chronic lymphocytic leukemia
(37). In colon cancer, the
expression of galectin-3 has proven to be correlated with the stage
of colon cancer (38). A positive
correlation between the expression of galectin-3 and tumor grade
has also been reported (39).
Galectins as diagnostic
biomarkers
As aforementioned, cancer typically exhibits altered
galectin expression. Since galectins are secreted, their
circulating levels may be taken as useful biomarkers to predict the
presence of malignancies. For example, a previous study revealed
that serum galectin-1 levels were increased in patients with lung
cancer compared with healthy subjects, as well as increased mRNA
expression levels of galectin-1 in tumor sections compared with
non-tumor sections (40). The
circulating levels and tissue levels of galectin-1 and −3 are
increased in thyroid cancer patients (41), with similar results observed in
clinical cases such as that for galectin-3 expression in melanoma
(42) and galectin-8 expression in
breast cancer (28). However, a study
of thyroid tumors has demonstrated a dissociation between
circulating galectin levels and tissue galectin levels (43). These observed discrepancies in the
literature may be due to a number of reasons, including the
non-classical secretion pathways of galectins and the abnormal
activity of cellular signaling pathways in tumor cells (7).
Galectins as biomarkers for prognosis
and treatment response
Circulating galectins may also be useful biomarkers
for predicting cancer prognosis and treatment response. For
example, in Hodgkin's lymphoma, the circulating galectin-1
expression level is well correlated with tumor burden, clinical
staging and other prognostic markers (44). Similarly, circulating galectin-3
expression level is a valuable prognostic marker in patients with
advanced melanoma (45). In breast
and gastrointestinal cancer, serum galectin-3 expression level is
significantly different between patients with metastatic and
non-metastatic diseases (46). In
colon cancer, serum galectin-3 and galectin-4 expression levels are
distinct between patients with metastatic and non-metastatic
disease (47).
Serum galectins are also useful for monitoring
therapeutic response in patients with cancer. In a previous study,
serum galectin-3 expression level decreased significantly following
surgery in patients with pancreatic cancer (48). Serum levels of galectin-1, −3 and −4
were increased in patients with colorectal cancer, and their serum
levels of galectin-1 and −4 decreased significantly following
curative surgery (49). In head and
neck squamous cell carcinoma, galectin-1 and galectin-3 also
decreased following surgical and chemotherapeutic treatments
(50).
Taken together, circulating galectin levels may be
potential biomarkers for detecting cancer, and for predicting
disease prognosis and therapeutic efficacy (7).
Roles of galectins in lung cancer
Overview
Lung cancer is one of the most common types of
cancer, as well as being the leading cause of cancer-associated
mortality worldwide (51,52). Lung cancer is classified as either
non-small cell lung cancer (NSCLC) or SCLC. NSCLC is traditionally
classified into squamous cell carcinoma, large cell carcinoma,
adenocarcinoma and other less frequently observed cell types
(53). Although NSCLC is often
attributed to cigarette smoking, adenocarcinoma is often observed
in individuals who have never smoked. Numerous compounds and
molecular mechanisms are involved in the formation and progression
of lung cancer; as for other cancer types, galectins serve
important roles in these processes (6). Previous studies have demonstrated that
galectin-1, −3, −4, −7, −8 and −9 are associated with lung cancer
(Table I) (27,40,54–62),
whereas the roles of galectin-2, 5, −6, −10, −11, −12, −13, −14 and
−15 in lung cancer are, to the best of our knowledge, yet to be
investigated.
 | Table I.Effects of each galectin identified
to affect lung cancer progression. |
Table I.
Effects of each galectin identified
to affect lung cancer progression.
