Introduction
The autophagic flux is a catabolic biological
process that maintains cellular homeostasis through various
functions, including the degradation and removal of damaged
substrates and macromolecules (1–3).
Autophagic activity increases under stress, with the formation of
autophagosomes regulated by several autophagy-related proteins
(ATGs) (4). Autophagy may play a
dual role, acting as either a tumor promoter or a tumor suppressor
in different human tumors (5,6).
Chordomas (CHs) are rare bone tumors that originate
from remnants of the notochord, likely driven by brachyury
activation, and account for approximately 1.5% of primary malignant
bone tumors (7). They can occur
along the midline of the spine, extending from the clivus to the
sacrum, and are located anterior to the spinal cord (8). CHs typically affect adults, although
some cases have been also reported in children. The most common
site for CHs is the sacrum-coccygeal region (50%), followed by the
clivus and spheno-occipital area (30–35%). The remaining 10–15% are
found in the vertebral body (9).
The physical findings and clinical signs associated with CHs depend
on their specific location. Clival and skull base CHs typically
present with headaches, cranial neuropathies, endocrinopathies,
and, rarely, rhinorrhea due to cerebrospinal fluid leaks (8). Vertebral and sacral CHs often exhibit
nonspecific localized pain and may lead to pathological fractures,
radiculopathy, or myelopathy, as well as dysfunction of the bladder
and bowel due to involvement of the autonomic nervous system
(8). The evaluation of CHs relies
on imaging techniques such as computed tomography (CT) and magnetic
resonance imaging (MRI). CT is more effective in demonstrating
lytic bone destruction and irregular dystrophic calcification
(8), while MRI provides better
delineation of the extent of CHs, typically showing lower signal
intensity on T1-weighted images and heterogeneous contrast
enhancement or hyperintensity with gadolinium contrast (10).
The main macroscopic findings of CHs consist of firm
masses that may contain fluid, gelatinous substances, and/or areas
of hemorrhage and necrosis. Additionally, calcification and
sequestered bone fragments may be present (11). Microscopically, CHs are
characterized by the presence of typical ‘physaliphorous’ cells,
which exhibit vacuoles in their bubbly cytoplasm (7,11).
Currently, three subtypes of CHs are recognized: conventional,
chondroid, and poorly differentiated/dedifferentiated, with the
latter being the least common and associated with the poorest
prognosis (8,11).
Although an association between the development of
CHs and autophagic flux can be hypothesized, similar to findings
documented in other intracranial and bone malignancies (2,12), the
role of ATGs is yet to be fully elucidated. Therefore, this study
aimed to investigate the immunoexpression of certain ATGs,
including microtubule-associated protein 1 light chain 3 (LC3A/B),
p62, and activating molecule in Beclin-1 regulated autophagy
(AMBRA-1), in our series of sporadic adult conventional clival
CHs.
Materials and methods
The analysis was conducted according to the Good
Clinical Practice guidelines and the Declaration of Helsinki (1975,
revised in 2013). Prior to the surgical procedures, all patients
provided written, anonymized, and informed consent. Pathology
reports and medical records were thoroughly reviewed. Patients'
initials and other personal identifiers were removed from all
images. The Institutional Review Board of the University Hospital
of Messina (Messina, Italy) approved this study (prot. N. 47/19;
May 2, 2019).
Case selection
A cohort of 10 cases of clival CHs removed through
neurological resection and collected between 2011 and 2022 was
obtained from the archives of the Department of Human Pathology of
Adult and Developmental Age at the University of Messina, Messina,
Italy. Clinical and pathological parameters, including age, sex,
tumor site, growth fraction, neurological status, imaging
appearance, surgical/radiotherapeutic treatment, and clinical
course, were collected for all cases of clival CHs. Follow-up data
were available for eight out of the 10 cases. All patients
underwent gadolinium contrast-enhanced T1, T2, FLAIR, and MPR brain
MRI, supplemented with CT scans for surgical navigation and when
intralesional calcifications were observed.
Immunohistochemistry
The immunohistochemical analysis was performed on
5-micron thick sections taken from paraffin-embedded tissue blocks.
