|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dasgupta P, Henshaw C, Youlden DR, Clark
PJ, Aitken JF and Baade PD: Global trends in incidence rates of
primary adult liver cancers: A systematic review and meta-analysis.
Front Oncol. 10:1712020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gavriilidis P, Pawlik TM and Azoulay D:
Comprehensive review of hepatocellular carcinoma with portal vein
tumor thrombus: State of art and future perspectives. Hepatobiliary
Pancreat Dis Int. 23:221–227. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng ZJ, Li L, Teng YX, Zhang YQ, Zhang
YX, Liu HT, Huang JL, Liu ZX, Ma L and Zhong JH: Treatments of
hepatocellular carcinoma with portal vein tumor thrombus: Current
status and controversy. J Clin Transl Hepatol. 10:147–158. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun J, Guo R, Bi X, Wu M, Tang Z, Lau WY,
Zheng S, Wang X, Yu J, Chen X, et al: Guidelines for diagnosis and
treatment of hepatocellular carcinoma with portal vein tumor
thrombus in China (2021 edition). Liver Cancer. 11:315–328. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan AR, Wei X and Xu X: Portal vein tumor
thrombosis and hepatocellular carcinoma-the changing tides. J
Hepatocell Carcinoma. 8:1089–1115. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng S, Chen M and Cai J; National
Research Cooperative Group for Diagnosis and Treatment of
Hepatocellular Carcinoma with Tumor Thrombus, : Chinese expert
consensus on multidisciplinary diagnosis and treatment of
hepatocellular carcinoma with portal vein tumor thrombus: 2016
Edition. Oncotarget. 8:8867–8876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yau T, Tang VYF, Yao TJ, Fan ST, Lo CM and
Poon RTP: Development of Hong Kong liver cancer staging system with
treatment stratification for patients with hepatocellular
carcinoma. Gastroenterology. 146:1691–1700.e3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kudo M, Matsui O, Izumi N, Iijima H,
Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et
al: JSH consensus-based clinical practice guidelines for the
management of hepatocellular carcinoma: 2014 Update by the liver
cancer study group of Japan. Liver Cancer. 3:458–468. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye JZ, Wang YY, Bai T, Chen J, Xiang BD,
Wu FX and Li LQ: Surgical resection for hepatocellular carcinoma
with portal vein tumor thrombus in the Asia-Pacific region beyond
the Barcelona clinic liver cancer treatment algorithms: A review
and update. Oncotarget. 8:93258–93278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakamoto K and Nagano H: Surgical
treatment for advanced hepatocellular carcinoma with portal vein
tumor thrombus. Hepatol Res. 47:957–962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Z, Zhao J, Bi X, Zhang Y, Zhou J, Li
Z, Huang Z, Zhao H and Cai J: Neoadjuvant radiotherapy for
resectable hepatocellular carcinoma with portal vein tumor
thrombus: A systematic review. Hepatobiliary Surg Nutr. 11:709–717.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anand AC and Acharya SK: New developments
in the treatment of hepatocellular carcinoma: The concept of
adjuvant and neoadjuvant chemotherapy. J Clin Exp Hepatol.
11:284–287. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seckler F, Doussot A, Colpart P, Turco C,
Calame P, Aubin F, Algros MP, Borg C, Nardin C and Heyd B:
Preoperative immunotherapy for resectable hepatocellular carcinoma:
Toward a paradigm shift? J Hepatol. 73:1588–1590. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang A, Yang XR, Chung WY, Dennison AR
and Zhou J: Targeted therapy for hepatocellular carcinoma. Signal
Transduct Target Ther. 5:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee M and Shin HP: Efficacy of
transarterial chemoembolization (TACE) for early-stage
hepatocellular carcinoma. Medicina (Kaunas). 59:21742023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu L, Chen L and Zhang W: Neoadjuvant
treatment strategies for hepatocellular carcinoma. World J
Gastrointest Surg. 13:1550–1566. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Kim JM, Rhu J, Choi GS, David Kwon
CH and Joh JW: Surgical resection is preferred in selected solitary
hepatocellular carcinoma with portal vein tumor thrombosis. Dig
Surg. 39:42–50. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng X, Zhang Y, Kwong JS, Zhang C, Li S,
Sun F, Niu Y and Du L: The methodological quality assessment tools
for preclinical and clinical studies, systematic review and
meta-analysis, and clinical practice guideline: A systematic
review. J Evid Based Med. 8:2–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao B, Shentu H, Sha S, Wang D, Chen X,
Huang Z, Dong N, Lai H, Xu J and Zhou X: Efficacy of IL-23
inhibitors and IL-12/23 inhibitors in the induction treatment of
Crohn's disease: A meta-analysis based on randomized controlled
trials. Cent Eur J Immunol. 48:301–310. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Dai M, Huang T, Chen B, Xiang Z,
Tang J, Zheng M and Guo L: Association between BMI and efficacy of
SGLT2 inhibitors in patients with heart failure or at risk of heart
failure: A meta-analysis based on randomized controlled trials.