| Galectin | Type | Effects in lung
cancer (Reference) |
|---|
| Galectin-1 | Dimeric |
Galectin-1/interleukin-10 functional axis
may be an important regulator in lung cancer-mediated immune
suppression (40). |
|
|
| Upregulates and
promotes migration and invasion (54). |
|
|
| Lymph node
metastasis of lung cancer (54). |
|
|
| Mediates tumor
progression and chemoresistance of non-small cell lung cancer
(55). |
| Galectin-3 | Chimeric | Enhances lung
cancer adhesion to extracellular matrix components, cell motility
and in vitro invasiveness (56). |
|
|
| Promotes cancer
stem cell formation, chemoresistance, tumorigenicity, tumor
initiation and sphere-forming capacity (57). |
|
|
| Promotes epidermal
growth factor receptor activation and enhances lung cancer stemness
through the EGFR/c-Myc/Sox2 axis (58). |
| Galectin-4 | Tandem | Expressed in the
cytoplasm, nucleus and membrane of lung adenocarcinomas (27). |
|
|
| May be an
independent predictor for lymph node metastasis (27). |
| Galectin-7 | Dimeric | May be associated
with the metastasis of other types of cancer to the lung (59). |
| Galectin-8 | Tandem | Associated with
metastatic progression of lung cancer (60). |
| Galectin-9 | Tandem | Suppresses
pulmonary metastasis by tumor cell, tumor attachment and tumor
invasion (61). |
|
|
| Promotes activation
of natural killer cells (62). |
Galectin-1
Galectin-1 was the first identified galectin and it
is overexpressed in numerous types of cancer, including lymphoma,
astrocytoma and melanoma, as well as oral, colon, liver,
pancreatic, bladder and ovarian cancers (63). A previous study demonstrated that the
galectin-1/IL-10 functional axis may be an important regulator in
lung cancer-mediated immune suppression (40). Galectin-1 is an immune modulator of
the development of monocytes, which is controlled via IL-10 and
granulocyte-colony stimulating factor (64). Galectin-1 is overexpressed in
highly-invasive lung cancer cell lines and is important in
promoting lung cancer cell invasion and migration (5). Galectin-1 derived from lung cancer
modifies dendritic cells to produce mitogenic and pro-invasive
factors, which subsequently promote tumorigenic potentiation of
dendritic cells by expressing heparin-binding epidermal growth
factor (EGF)-like growth factor (65). Galectin-1 also possesses the potential
to be a prognostic marker in early-stage NSCLC (66). Patients with tumors exhibiting
increased galectin-1 expression experience poorer clinical outcomes
(67). Galectin-1 is upregulated in
CD133+ lung adenocarcinoma cells, occasionally
designated as cancer stem cells, which promotes the growth of
CD133+ lung adenocarcinoma (54). Serum galectin-1 level is positively
correlated to the stage and prognosis of patients with lung
adenocarcinoma (54), and has also
been revealed to mediate the tumor progression and chemo-resistance
of NSCLCs by activation of p38 mitogen-activated protein kinase,
extracellular signal-regulated kinase and
prostaglandin-endoperoxidase synthase 2 (55). In NSCLC, tumor-associated galectin-1
secretion mediates radiotherapy-associated systemic lymphopenia and
may affect tumor progression (68).
Galectin-3
The role of galectin-3 in lung cancer has also been
extensively studied. Expression of galectin-3 has been observed
extensively in normal lung cells, including bronchial epithelial
cells, chondrocytes of the bronchial cartilage, pneumocytes of the
alveolar wall, alveolar macrophages and interstitial fibroblasts
(69). NSCLC generally possesses
significantly increased galectin-3 expression compared with SCLC
(3). Galectin-3 was found in
extracellular and intracellular compartments of NSCLC; in squamous
cell carcinoma, galectin-3 was primarily identified in the
cytoplasm, whereas it was observed in the cytoplasm and nucleus in
adenocarcinoma (3). Overexpression of
galectin-3 in DLKP lung cancer cells enhances their adhesion to
extracellular matrix components, increasing cell motility and
invasiveness (56). Overexpression of
galectin-3 in A549 cells promotes their sphere-forming capacity (a
character of cancer stem cells), and suppression of galectin-3 in
H1299 cells in vitro decreases the expression of
stemness-associated genes, their sphere-forming capacity,
tumorigenicity, chemo-resistance; in vivo, suppression of
galectin-3 in H1299 cells also decreases tumor-initiating capacity
in NOD/SCID mice (57). In Lewis lung
carcinoma-bearing mice, increased expression of galectin-3 may
promote the migration of myeloid-derived suppressor cells to the
tumor microenvironment following exposure to cisplatin,
contributing to the immuno-suppressive status of the tumor
microenvironment (70). Galectin-3
may also be used as a prognostic marker; nuclear galectin-3
expression has been revealed as an independent predictive factor
for the recurrence of NSCLC (55).
Detection of galectin-3 and CD82 may be associated with the
initiation, development and metastasis of NSCLC (71). Galectin-3 has also been demonstrated
to promote EGF receptor activation in lung cancer, leading to the
upregulation of sex determining region Y-box 2 expression and
sphere formation (58).