The sections were deparaffinized and washed using a descending
alcohol gradient. A treatment with 3% hydrogen peroxide for 10 min
was employed to eliminate endogenous peroxidase activity. After
three rinses in deionized water, the sections were incubated for 30
min at room temperature with normal sheep serum to prevent
nonspecific protein binding. Subsequently, the sections were
incubated for 30 min at 37°C with primary polyclonal rabbit
anti-human antisera against p62 (working dilution 1:250; Abcam),
AMBRA-1 (working dilution 1:250; Abcam), and LC3A/B (working
dilution 1:100; Abcam). Following three rinses with
phosphate-buffered saline (PBS), the sections were incubated with a
biotinylated goat anti-rabbit IgG secondary antibody (1:300; Abcam)
for 20 min at room temperature. Finally, the sections were
incubated with a horseradish peroxidase-labeled secondary antibody
for 30 min, and the immunoreaction was visualized using
diaminobenzidine tetrahydrochloride and counterstained with
hematoxylin, utilizing the ULTRA Staining System (Ventana Medical
Systems). Specific primary antisera were omitted and replaced with
PBS to serve as negative controls.
The intensity of positive cells for p62, AMBRA-1,
and LC3A/B was recorded according to previously reported
immunohistochemical methods. The cytoplasmic staining intensity was
rated as follows: 0, negative; 1, weak; and 2, strong. The
percentage of positive cells was scored as follows: grade 0, 0–5%;
grade 1, >5-25%; grade 2, >25-50%; grade 3, >50-75%; and
grade 4, >75-100% for all ATGs. Immunohistochemical scores were
independently assigned by two pathologists (AI and GT), who were
blinded to patient information and other clinical characteristics.
Scoring was conducted using a Zeiss Axioskop microscope (Carl Zeiss
Microscopy GmbH) at 40× objective magnification. A kappa value
ranging from 0.73 to 0.80 (substantial agreement) was documented
for interobserver agreement.
Multiplying the staining intensity by the percentage
of positive cells yielded a final score ranging from 0 to 6. Cases
with an immunoreactive score of 0 to 3 were considered negative,
while those with a score of 4 to 6 were classified as positive.
Ki-67 antiserum (clone MIB-1, dilution 1:100, Dako
Corp., Glostrup, Denmark) was applied to slides for 30 min at room
temperature to determine the growth fraction of meningiomas. This
was preceded by antigen retrieval, which was performed three times
in 0.01 M citrate buffer (pH: 6.0) using a microwave oven set to
750 W. The Ki-67 labeling index (LI) was calculated as the mean
percentage of stained nuclei by counting 1,000 tumor cells across
three representative neoplastic fields. A median Ki-67 LI value of
3% was established as the cut-off point to differentiate between
low and high Ki-67 expression.
Statistical analysis
Statistical evaluation was performed using the SPSS
version 13.0 software package (SPSS, Inc.). The Chi-square
(χ2) test or Fisher's exact test was employed to analyze
the relationship between autophagic immunoexpression markers
(LC3A/B, p62, and AMBRA-1) and various clinicopathological
parameters (age, sex, growth fraction, neoplastic volume, and
surgical treatment), in relation to recurrence. A p-value of less
than 0.05 was considered statistically significant.
Results
The clinico-surgical, pathological, and therapeutic
features of clival CHs in this study are summarized in Table I.
 | Table I.Clinical, surgical, pathological and
therapeutic features of CHs. |
Table I.
Clinical, surgical, pathological and
therapeutic features of CHs.