Cardiology. 149:104–116. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin J, Wang D, He Y, Sha H, Zhang W and
Huang W: The safety of not implementing endoscopic nasobiliary
drainage after elective clearance of choledocholithiasis: A
systematic review and meta-analysis. BMC Surg. 24:2392024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
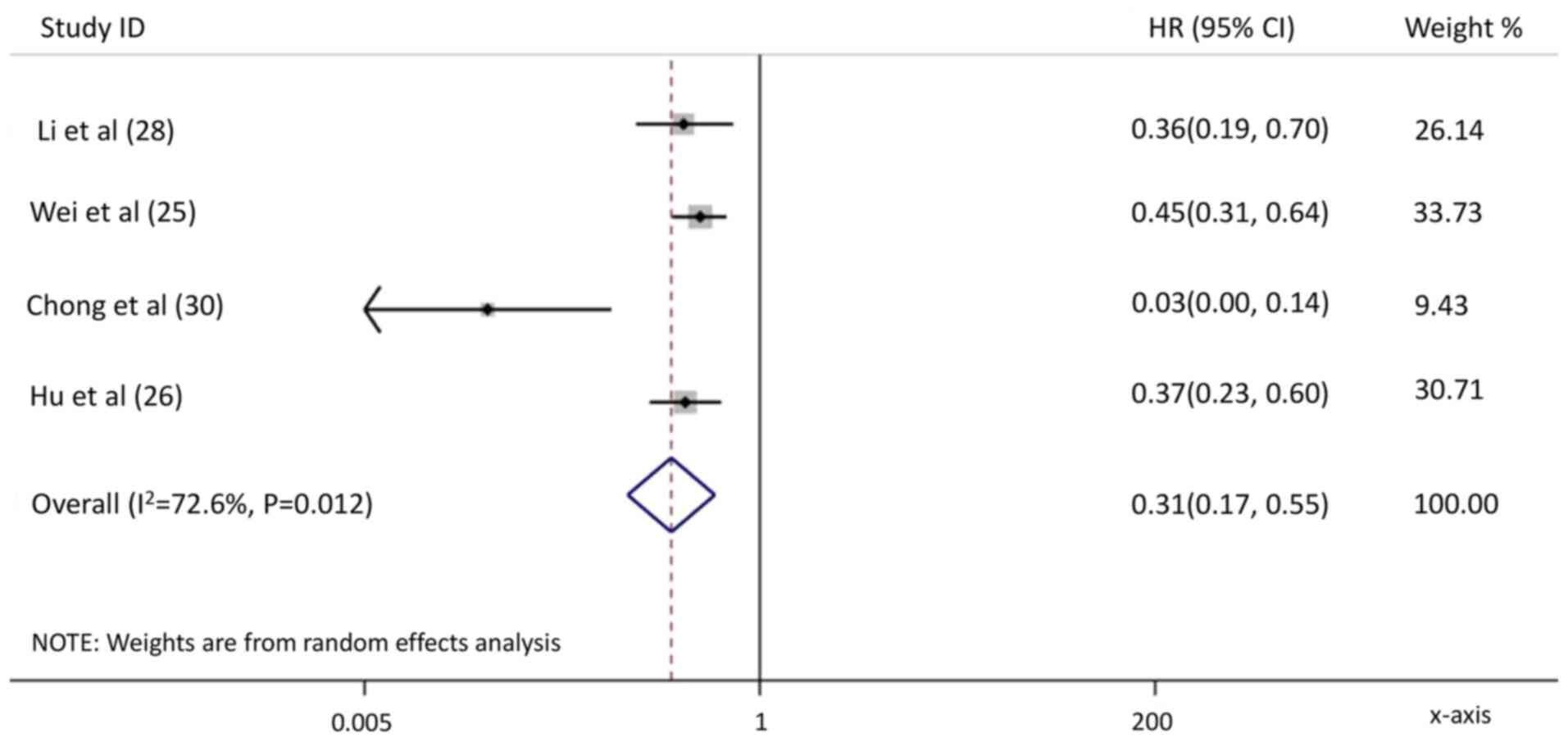
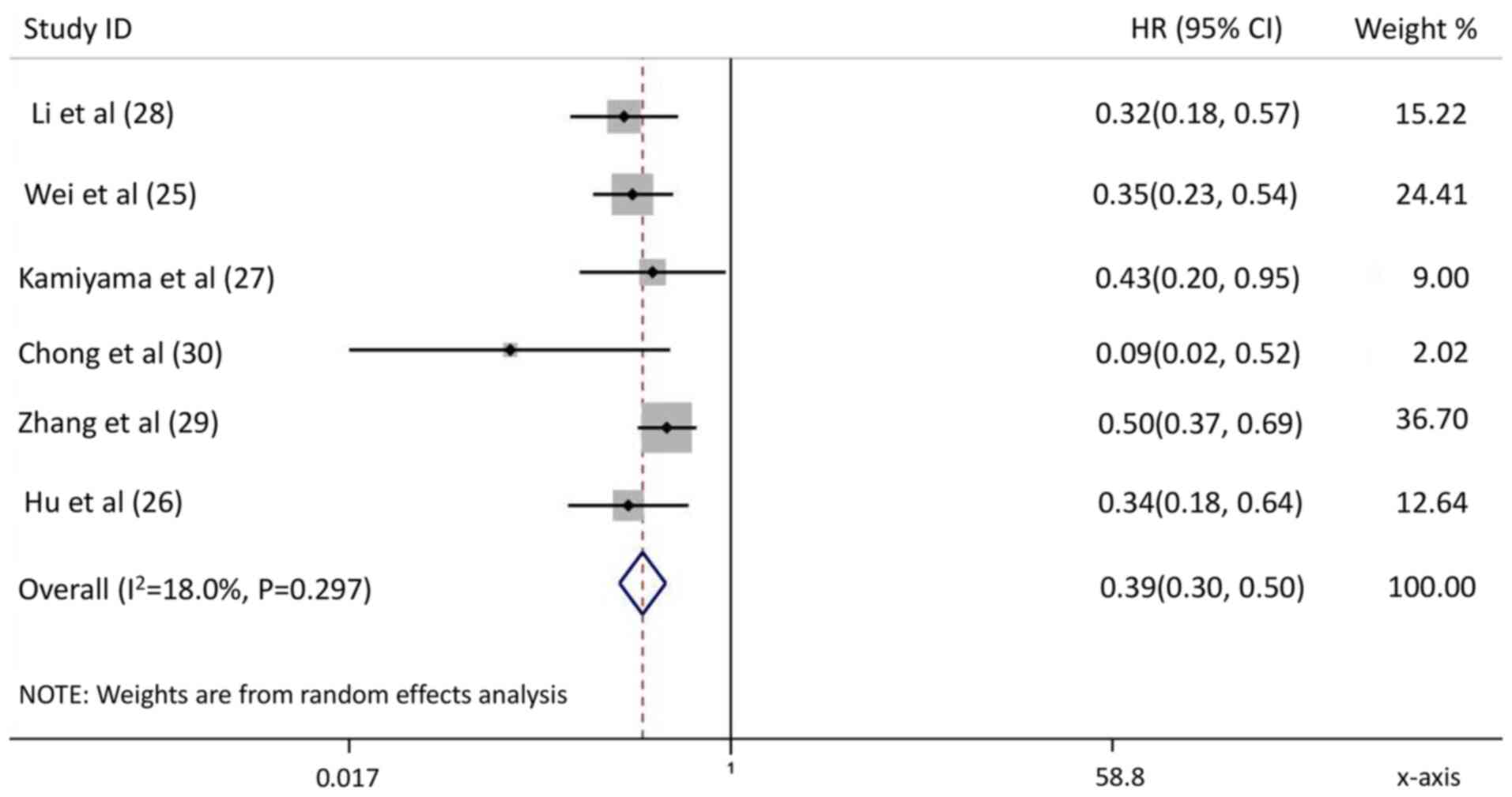