Galectin-4
In contrast to its almost exclusive distribution in
the cytoplasm of breast cancer and colon cancer cells, galectin-4
is extensively detected in the cytoplasm, nucleus and membranes of
lung adenocarcinoma (72). In lung
adenocarcinoma, the expression of galectin-4 is associated with
clinicopathological variables of disease progression, including
tumor size, nodal status, pleural or venous invasion, and
tumor-node-metastasis staging, and may be an independent predictor
for lymph node metastasis of intermediate power (27). Unlike gastric carcinoid tumors, which
exhibited increased galectin-4 expression, a limited number of
pulmonary carcinoids possessed decreased galectin-4 expression
(73).
Galectin-7
With regard to cancer progression, galectin-7 has
distinct functions in various types of cancer. Galectin-7 has been
revealed to be associated with the sensitivity of human cervical
carcinoma cells to chemotherapy-induced apoptosis (74). In melanoma, galectin-7 has been found
in the primary site and the lung metastasis site (75). Although no convincing evidence has
demonstrated that galectin-7 is directly associated with lung
cancer, it may be associated with cancer metastasis to the lung;
for example, it has been demonstrated to promote the bone and lung
metastasis of breast cancer (59).
Galectin-8
Human galectin-8, encoded from the lectin
galactoside-binding soluble 8 gene, has a variety of isoforms as a
result of alternative splicing. Galectin-8 was initially isolated
from the prostate [prostate carcinoma tumor antigen (PCTA)-1] and
lung [Po66 carbohydrate-binding protein (Po66-CBP)] (76–78).
PCTA-1 and Po66-CBP share 98.7% of their amino acid sequence
(79). Galetin-8 is widely expressed
in normal tissue, as well as in tumor tissue (79); however, it is overexpressed in
squamous cell carcinoma, with its expression level associated with
the degree of tumor differentiation (76,80). Using
cell-extracellular matrix microarrays, galectin-8, in combination
with fibronectin, has been demonstrated to be associated with the
metastatic progression of lung adenocarcinoma (60). The marked difference in galectin-8
expression levels between lung cancer cells and normal lung tissues
may provide the opportunity to use monoclonal antibodies for
preventing and treating lung cancer (79,81).
Galectin-9
A limited number of studies have investigated the
role of galectin-9 in lung cancer, however, it has been widely
studied in the field of immunity and inflammation. Increased
cytoplasmic galectin-9 expression in tumor cells may suppress
pulmonary metastasis and recurrence of malignant melanoma and
breast cancer, which may be associated with the suppressive effect
of galectin-9 on the attachment and invasion of tumor cells
(61). Galectin-9 is also important
in anticancer immunity. For example, galectin-9 has been revealed
to increase T-cell immunoglobulin and mucin-domain containing
(TIM)-3+ dendritic cells and CD8+ cytotoxic T
cells, and to enhance anticancer immunity through its interaction
with TIM-3 in MethA cell-bearing mice (82). In a study using Lewis lung carcinoma
cell-bearing mice, galectin-9 was demonstrated to induce macrophage
differentiation into plasmacytoid dendritic cell-like macrophages,
which may augment the activation of NK cells that prolong the
survival of tumor-bearing mice (62).
Conclusions and future perspectives
Each galectin possesses specific functions and is
observed at distinct locations. Galectins serve important roles in
cell survival, cellular proliferation, adhesion and migration
(83), and they also participate in
cell-cell and cell-matrix interactions (84). These characteristics may suggest that
galectins may be an important component of the tumor
microenvironment. The tumor microenvironment has been recognized as
an important contributor to cancer progression, chemo-resistance
(85), invasion and metastasis
(86). The majority of galectins have
been revealed to be associated with lung cancer, including
galectin-1, −3, −4, −7 and −8, which are associated with the
progression of lung cancer, while galectin-9 may decrease
metastasis and enhance anticancer immunity. However, with the
exception of galectin-1 and −3, the roles of galectins in lung
cancer are not fully understood and require further investigation.
The roles of galectins in lung cancer are much more complicated
than suggested thus far, since numerous types of cell, particularly
immune cells, in the tumor microenvironment may participate in
their interactions (Fig. 3). Future
studies to improve our understanding of the roles of galectins in
the carcinogenesis, progression, invasion, metastasis and
chemo-resistance of lung cancer are required in order to identify
potential therapeutic targets for novel anticancer treatments.
Acknowledgements
The present study was supported by the Ministry of
Science and Technology (grant no. MOST 104-2320-B-037-014-MY3) and
the Kaohsiung Medical University ‘Aim for the Top Journals Grant’
(grant no. KMU-DT106005).