| Sex | Age | Site | Associated
diseases | Neurological
status | MRI | Size (cm) | Volume
(cm3) | Treatment Endoscopic
endonasal | RT | Recurrence |
|---|
| F | 70 | Clivus | Hypertension, | Strabismus,
dysarthria, | Non-homogenous, | 2.2×2.3×2.5 | 17 | Partial removal | No | Yes |
|
|
|
| Diabetes II | left hemiparesis | discrete
enhancement |
|
|
|
|
|
| M | 48 | Clivus | - | Dysphagia | Non-enhancing | 1.7×1.5×1 | 3 | Complete removal | No | No |
| F | 77 | Clivus with
retro | Hypertension, | Left hemiparesis | Non-homogenous | 2×2×1 | 8 | Partial removal | Yes | Yes |
|
|
| and intrasellar | Diabetes II,
Goiter |
| weak enhancement |
|
|
|
|
|
|
|
|
|
|
| with
calcifications |
|
|
|
|
|
| M | 26 | Clivus with | - | Diplopia Right VI
cn | Non-homogenous | 3×3×2 | 18 | Complete removal | No | NA |
|
|
| retroclival and
intrasellar |
| paresis | weak enhancement |
|
|
|
|
|
| M | 40 | Middle clivus, | Esophageal
achalasia, | Negative | Non-homogenous | 2×1×2 | 3 | Complete removal | No | No |
|
|
| retroclival and | sarcoma, bladder |
| weak enhancement |
|
|
|
|
|
|
|
| intrasellar | carcinoma |
|
|
|
|
|
|
|
| M | 63 | Inferior
clivus | Hypertension | Arms
paresthesia | Isointense | 4×3×4 | 48 | Partial
removal | Yes | Yes |
|
|
|
| Diabetes II |
| non-enhancing, |
|
|
|
|
|
|
|
|
|
|
| calcifications |
|
|
|
|
|
| F | 58 | Clivus | Charcot-Marie
Tooth | Walking deficits
Left | Non-enhancing | 4×4×3 | 18 | Partial
removal | Yes | No |
|
|
|
| 1A Diabetes II Bell
paralysis | hemiparesis |
|
|
|
|
|
|
| M | 68 | Clivus | - | Left
hemiparesis | Non-homogenous | 3×2×2 | 10 | Partial
removal | Yes | Yes |
|
|
|
|
|
| weak
enhancement |
|
|
|
|
|
| F | 75 | Inferior
clivus | Hypertension | Negative | Isointense
non-enhancing, | 2×2×1 | 6 | Complete
removal | No | No |
|
|
|
| Diabetes II |
| calcifications |
|
|
|
|
|
| M | 65 | Clivus | - | Diplopia | Non-homogenous
weak | 1.9×1.8×1 | 4 | Complete
removal | No | No |
|
|
|
|
|
| enhancement |
|
|
|
|
|
The patient ages ranged from 26 to 77 years (mean 59
years), with a male-to-female (M:F) ratio of 6:4. All tumors were
located in the clivus, occasionally extending into the middle or
inferior portions with retroclival involvement. Upon admission,