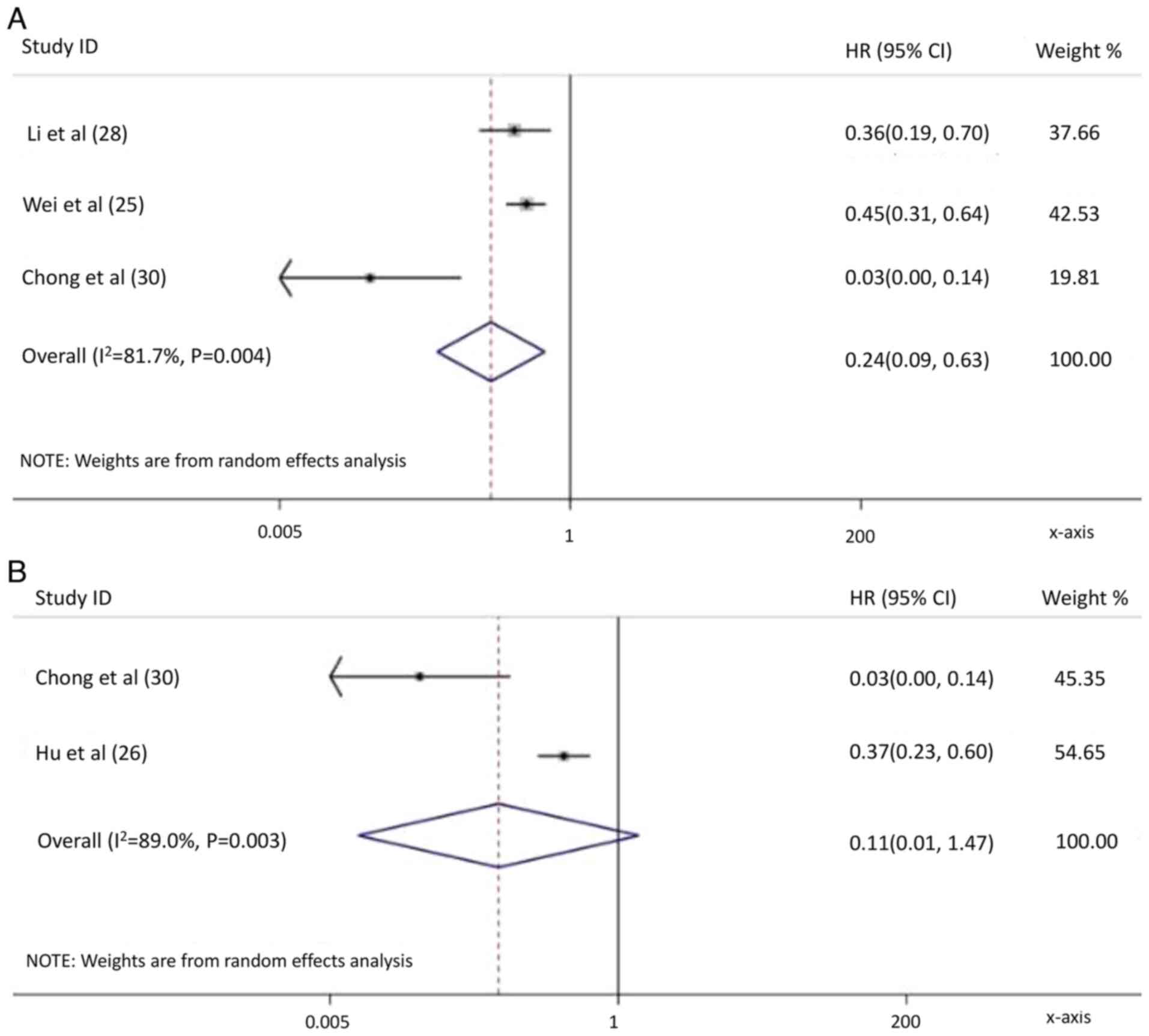
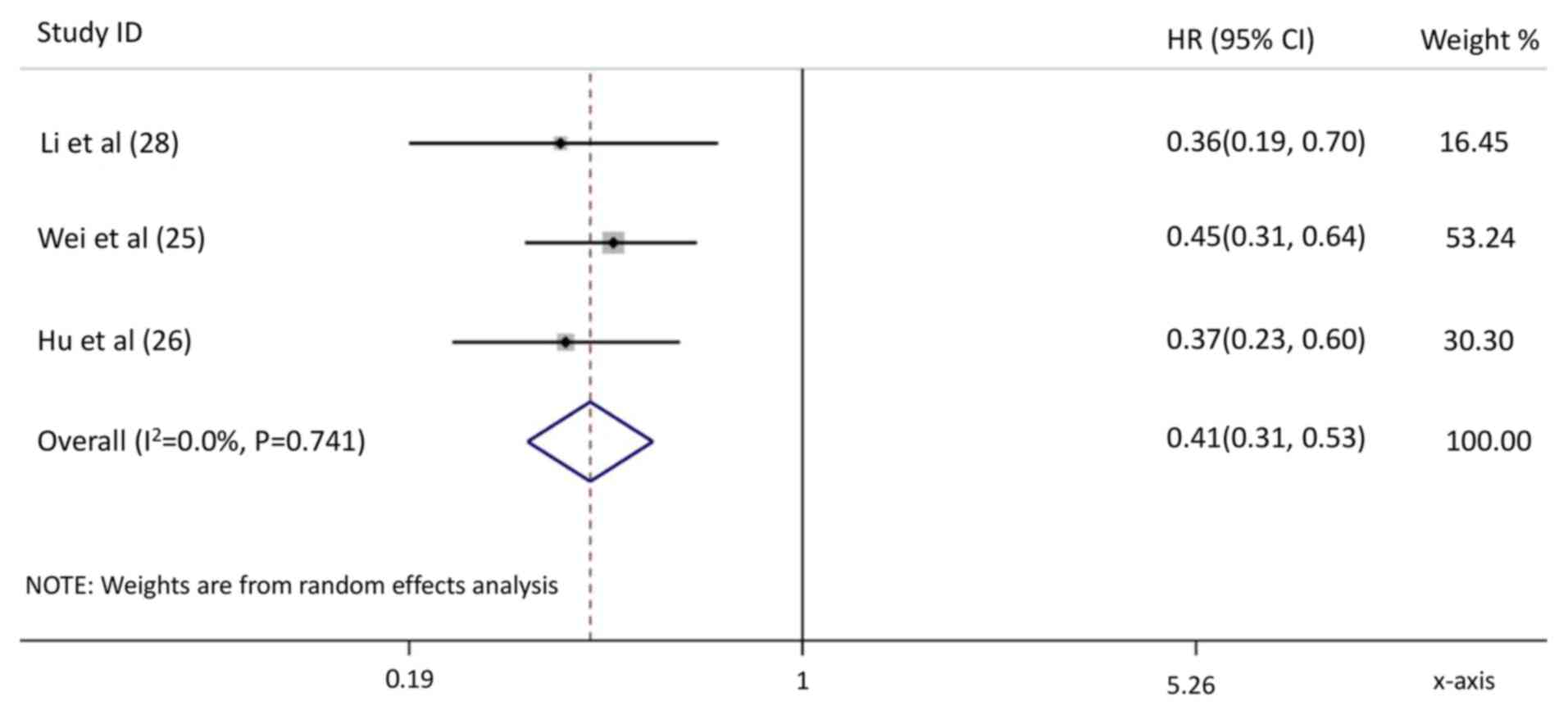
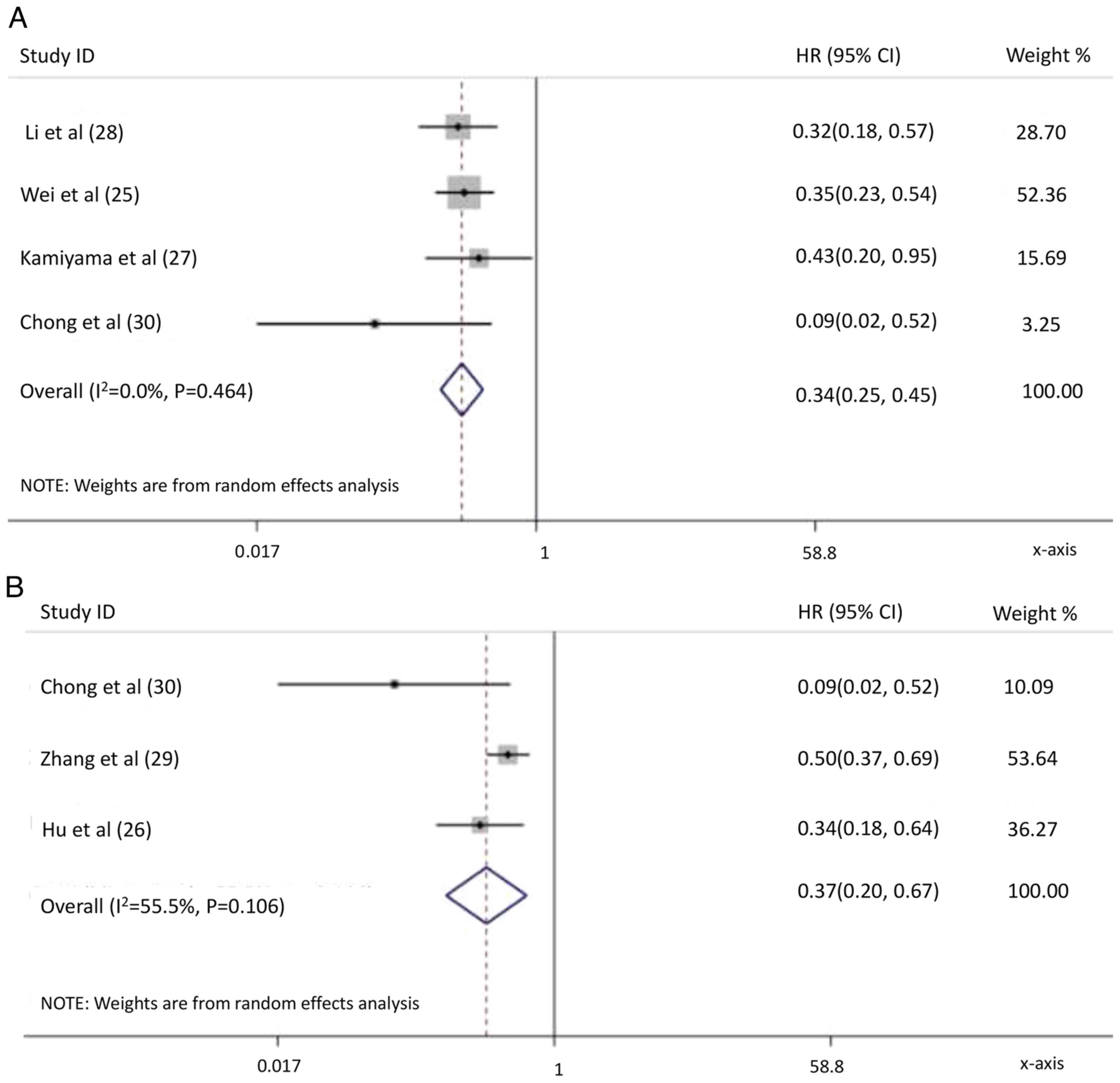
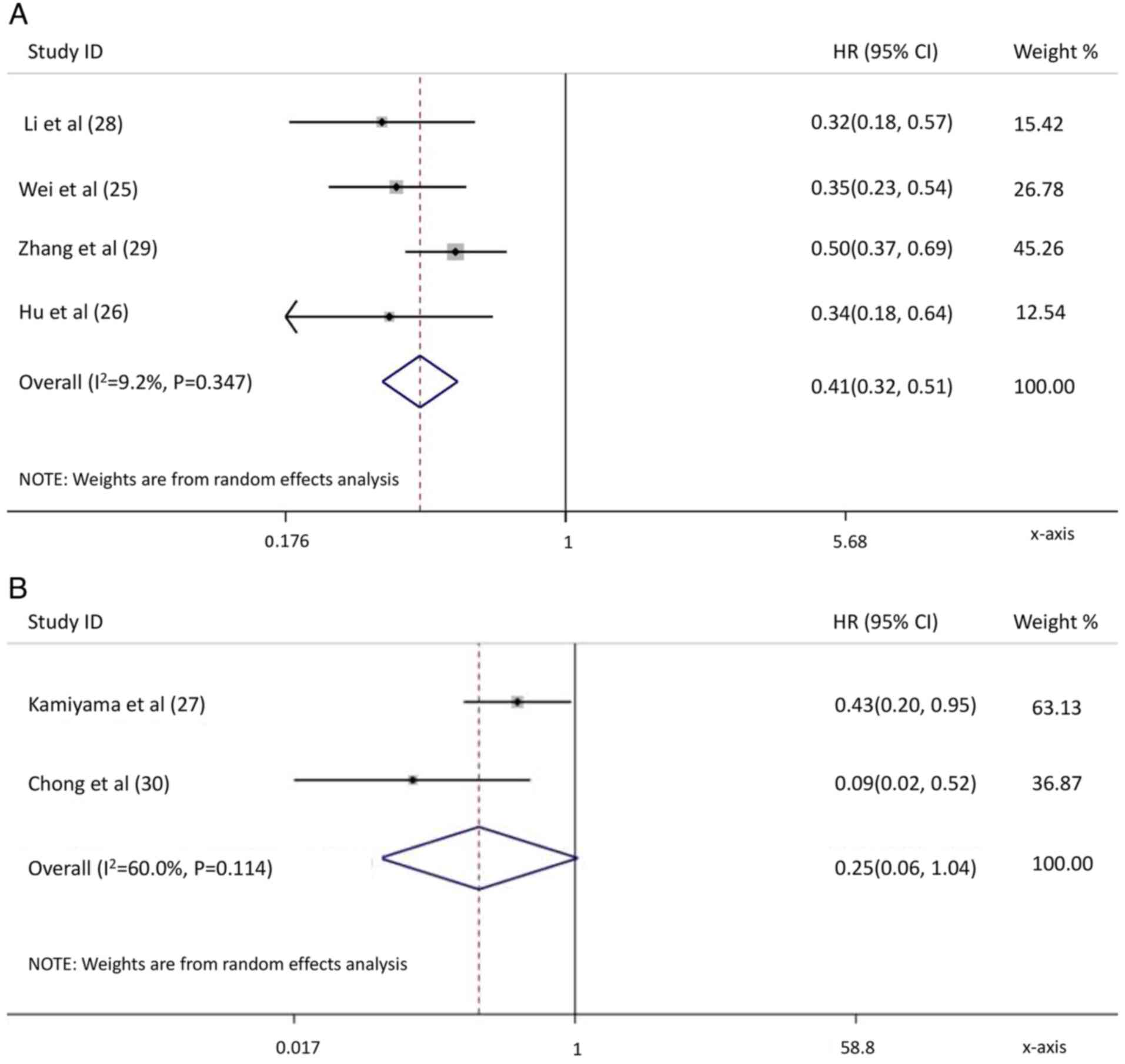
Wei X, Jiang Y, Zhang X, Feng S, Zhou B,
Ye X, Xing H, Xu Y, Shi J, Guo W, et al: Neoadjuvant
three-dimensional conformal radiotherapy for resectable
hepatocellular carcinoma with portal vein tumor thrombus: A
randomized, open-label, multicenter controlled study. J Clin Oncol.