References
|
1
|
Barondes SH, Cooper DN, Gitt MA and
Leffler H: Galectins. Structure and function of a large family of
animal lectins. J Biol Chem. 269:20807–20810. 1994.PubMed/NCBI
|
|
2
|
Powell JT and Whitney PL: Postnatal
development of rat lung. Changes in lung lectin, elastin,
acetylcholinesterase and other enzymes. Biochem J. 188:1–8. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buttery R, Monaghan H, Salter DM and Sethi
T: Galectin-3: Differential expression between small-cell and
non-small-cell lung cancer. Histopathology. 44:339–344. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cummings RD and Liu FT: Chapter 33
GalectinsEssentials of Glycobiology. 2nd edition. Varki A, Cummings
RD, Esko JD, et al: Cold Spring Harbor Laboratory Press; La Jolla,
California: 2009
|
|
5
|
Hsu YL, Wu CY, Hung JY, Lin YS, Huang MS
and Kuo PL: Galectin-1 promotes lung cancer tumor metastasis by
potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway.
Carcinogenesis. 34:1370–1381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebrahim AH, Alalawi Z, Mirandola L,
Rakhshanda R, Dahlbeck S, Nguyen D, Jenkins M, Grizzi F, Cobos E,
Figueroa JA and Chiriva-Internati M: Galectins in cancer:
Carcinogenesis, diagnosis and therapy. Ann Transl Med.
2:882014.PubMed/NCBI
|
|
7
|
Thijssen VL, Heusschen R, Caers J and
Griffioen AW: Galectin expression in cancer diagnosis and
prognosis: A systematic review. Biochim Biophys Acta. 1855:235–247.
2015.PubMed/NCBI
|
|
8
|
Elola MT, Wolfenstein-Todel C, Troncoso
MF, Vasta GR and Rabinovich GA: Galectins: Matricellular
glycan-binding proteins linking cell adhesion, migration and
survival. Cell Mol Life Sci. 64:1679–1700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Compagno D, Jaworski FM, Gentilini L,
Contrufo G, Pérez González I, Elola MT, Pregi N, Rabinovich GA and
Laderach DJ: Galectins: Major signaling modulators inside and
outside the cell. Curr Mol Med. 14:630–651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varki A, Cummings RD, Esko JD, Freeze HH,
Stanley P, Bertozzi CR, Hart GW and Etzler ME: Essentials of
Glycobiology. 2nd. (eds). Cold Spring Harbor Laboratory Press; Cold
Spring Harbor (NY): 2019
|
|
11
|
Belo AI, van der Sar AM, Tefsen B and van
Die I: Galectin-4 reduces migration and metastasis formation of
pancreatic cancer cells. PLoS One. 8:e659572013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Irie A, Yamauchi A, Kontani K, Kihara M,
Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H and
Hirashima M: Galectin-9 as a prognostic factor with antimetastatic
potential in breast cancer. Clin Cancer Res. 11:2962–2968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choufani G, Nagy N, Saussez S, Marchant H,
Bisschop P, Burchert M, Danguy A, Louryan S, Salmon I, Gabius HJ,
et al: The levels of expression of galectin-1, galectin-3, and the
Thomsen-Friedenreich antigen and their binding sites decrease as
clinical aggressiveness increases in head and neck cancers. Cancer.
86:2353–2363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gillenwater A, Xu XC, el-Naggar AK,
Clayman GL and Lotan R: Expression of galectins in head and neck
squamous cell carcinoma. Head Neck. 18:422–432. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cindolo L, Benvenuto G, Salvatore P, Pero
R, Salvatore G, Mirone V, Prezioso D, Altieri V, Bruni CB and
Chiariotti L: Galectin-1 and galectin-3 expression in human bladder
transitional-cell carcinomas. Int J Cancer. 84:39–43. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van den Brule FA, Buicu C, Berchuck A,
Bast RC, Deprez M, Liu FT, Cooper DN, Pieters C, Sobel ME and
Castronovo V: Expression of the 67-kD laminin receptor, galectin-1,
and galectin-3 in advanced human uterine adenocarcinoma. Hum
Pathol. 27:1185–1191. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Zhu L, Cai Y, Suo J and Jin J:
Role of downregulation of galectin-9 in the tumorigenesis of
gastric cancer. Int J Oncol. 45:1313–1320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia
Z, Wang YP, Suo J and Cao X: Decreased galectin-9 and increased
Tim-3 expression are related to poor prognosis in gastric cancer.