patients presented with a wide array of neurological symptoms,
ranging from asymptomatic cases (where the lesion was discovered
during a CT scan for oncological follow-up of urothelial carcinoma)
to cranial nerve deficits (diplopia, dysphagia, and strabismus),
arm paresthesia, and walking deficits.
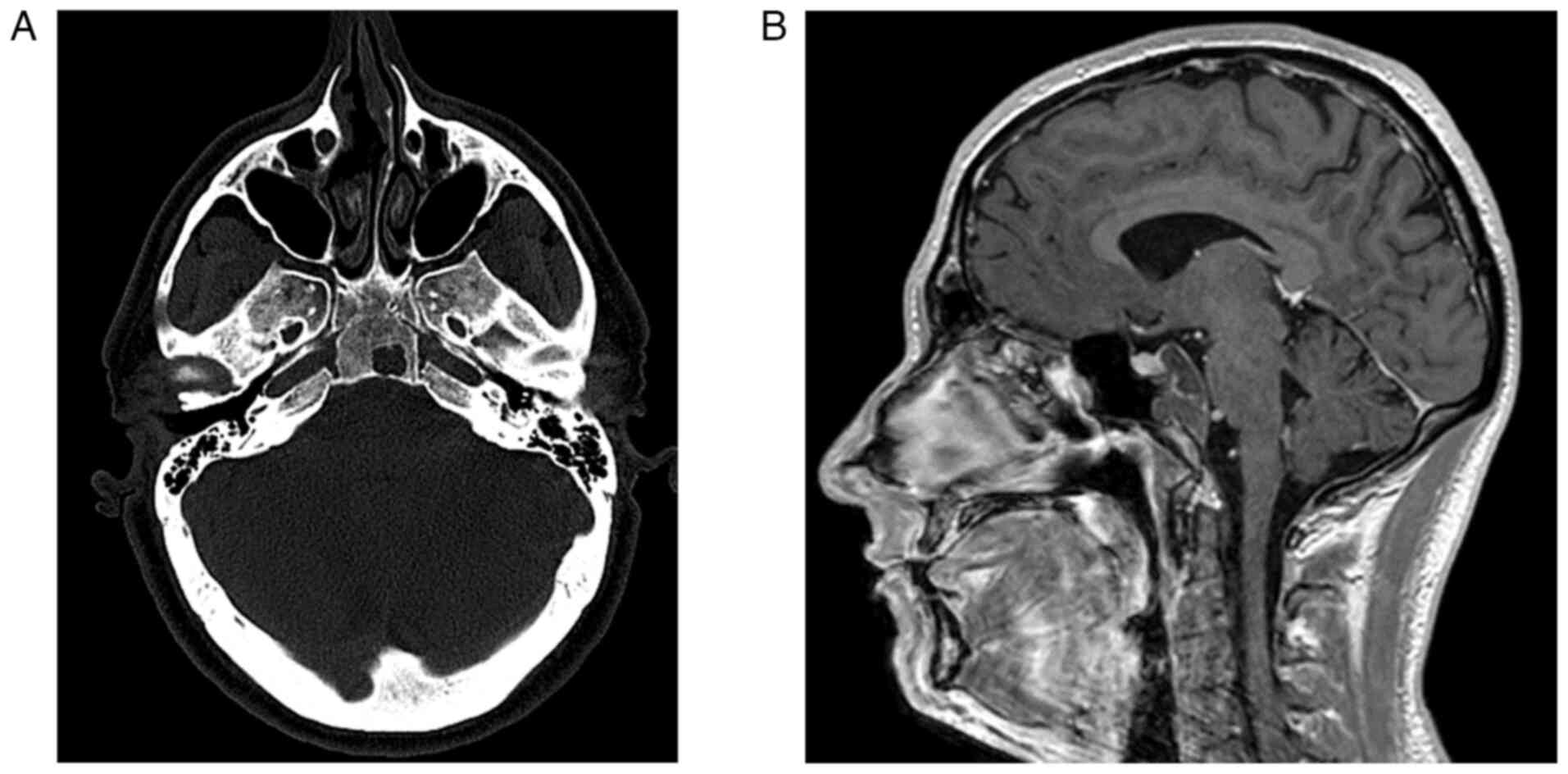
Lesions appeared as solid masses isointense to brain
parenchyma in both standard T1 and T2-weighted images (Fig. 1A and B). Following contrast
administration, a wide range of enhancement was observed, from
non-enhancement in three cases, weak enhancement in three cases, to
discrete enhancement in the last case. Calcifications were noted in
two cases. In one case, the CH remained confined to the occipital
bone, while in the remaining cases, it extended extradurally in all
directions, including the cervical spine at the level of the
extracanalar laterocervical area, the posterior cranial fossa,
cavernous sinus, Meckel's cave, sphenoidal sinus, and intrasellar
region, resulting in compression of the pituitary gland. The mean
tumor size was 2,61 cm (range: 1,7-4 cm), with the mean neoplastic
volume measured at 13,5 cm3 (range: 3–48
cm3). MRI findings were available for all cases. The
follow-up period for patients ranged from one to 60 months, with a
mean follow-up of 18.8 months. The associated disease, neurological
status, and magnetic resonance features are described in Table I. Surgical treatment was performed
via endonasal endoscopy in all cases, achieving complete removal of
the clival CH in five cases. Radiotherapy was not administered in
six cases, while three cases received stereotactic radiotherapy
with a 30 Gy isodose delivered in five fractions, and one case
underwent proton beam therapy at 74 Gy. During the follow-up
period, no patients died from the disease; however, one patient was
lost to follow-up, and there was no record of ongoing therapy.
Another patient refused radiotherapy and experienced an increase in
the residual lesion a few months postoperatively. Four patients
experienced disease recurrence at one, nine, and 17 months,
respectively.
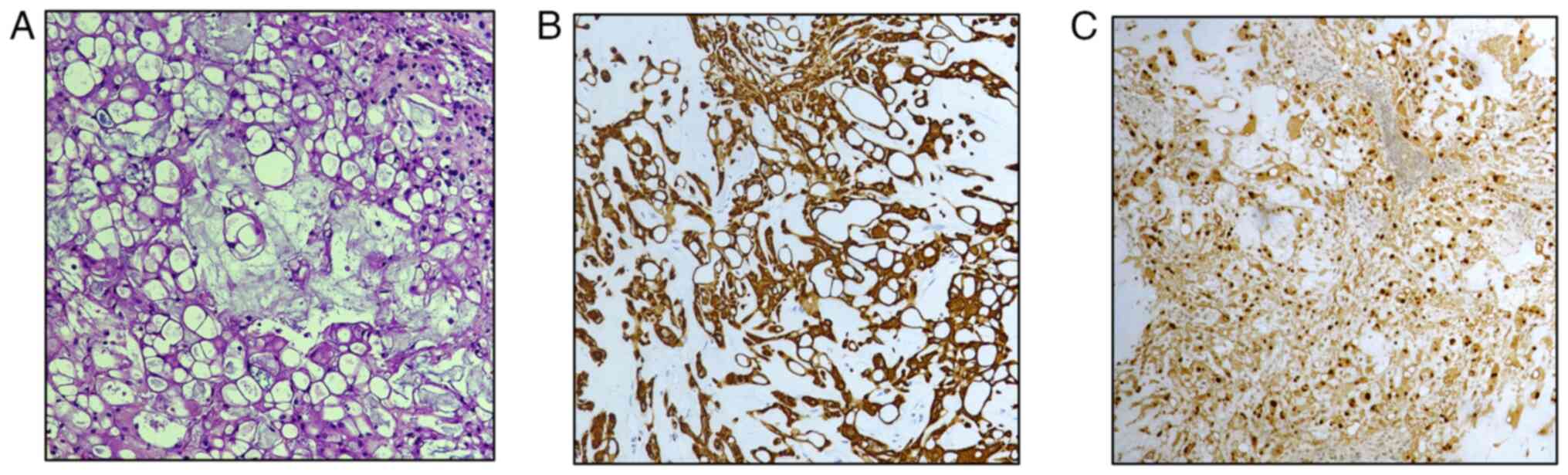
All cases exhibited the classical appearance of CH
with typical physaliphorous cells (Fig.
