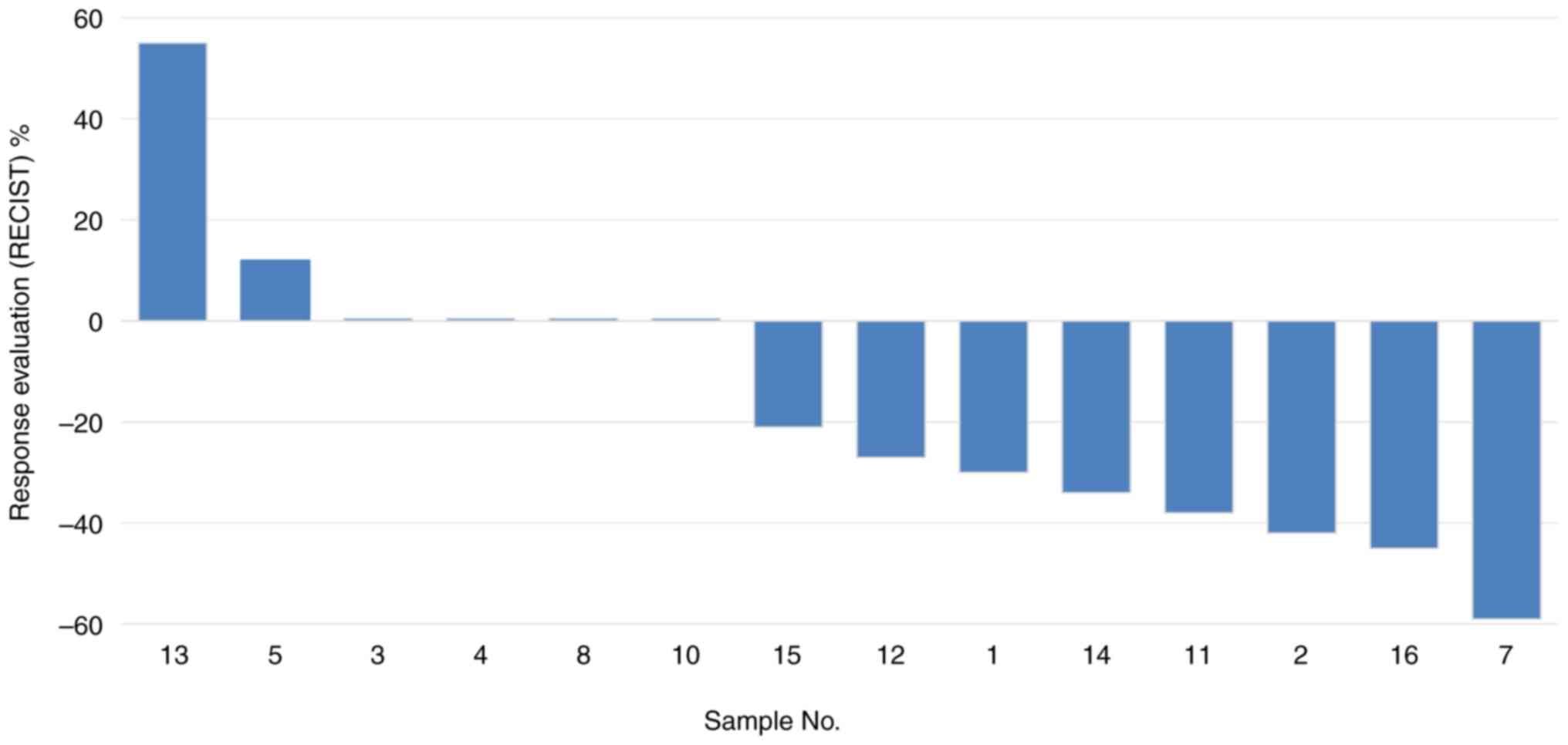
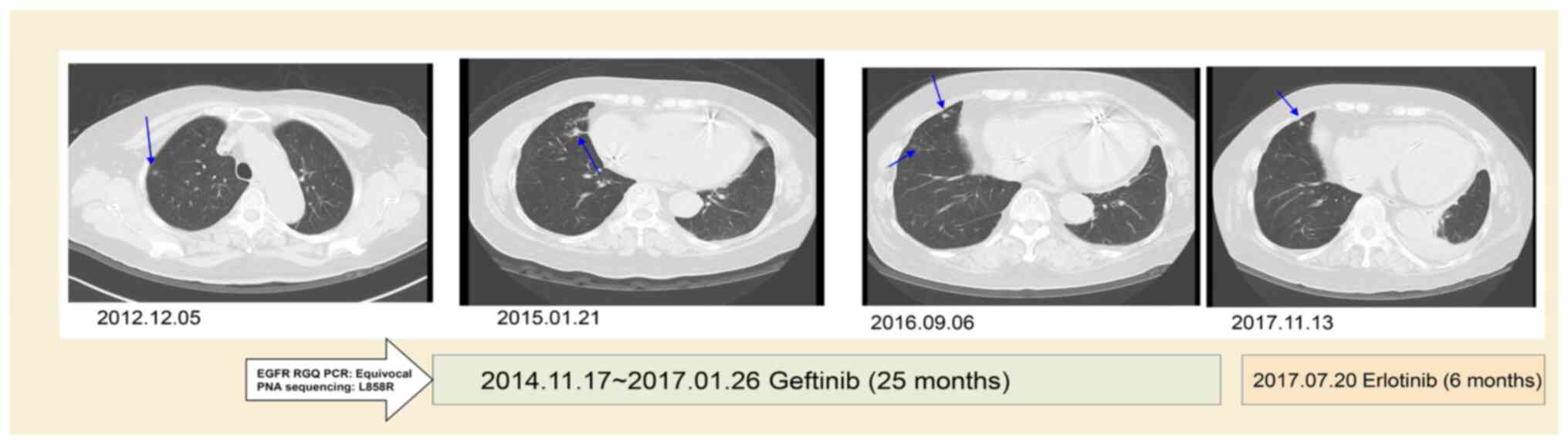
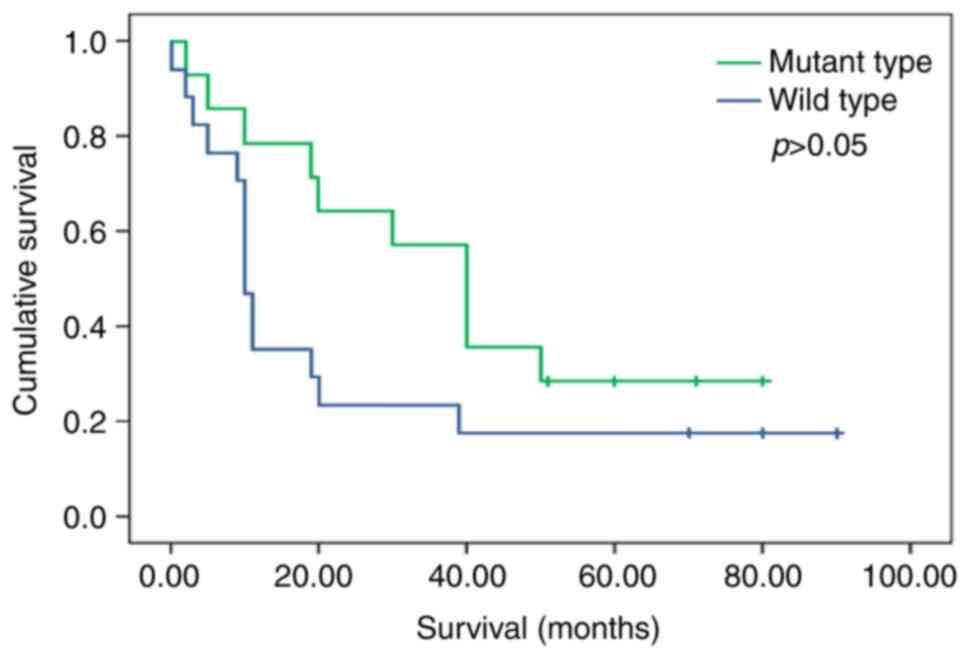
|
1
|
Midha A, Dearden S and McCormack R: EGFR
mutation incidence in non-small-cell lung cancer of adenocarcinoma
histology: A systematic review and global map by ethnicity
(mutMapII). Am J Cancer Res. 5:2892–2911. 2015.PubMed/NCBI
|
|
2
|
Hsu PC, Jablons DM, Yang CT and You L:
Epidermal growth factor receptor (EGFR) pathway, yes-associated
protein (YAP) and the regulation of programmed death-ligand 1
(PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci.
20:38212019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He J, Huang Z, Han L, Gong Y and Xie C:
Mechanisms and management of 3rd-generation EGFR-TKI resistance in
advanced non-small cell lung cancer (Review). Int J Oncol.
59:902021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gelatti ACZ, Drilon A and Santini FC:
Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in
epidermal growth factor receptor (EGFR) mutation-positive non-small
cell lung cancer (NSCLC). Lung Cancer. 137:113–122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo Y, Song J, Wang Y, Huang L, Sun L,
Zhao J, Zhang S, Jing W, Ma J and Han C: Concurrent genetic
alterations and other biomarkers predict treatment efficacy of
EGFR-TKIs in EGFR-mutant non-small cell lung cancer: A review.