PLoS One. 8:e817992013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okada K, Shimura T, Suehiro T, Mochiki E
and Kuwano H: Reduced galectin-3 expression is an indicator of
unfavorable prognosis in gastric cancer. Anticancer Res.
26:1369–1376. 2006.PubMed/NCBI
|
|
20
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu FT and Rabinovich GA: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dimitroff CJ: Galectin-Binding
O-glycosylations as regulators of malignancy. Cancer Res.
75:3195–3202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Campion CG, Labrie M, Lavoie G and
St-Pierre Y: Expression of galectin-7 is induced in breast cancer
cells by mutant p53. PLoS One. 8:e724682013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu X, Ding M, Yu ML, Feng MX, Tan LJ and
Zhao FK: Identification of galectin-7 as a potential biomarker for
esophageal squamous cell carcinoma by proteomic analysis. BMC
Cancer. 10:2902010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai CJ, Sulman EP, Eifel PJ, Jhingran A,
Allen PK, Deavers MT and Klopp AH: Galectin-7 levels predict
radiation response in squamous cell carcinoma of the cervix.
Gynecol Oncol. 131:645–649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SJ, Hwang JA, Ro JY, Lee YS and Chun
KH: Galectin-7 is epigenetically-regulated tumor suppressor in
gastric cancer. Oncotarget. 4:1461–1471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi T, Saito T, Fujimura T, Hara K,
Takamochi K, Mitani K, Mineki R, Kazuno S, Oh S, Ueno T, et al:
Galectin-4, a novel predictor for lymph node metastasis in lung
adenocarcinoma. PLoS One. 8:e818832013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barrow H, Guo X, Wandall HH, Pedersen JW,
Fu B, Zhao Q, Chen C, Rhodes JM and Yu LG: Serum galectin-2, −4 and
−8 are greatly increased in colon and breast cancer patients and
promote cancer cell adhesion to blood vascular endothelium. Clin
Cancer Res. 17:7035–7046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding YM, Dong JH, Chen LL and Zhang HD:
Increased expression of galectin-1 is associated with human oral
squamous cell carcinoma development. Oncol Rep. 21:983–987.
2009.PubMed/NCBI
|
|
30
|
Chung H, Kim B, Jung SH, Won KJ, Jiang X,
Lee CK, Lim SD, Yang SK, Song KH and Kim HS: Does phosphorylation
of cofilin affect the progression of human bladder cancer? BMC
Cancer. 13:452013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Makino K, Kawamura K, Sato W, Kawamura N,
Fujimoto T and Terada Y: Inhibition of uterine sarcoma cell growth
through suppression of endogenous tyrosine kinase B signaling. PLoS
One. 7:e410492012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Camby I, Belot N, Rorive S, Lefranc F,
Maurage CA, Lahm H, Kaltner H, Hadari Y, Ruchoux MM, Brotchi J, et
al: Galectins are differentially expressed in supratentorial
pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and
glioblastomas and significantly modulate tumor astrocyte migration.
Brain Pathol. 11:12–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng CL, Hou HA, Lee MC, Liu CY, Jhuang
JY, Lai YJ, Lin CW, Chen HY, Liu FT, Chou WC, et al: Higher bone
marrow LGALS3 expression is an independent unfavorable prognostic
factor for overall survival in patients with acute myeloid
leukemia. Blood. 121:3172–3180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Endo K, Kohnoe S, Tsujita E, Watanabe A,
Nakashima H, Baba H and Maehara Y: Galectin-3 expression is a
potent prognostic marker in colorectal cancer. Anticancer Res.
25:3117–3121. 2005.PubMed/NCBI
|
|
35
|
Chen J, Zhou SJ, Zhang Y, Zhang GQ, Zha
TZ, Feng YZ and Zhang K: Clinicopathological and prognostic
significance of galectin-1 and vascular endothelial growth factor
expression in gastric cancer. World J Gastroenterol. 19:2073–2079.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim HJ, Jeon HK, Cho YJ, Park YA, Choi JJ,
Do IG, Song SY, Lee YY, Choi CH, Kim TJ, et al: High galectin-1
expression correlates with poor prognosis and is involved in
epithelial ovarian cancer proliferation and invasion. Eur J Cancer.
48:1914–1921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Croci DO, Morande PE, Dergan-Dylon S,
Borge M, Toscano MA, Stupirski JC, Bezares RF, Avalos JS, Narbaitz
M, Gamberale R, et al: Nurse-like cells control the activity of
chronic lymphocytic leukemia B cells via galectin-1. Leukemia.