2A) and consistent immunoexpression of cytokeratin (CK) AE1/AE3
(Fig. 2B), CK 8, CK 18, CK 19,
epithelial membrane antigen (EMA), and S100 protein. In contrast,
they were negative for CK 7 and CK 20. Additionally, a clear
nuclear expression of Brachyury was demonstrated (Fig. 2C), as reported in other studies on
CHs (11,13).
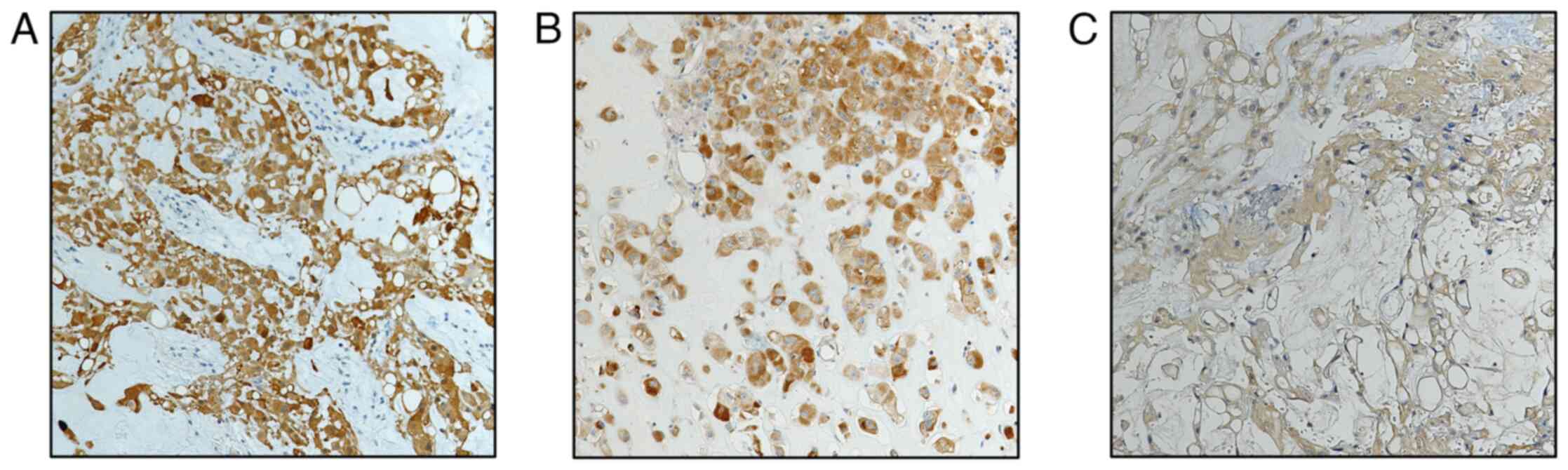
Immunostaining for LC3A/B (Fig. 3A), p62 (Fig. 3B), and AMBRA-1 (Fig. 3C) was exclusively observed in
neoplastic cells, with no expression detected in the surrounding
stromal cells. Both LC3A/B and p62 were expressed in the cytoplasm
and nucleus of neoplastic cells (Fig.