Front Oncol. 10:6109232020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vallée A, Le Loupp AG and Denis MG:
Efficiency of the Therascreen® RGQ PCR kit for the
detection of EGFR mutations in non-small cell lung carcinomas. Clin
Chim Acta. 429:8–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiede C, Bayerdörffer E, Blasczyk R,
Wittig B and Neubauer A: Simple and sensitive detection of
mutations in the ras proto-oncogenes using PNA-mediated PCR
clamping. Nucleic Acids Res. 24:983–984. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sano R, Takahashi Y, Nakajima T, Yoshii M,
Kubo R, Takahashi K, Kominato Y, Takeshita H, Yasuda T, Tsuneyama
H, et al: ABO chimerism with a minor allele detected by the peptide
nucleic acid-mediated polymerase chain reaction clamping method.
Blood Transfus. 12:431–434. 2014.PubMed/NCBI
|
|
9
|
Chen YL, Lee CT, Lu CC, Yang SC, Chen WL,
Lee YC, Yang CH, Peng SL, Su WC, Chow NH and Ho CL: Epidermal
growth factor receptor mutation and anaplastic lymphoma kinase gene
fusion: Detection in malignant pleural effusion by RNA or PNA
analysis. PLoS One. 11:e01581252016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YL, Lu CC, Yang SC, Su WP, Lin YL,
Chen WL, Huang W, Su WC, Chow NH and Ho CL: Verification of
wild-type EGFR status in non-small cell lung carcinomas using a
mutant-enriched PCR on selected cases. J Mol Diagn. 16:486–494.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rotow J and Bivona TG: Understanding and
targeting resistance mechanisms in NSCLC. Nat Rev Cancer.
17:637–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeo WL, Riely GJ, Yeap BY, Lau MW, Warner
JL, Bodio K, Huberman MS, Kris MG, Tenen DG, Pao W, et al:
Erlotinib at a dose of 25 mg daily for non-small cell lung cancers
with EGFR mutations. J Thorac Oncol. 5:1048–1053. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan CS, Gilligan D and Pacey S: Treatment
approaches for EGFR-inhibitor-resistant patients with
non-small-cell lung cancer. Lancet Oncol. 16:e447–e459. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ricciardi S, Tomao S and de Marinis F:
Pemetrexed as first-line therapy for non-squamous non-small cell
lung cancer. Ther Clin Risk Manag. 5:781–787. 2009.PubMed/NCBI
|
|
17
|
Okamoto I, Aoe K, Kato T, Hosomi Y,
Yokoyama A, Imamura F, Kiura K, Hirashima T, Nishio M, Nogami N, et
al: Pemetrexed and carboplatin followed by pemetrexed maintenance
therapy in chemo-naive patients with advanced nonsquamous
non-small-cell lung cancer. Invest New Drugs. 31:1275–1282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeon SH, Kim HW, Kim BN, Kang N, Yeo CD,
Park CK, Kim YK, Lee YH, Kim TJ, Lee KY, et al: Comparison of PNA
clamping-assisted fluorescence melting curve analysis and PNA
clamping in detecting EGFR mutations in matched tumor tissue, cell
block, pleural effusion and blood of lung cancer patients with
malignant pleural effusion. In Vivo. 33:595–603. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fouz MF and Appella DH: PNA clamping in
nucleic acid amplification protocols to detect single nucleotide
mutations related to cancer. Molecules. 25:7862020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Billaud A, Verriele V, Dauvé J, Chevalier
LM and Morel A: Non-small-cell lung cancer-sensitive detection of
the p.Thr790Met EGFR alteration by preamplification before
PNA-mediated PCR clamping and pyrosequencing. Diagnostics (Basel).
10:5272020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Chen Z, Huang C, Ding C, Li C,
Chen J, Zhao J and Miao L: Ultrasensitive and quantitative
detection of EGFR mutations in plasma samples from patients with
non-small-cell lung cancer using a dual PNA clamping-mediated
LNA-PNA PCR clamp. Analyst. 144:1718–1724. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song JU and Lee J: Peptide nucleic acid
clamping and direct sequencing in the detection of oncogenic
alterations in lung cancer: Systematic review and meta-analysis.
Yonsei Med J. 59:211–218. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Orum H: PCR clamping. Curr Issues Mol
Biol. 2:27–30. 2000.PubMed/NCBI
|
|
24
|
Zhang T, Wan B, Zhao Y, Li C, Liu H, Lv T,
Zhan P and Song Y: Treatment of uncommon EGFR mutations in
non-small cell lung cancer: New evidence and treatment. Transl Lung
Cancer Res. 8:302–316. 2019. View Article : Google Scholar : PubMed/NCBI
|