27:1413–1416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakamura M, Inufusa H, Adachi T, Aga M,
Kurimoto M, Nakatani Y, Wakano T, Nakajima A, Hida JI, Miyake M, et
al: Involvement of galectin-3 expression in colorectal cancer
progression and metastasis. Int J Oncol. 15:143–148.
1999.PubMed/NCBI
|
|
39
|
Strik HM, Deininger MH, Frank B,
Schluesener HJ and Meyermann R: Galectin-3: Cellular distribution
and correlation with WHO-grade in human gliomas. J Neurooncol.
53:13–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuo PL, Hung JY, Huang SK, Chou SH, Cheng
DE, Jong YJ, Hung CH, Yang CJ, Tsai YM, Hsu YL and Huang MS: Lung
cancer-derived galectin-1 mediates dendritic cell anergy through
inhibitor of DNA binding 3/IL-10 signaling pathway. J Immunol.
186:1521–1530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Saussez S, Glinoer D, Chantrain G, Pattou
F, Carnaille B, André S, Gabius HJ and Laurent G: Serum galectin-1
and galectin-3 levels in benign and malignant nodular thyroid
disease. Thyroid. 18:705–712. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vereecken P, Boudjeltia Zouaoui K, Debray
C, Awada A, Legssyer I, Sales F, Petein M, Vanhaeverbeek M, Ghanem
G and Heenen M: High serum galectin-3 in advanced melanoma:
Preliminary results. Clin Exp Dermatol. 31:105–109. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Išić T, Savin S, Cvejić D, Marečko I,
Tatić S, Havelka M and Paunović I: Serum Cyfra 21.1 and galectin-3
protein levels in relation to immunohistochemical cytokeratin 19
and galectin-3 expression in patients with thyroid tumors. J Cancer
Res Clin Oncol. 136:1805–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ouyang J, Plütschow A, von Strandmann
Pogge E, Reiners KS, Ponader S, Rabinovich GA, Neuberg D, Engert A
and Shipp MA: Galectin-1 serum levels reflect tumor burden and
adverse clinical features in classical Hodgkin lymphoma. Blood.
121:3431–3433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vereecken P, Awada A, Suciu S, Castro G,
Morandini R, Litynska A, Lienard D, Ezzedine K, Ghanem G and Heenen
M: Evaluation of the prognostic significance of serum galectin-3 in
American Joint Committee on Cancer stage III and stage IV melanoma
patients. Melanoma Res. 19:316–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Iurisci I, Tinari N, Natoli C, Angelucci
D, Cianchetti E and Iacobelli S: Concentrations of galectin-3 in
the sera of normal controls and cancer patients. Clin Cancer Res.
6:1389–1393. 2000.PubMed/NCBI
|
|
47
|
Barrow H, Rhodes JM and Yu LG:
Simultaneous determination of serum galectin-3 and −4 levels
detects metastases in colorectal cancer patients. Cell Oncol
(Dordr). 36:9–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gaida MM, Bach ST, Günther F, Baseras B,
Tschaharganeh DF, Welsch T, Felix K, Bergmann F, Hänsch GM and
Wente MN: Expression of galectin-3 in pancreatic ductal
adenocarcinoma. Pathol Oncol Res. 18:299–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Watanabe M, Takemasa I, Kaneko N, Yokoyama
Y, Matsuo E, Iwasa S, Mori M, Matsuura N, Monden M and Nishimura O:
Clinical significance of circulating galectins as colorectal cancer
markers. Oncol Rep. 25:1217–1226. 2011.PubMed/NCBI
|
|
50
|
Saussez S, Lorfevre F, Lequeux T, Laurent
G, Chantrain G, Vertongen F, Toubeau G, Decaestecker C and Kiss R:
The determination of the levels of circulating galectin-1 and −3 in
HNSCC patients could be used to monitor tumor progression and/or
responses to therapy. Oral Oncol. 44:86–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tsai MJ, Yang CJ, Kung YT, Sheu CC, Shen
YT, Chang PY, Huang MS and Chiu HC: Metformin decreases lung cancer
risk in diabetic patients in a dose-dependent manner. Lung Cancer.