3A and B), while AMBRA-1 was predominantly localized in the
cytoplasm (Fig. 3C). All CH cases
exhibited a constant high immunoreactivity for p62. In contrast,
low LC3A/B staining was found in five out of 10 cases (50%), while
five cases demonstrated high LC3A/B immunoexpression; four of these
cases were characterized by neoplastic recurrence and partial
removal. Finally, AMBRA-1 low immunoreactivity was noted in seven
out of 10 cases (70%), while three recurrent cases exhibited high
AMBRA-1 immunostaining. In terms of growth fraction, five cases
were considered high Ki-67 expression, with a Ki-67 LI >3%, four
of which had undergone partial neoplastic removal and showed
evidence of recurrence.
Utilizing Fisher's exact test revealed significant
P-values for LC3A/B (0.048), AMBRA-1 (0.033), Ki-67 (0.048), and
surgical treatment (0.048) (Table
II).
 | Table II.Clinico-pathological and
immunohistochemical features of autophagic proteins (p62, LC3A/B
and AMBRA 1) in relation to neoplastic recurrence in chordomas. |
Table II.
Clinico-pathological and
immunohistochemical features of autophagic proteins (p62, LC3A/B
and AMBRA 1) in relation to neoplastic recurrence in chordomas.
| Feature | No recurrence | Recurrence | P-value | OR (95% CI) |
|---|
| Age |
|
| 1.00 |
|
|
<60 | 4 | 0 |
| 0.500 (0.037 to
6.680) |
|
≥60 | 2 | 4 |
|
|
| Sex |
|
| 0.076 |
|
|
Male | 4 | 2 |
| 0.062 (0.001 to
1.684) |
|
Female | 2 | 2 |
|
|
| p62 |
|
| 1.00 |
|
|
Low | 0 | 0 |
| 1.444 (0.024 to
87.250) |
|
High | 6 | 4 |
|
|
| LC3A/B |
|
| 0.048 |
|
|
Low | 5 | 0 |
| 0.030 (0.001 to
0.940) |
|
High | 1 | 4 |
|
|
| AMBRA 1 |
|
| 0.033 |
|
|
Low | 6 | 1 |
| 0.032 (0.001 to
1.044) |
|
High | 0 | 3 |
|
|
| Ki67 |
|
| 0.048 |
|
|
<3% | 5 | 0 |
| 0.030 (0.001 to
0.940) |
|
≥3% | 1 | 4 |
|
|
| Volume |
|
| 1.00 |
|
|
<13.5 cm | 4 | 2 |
| 0.500 (0.037 to
6.680) |
| ≥13.5
cm | 2 | 2 |
|
|
| Resection |
|
| 0.048 |
|
|
Partial | 1 | 4 |
| 33 (1.063 to
1.204) |
|
Total | 5 | 0 |
|
|
Discussion
The molecular mechanisms of autophagy and the role
of autophagy-targeting agents in human brain neoplasms,
particularly gliomas, have been previously investigated (1,2).
However, the expression of ATGs has been reported in CHs (7), in which diffuse and strong
immunohistochemical expression of p62 has been highlighted,
consistent with the findings of the present investigation. The
significance of such prominent p62 expression may be related to
blocked autophagic degradation, as suggested by Karpathiou et
al (7). On the other hand,
another significant ATG, LC3A/B, is considered a reliable marker of
autophagy and a prognostic factor in various malignant neoplastic
conditions (13–15). In CHs, LC3A/B expression has been
analyzed, showing negative, mild, moderate, and strong expression
based on the number of dots per cell (7); however, no relationships emerged
between immunohistochemical findings and neoplastic recurrence.
Similarly, AMBRA-1 has been implicated in the autophagy machinery,
and its poor prognostic value has been documented in
cholangio-pancreatic and prostatic adenocarcinomas, where it is
associated with depth of invasion and lymph node metastasis
(16,17). Nevertheless, to date, no data
regarding AMBRA-1 immunoexpression in CHs have been previously
described.
In the present study, we utilized the three
aforementioned ATGs and employed a quantitative scoring system
(0–3=negative; 4–6=positive) to explore the potential autophagic
signature in CHs. Notably, p62 was highly positive in all CH cases,
while LC3A/B and AMBRA-1 expressions were positive in 50 and 33% of
CH cases, respectively. Interestingly, cases with elevated LC3A/B
and AMBRA-1 immunoexpression were significantly associated with
tumor recurrence, while p62 was evenly distributed between
recurrent and non-recurrent cases. Therefore, the association
between LC3A/B and AMBRA-1 allowed us to identify CH cases
characterized by tumor recurrence, which also revealed an increased
growth fraction (Ki-67 >3) and partial neoplastic resection.
Consequently, a negative prognostic role for these two ATGs may be
hypothesized in the development of CHs. Although the exact
mechanism of activation in CHs remains poorly defined, a possible
association between autophagic flux and brachyury activation
(13,18) is a well-known molecular abnormality
in CHs, similar to that reported in brain gliomas, where autophagy
represents a significant phenomenon (1,2).
Additionally, we demonstrated that autophagic factors, such as
LC3A/B and AMBRA-1, are frequently present in CHs, associated with
a strong and diffuse expression of p62, suggesting a blocked
autophagic flow in contrast to their normal tissue
counterparts.
In general, it is important to note that the present
study had certain biases and limitations, primarily due to its
retrospective nature. The main constraint was the investigation of
specific ATGs exclusively through immunohistochemistry, though
autophagy may represent a flux when more adequately and
functionally analyzed. Furthermore, autophagy plays a crucial role
in the immune tumor microenvironment and is considered significant
for all tumors, including rare neoplasms such as sarcomas and CHs.
However, only controversial data have been reported regarding the
immune microenvironment in CHs (19–21),
despite the potential for immunotherapy as a treatment option for
these tumors (22). In detail, the
analysis of the immune microenvironment in CHs has only recently
begun, revealing that macrophages and T-lymphocytes are the most
prevalent immune cells and may play critical roles in tumor immune
regulation (23,24). Additionally, various cytokines and
chemokines, along with other immune checkpoints such as PD-1/PD-L1,
CD47/SIRPα, TIM3, and CTLA4, are expressed on the surface of both
CH cells and immune cells (23,24).
However, some contradictory results require further investigation
and interpretation.
Further evidence characterizing the role of the
immune tumor microenvironment is essential to enhance our
understanding of identifying more aggressive subtypes of CHs after
resection. This knowledge could inform decision-making regarding
repeated resections for partial removal and adjuvant
chemoradiotherapy. In conclusion, while our study represents one of
the few reports on the immunohistochemical expression of ATGs in
CHs, we hope that a larger series of CH tissue samples will be
investigated for autophagic proteins. This investigation should
compare their expression with the tumor immune microenvironment,
particularly focusing on B cells, considering that PD-L1+ immune
cells also express LC3A/B.
Acknowledgements
Not applicable.
Funding
Funding: No funds were received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CP, AI and GT developed the study design and the
manuscript draft. CP, AC, VF, MM and AG were involved in data
acquisition and interpretation. AI and GT reviewed the manuscript.
AG and GT confirmed the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The analysis was conducted according to the Good
Clinical Practice guidelines and the Declaration of Helsinki (1975,
revised in 2013). Prior to surgical procedures, all patients
provided written, anonymized and informed consent. Pathology
reports and medical records were thoroughly reviewed. Patients'
initials or other personal identifiers were removed from all
images. The Institutional Review Board of the University Hospital
of Messina (Messina, Italy) approved this study (approval no. N.
47/19; May 2, 2019).
Patient consent for publication
Written informed consent was obtained from all
patients for the publication of their data.
Competing interests
No competing interests have been declared.
References
|
1
|
Ieni A, Pizzimenti C, Broggi G, Caltabiano
R, Germanò A, Barbagallo GMV, Vigneri P, Giuffrè G and Tuccari G:
Immunoexpression of p62/SQSTM1/Sequestosome-1 in human primary and
recurrent IDH1/2 wild-type glioblastoma: A pilot study. Oncol Lett.
24:3362022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pizzimenti C, Fiorentino V, Franchina M,
Martini M, Giuffrè G, Lentini M, Silvestris N, Di Pietro M, Fadda
G, Tuccari G and Ieni A: Autophagic-related proteins in brain
gliomas: Role, mechanisms, and targeting agents. Cancers (Basel).
15:26222023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Broggi G, Ieni A, Russo D, Varricchi S,
Puzzo L, Russo A, Reibaldi M, Longo A, Tuccari G, Staibano S and
Caltabiano R: The macro-autophagy-related protein beclin-1
immunohistochemical expression correlates with tumor cell type and
clinical behavior of uveal melanoma. Front Oncol. 10:5898492020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yun CW and Lee SH: The roles of autophagy
in cancer. Int J Mol Sci. 19:34662018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yun CW, Jeon J, Go G, Lee JH and Lee SH:
The dual role of autophagy in cancer development and a therapeutic
strategy for cancer by targeting autophagy. Int J Mol Sci.