86:137–143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Y, Wang H, Wang J, Bao L, Wang L,
Huo J and Wang X: Global analysis of chromosome 1 genes among
patients with lung adenocarcinoma, squamous carcinoma, large-cell
carcinoma, small-cell carcinoma, or non-cancer. Cancer Metastasis
Rev. 34:249–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou X, Li D, Wang X, Zhang B, Zhu H and
Zhao J: Galectin-1 is overexpressed in CD133+ human lung
adenocarcinoma cells and promotes their growth and invasiveness.
Oncotarget. 6:3111–3122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chung LY, Tang SJ, Sun GH, Chou TY, Yeh
TS, Yu SL and Sun KH: Galectin-1 promotes lung cancer progression
and chemoresistance by upregulating p38 MAPK, ERK, and
cyclooxygenase-2. Clin Cancer Res. 18:4037–4047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
O'Driscoll L, Linehan R, Liang YH, Joyce
H, Oglesby I and Clynes M: Galectin-3 expression alters adhesion,
motility and invasion in a lung cell line (DLKP), in vitro.
Anticancer Res. 22:3117–3125. 2002.PubMed/NCBI
|
|
57
|
Chung LY, Tang SJ, Wu YC, Sun GH, Liu HY
and Sun KH: Galectin-3 augments tumor initiating property and
tumorigenicity of lung cancer through interaction with β-catenin.
Oncotarget. 6:4936–4952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kuo HY, Hsu HT, Chen YC, Chang YW, Liu FT
and Wu CW: Galectin-3 modulates the EGFR signalling-mediated
regulation of Sox2 expression via c-Myc in lung cancer.
Glycobiology. 26:155–165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Demers M, Rose AA, Grosset AA, Biron-Pain
K, Gaboury L, Siegel PM and St-Pierre Y: Overexpression of
galectin-7, a myoepithelial cell marker, enhances spontaneous
metastasis of breast cancer cells. Am J Pathol. 176:3023–3031.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Reticker-Flynn NE, Malta DF, Winslow MM,
Lamar JM, Xu MJ, Underhill GH, Hynes RO, Jacks TE and Bhatia SN: A
combinatorial extracellular matrix platform identifies
cell-extracellular matrix interactions that correlate with
metastasis. Nat Commun. 3:11222012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nobumoto A, Nagahara K, Oomizu S, Katoh S,
Nishi N, Takeshita K, Niki T, Tominaga A, Yamauchi A and Hirashima
M: Galectin-9 suppresses tumor metastasis by blocking adhesion to
endothelium and extracellular matrices. Glycobiology. 18:735–744.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kadowaki T, Arikawa T, Shinonaga R, Oomizu
S, Inagawa H, Soma G, Niki T and Hirashima M: Galectin-9 signaling
prolongs survival in murine lung-cancer by inducing macrophages to
differentiate into plasmacytoid dendritic cell-like macrophages.
Clin Immunol. 142:296–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Danguy A, Camby I and Kiss R: Galectins
and cancer. Biochim Biophys Acta. 1572:285–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cheng DE, Chang WA, Hung JY, Huang MS and
Kuo PL: Involvement of IL10 and granulocyte colonystimulating
factor in the fate of monocytes controlled by galectin-1. Mol Med
Rep. 10:2389–2394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kuo PL, Huang MS, Cheng DE, Hung JY, Yang
CJ and Chou SH: Lung cancer-derived galectin-1 enhances tumorigenic
potentiation of tumor-associated dendritic cells by expressing
heparin-binding EGF-like growth factor. J Biol Chem. 287:9753–9764.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Schulkens IA, Heusschen R, van den
Boogaart V, van Suylen RJ, Dingemans AM, Griffioen AW and Thijssen
VL: Galectin expression profiling identifies galectin-1 and
Galectin-9Δ5 as prognostic factors in stage I/II non-small cell
lung cancer. PLoS One. 9:e1079882014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Carlini MJ, Roitman P, Nuñez M, Pallotta
MG, Boggio G, Smith D, Salatino M, Joffé ED, Rabinovich GA and
Puricelli LI: Clinical relevance of galectin-1 expression in
non-small cell lung cancer patients. Lung Cancer. 84:73–78. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kuo P, Bratman SV, Shultz DB, von Eyben R,
Chan C, Wang Z, Say C, Gupta A, Loo BW Jr, Giaccia AJ, et al:
Galectin-1 mediates radiation-related lymphopenia and attenuates
NSCLC radiation response. Clin Cancer Res. 20:5558–5569. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mathieu A, Saal I, Vuckovic A, Ransy V,
Vereerstraten P, Kaltner H, Gabius HJ, Kiss R, Decaestecker C,
Salmon I and Remmelink M: Nuclear galectin-3 expression is an
independent predictive factor of recurrence for adenocarcinoma and
squamous cell carcinoma of the lung. Mod Pathol. 18:1264–1271.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang T, Chu Z, Lin H, Jiang J, Zhou X and
Liang X: Galectin-3 contributes to cisplatin-induced myeloid
derived suppressor cells (MDSCs) recruitment in Lewis lung
cancer-bearing mice. Mol Biol Rep. 41:4069–4076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wu SW, Yu L, Zhou L, Cheng ZN and Tao YS:
Expression of Gal-3 and CD82/KAI1 proteins in non-small cell lung
cancer and their clinical significance. Zhonghua Zhong Liu Za Zhi.
35:124–128. 2013.(In Chinese). PubMed/NCBI
|
|
72
|
Huflejt ME and Leffler H: Galectin-4 in
normal tissues and cancer. Glycoconj J. 20:247–255. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rumilla KM, Erickson LA, Erickson AK and
Lloyd RV: Galectin-4 expression in carcinoid tumors. Endocr Pathol.
17:243–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhu H, Pei HP, Zeng S, Chen J, Shen LF,
Zhong MZ, Yao RJ and Shen H: Profiling protein markers associated
with the sensitivity to concurrent chemoradiotherapy in human
cervical carcinoma. J Proteome Res. 8:3969–3976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Biron-Pain K, Grosset AA, Poirier F,
Gaboury L and St-Pierre Y: Expression and functions of galectin-7
in human and murine melanomas. PLoS One. 8:e633072013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Caulet-Maugendre S, Birolleau S, Corbineau
H, Bassen R, Desrues B, Bidon N, Delaval P, Ramée MP, Brichory F
and Dazord L: Immunohistochemical expression of the intracellular
component of galectin-8 in squamous cell metaplasia of the
bronchial epithelium in neoplastic and benign processes. Pathol Res
Pract. 197:797–801. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gopalkrishnan RV, Roberts T, Tuli S, Kang
D, Christiansen KA and Fisher PB: Molecular characterization of
prostate carcinoma tumor antigen-1, PCTA-1, a human galectin-8
related gene. Oncogene. 19:4405–4416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bassen R, Brichory F, Caulet-Maugendre S,
Bidon N, Delaval P, Desrues B and Dazord L: Expression of Po66-CBP,
a type-8 galectin, in different healthy, tumoral and peritumoral
tissues. Anticancer Res. 19:5429–5433. 1999.PubMed/NCBI
|
|
79
|
Bidon-Wagner N and Le Pennec JP: Human
galectin-8 isoforms and cancer. Glycoconj J. 19:557–563. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Henno S, Brichory F, Langanay T, Desrues
B, Bidon N, Delaval P, Ramee MP, Dazord L and Caulet-Maugendre S:
Expression of Po66-CBP, a galectin-8, in different types of primary
and secondary broncho-pulmonary tumors. Oncol Rep. 9:177–180.
2002.PubMed/NCBI
|
|
81
|
Dazord L, Bourel D, Martin A, Lecorre R,
Bourguet P, Bohy J, Saccavini JC, Delaval P, Louvet M and Toujas L:
A monoclonal antibody (Po66) directed against human lung squamous
cell carcinoma immunolocalization of tumour xenografts in nude
mice. Cancer Immunol Immunother. 24:263–268. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nagahara K, Arikawa T, Oomizu S, Kontani
K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, et
al: Galectin-9 increases Tim-3+ dendritic cells and
CD8+ T cells and enhances antitumor immunity via
galectin-9-Tim-3 interactions. J Immunol. 181:7660–7669. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Perillo NL, Marcus ME and Baum LG:
Galectins: Versatile modulators of cell adhesion, cell
proliferation and cell death. J Mol Med (Berl). 76:402–412. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hughes RC: Galectins as modulators of cell
adhesion. Biochimie. 83:667–676. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Heinrich EL, Walser TC, Krysan K, Liclican
EL, Grant JL, Rodriguez NL and Dubinett SM: The inflammatory tumor
microenvironment, epithelial mesenchymal transition and lung
carcinogenesis. Cancer Microenviron. 5:5–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Tsai MJ, Chang WA, Huang MS and Kuo PL:
Tumor microenvironment: A new treatment target for cancer. ISRN
Biochem. 2014:3519592014. View Article : Google Scholar : PubMed/NCBI
|