22:1792020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SS, Vats S, Chia AYQ, Tan TZ, Deng
S, Ong MS, Arfuso F, Yap CT, Goh BC, Sethi G, et al: Dual role of
autophagy in hallmarks of cancer. Oncogene. 37:1142–1158. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karpathiou G, Dumollard JM, Dridi M, Dal
Col P, Barral FG, Boutonnat J and Peoc'h M: Chordomas: A review
with emphasis on their pathophysiology, pathology, molecular
biology, and genetics. Pathol Res Pract. 216:1530892020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fletcher C, Bridge J, Hogendoom P and
Mertens F: Notochordal tumours. WHO Classification of Tumours. Soft
Tissue and Bone Tumors. 5th edition. IARC; Lyon, France: pp.
449–456. 2020
|
|
9
|
George B, Bresson D, Herman P and Froelich
S: Chordomas: A review. Neurosurg Clin N Am. 26:437–452. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Santegoeds RGC, Temel Y,
Beckervordersandforth JC, Van Overbeeke JJ and Hoeberigs CM:
State-of-the-art imaging in human chordoma of the skull base. Curr
Radiol Rep. 6:162018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ulici V and Hart J: Chordoma. Arch Pathol
Lab Med. 146:386–395. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schläfli AM, Adams O, Galván JA, Gugger M,
Savic S, Bubendorf L, Schmid RA, Becker KF, Tschan MP, Langer R and
Berezowska S: Prognostic value of the autophagy markers LC3 and
p62/SQSTM1 in early-stage non-small cell lung cancer. Oncotarget.
7:39544–39555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niu J, Yan T, Guo W, Wang W and Zhao Z:
Insight into the role of autophagy in osteosarcoma and its
therapeutic implication. Front Oncol. 9:12322019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barresi V, Ieni A, Branca G and Tuccari G:
Brachyury: A diagnostic marker for the differential diagnosis of
chordoma and hemangioblastoma versus neoplastic histological
mimickers. Dis Markers. 2014:5147532014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jovanović L, Nikolić A, Dragičević S,
Jović M and Janković R: Prognostic relevance of autophagy-related
markers p62, LC3, and Beclin1 in ovarian cancer. Croat Med J.
63:453–460. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lazova R, Camp RL, Klump V, Siddiqui SF,
Amaravadi RK and Pawelek JM: Punctate LC3B expression is a common
feature of solid tumors and associated with proliferation,
metastasis, and poor outcome. Clin Cancer Res. 18:370–379. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gillson J, Abd El-Aziz YS, Leck LYW,
Jansson PJ, Pavlakis N, Samra JS, Mittal A and Sahni S: Autophagy:
A key player in pancreatic cancer progression and a potential drug
target. Cancers (Basel). 14:35282022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Chen Z, Guo J, Wang L and Liu X:
Ambra1 induces autophagy and desensitizes human prostate cancer
cells to cisplatin. Biosci Rep. 39:BSR201707702019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yurube T, Hirata H, Ito M, Terashima Y,
Kakiuchi Y, Kuroda R and Kakutani K: Involvement of autophagy in
rat tail static compression-induced intervertebral disc
degeneration and notochordal cell disappearance. Int J Mol Sci.
22:56482021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng Y, Shen J, Gao Y, Liao Y, Cote G,
Choy E, Chebib I, Mankin H, Hornicek F and Duan Z: Expression of
programmed cell death ligand 1 (PD-L1) and prevalence of
tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget.
6:11139–11149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mathios D, Ruzevick J, Jackson CM, Xu H,
Shah SR, Taube JM, Burger PC, McCarthy EF, Quinones-Hinojosa A,
Pardoll DM and Lim M: PD-1, PD-L1, PD-L2 expression in the chordoma
microenvironment. J Neurooncol. 121:251–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou M, Pan Y, Huang W, Zhang TL, Escobar
D, Wang XB, Jiang Y, She XL, Lv GH and Li J: A four-factor immune
risk score signature predicts the clinical outcome of patients with
spinal chordoma. Clin Transl Med. 10:224–237. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Shi Q, Wang B, Ji T, Guo W, Ren T
and Tang X: The role of tumor immune microenvironment in chordoma:
Promising immunotherapy strategies. Front Immunol. 14:12572542023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y and Zhang H: Immune
microenvironment and immunotherapy for chordoma. Front Oncol.
14:13742492024. View Article : Google Scholar : PubMed/NCBI
|