37:2141–2151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu Z, Yang Z, Wang J, Fu Y, Hu Z, Zhou Z,
Chen M and Zhang Y: Survival benefit of neoadjuvant hepatic
arterial infusion chemotherapy followed by hepatectomy for
hepatocellular carcinoma with portal vein tumor thrombus. Front
Pharmacol. 14:12236322023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamiyama T, Nakanishi K, Yokoo H, Tahara
M, Nakagawa T, Kamachi H, Taguchi H, Shirato H, Matsushita M and
Todo S: Efficacy of preoperative radiotherapy to portal vein tumor
thrombus in the main trunk or first branch in patients with
hepatocellular carcinoma. Int J Clin Oncol. 12:363–368. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li N, Feng S, Xue J, Wei XB, Shi J, Guo
WX, Lau WY, Wu MC, Cheng SQ and Meng Y: Hepatocellular carcinoma
with main portal vein tumor thrombus: A comparative study comparing
hepatectomy with or without neoadjuvant radiotherapy. HPB (Oxford).
18:549–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang YF, Guo RP, Zou RH, Shen JX, Wei W,
Li SH, OuYang HY, Zhu HB, Xu L, Lao XM and Shi M: Efficacy and
safety of preoperative chemoembolization for resectable
hepatocellular carcinoma with portal vein invasion: A prospective
comparative study. Eur Radiol. 26:2078–2088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chong JU, Choi GH, Han DH, Kim KS, Seong
J, Han KH and Choi JS: Downstaging with localized concurrent
chemoradiotherapy can identify optimal surgical candidates in
hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg
Oncol. 25:3308–3315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Durand F and Valla D: Assessment of the
prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 42
(Suppl):S100–S107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Trevisani F, Vitale A, Kudo M, Kulik L,
Park JW, Pinato DJ and Cillo U: Merits and boundaries of the BCLC
staging and treatment algorithm: Learning from the past to improve
the future with a novel proposal. J Hepatol. 80:661–669. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yau T, Yao TJ, Chan P, Ng K, Fan ST and
Poon RTP: A new prognostic score system in patients with advanced
hepatocellular carcinoma not amendable to locoregional therapy:
Implication for patient selection in systemic therapy trials.
Cancer. 113:2742–2751. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Govalan R, Lauzon M, Luu M, Ahn JC, Kosari
K, Todo T, Kim IK, Noureddin M, Kuo A, Walid AS, et al: Comparison
of surgical resection and systemic treatment for hepatocellular
carcinoma with vascular invasion: National cancer database
analysis. Liver Cancer. 10:407–418. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ho MC, Hasegawa K, Chen XP, Nagano H, Lee
YJ, Chau GY, Zhou J, Wang CC, Choi YR, Poon RT and Kokudo N:
Surgery for intermediate and advanced hepatocellular carcinoma: A
consensus report from the 5th Asia-Pacific primary liver cancer
expert meeting (APPLE 2014). Liver Cancer. 5:245–256. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Torzilli G, Belghiti J, Kokudo N, Takayama
T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E,
Donadon M, et al: A snapshot of the effective indications and
results of surgery for hepatocellular carcinoma in tertiary
referral centers: Is it adherent to the EASL/AASLD
recommendations?: An observational study of the HCC East-West study
group. Ann Surg. 257:929–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kokudo T, Hasegawa K, Matsuyama Y,
Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima
O, et al: Survival benefit of liver resection for hepatocellular
carcinoma associated with portal vein invasion. J Hepatol.
65:938–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cheng S, Yang J, Shen F, Zhou W, Wang Y,
Cong W, Yang GS, Cheng H, Hu H, Gao C, et al: Multidisciplinary
management of hepatocellular carcinoma with portal vein tumor
thrombus-Eastern hepatobiliary surgical hospital consensus
statement. Oncotarget. 7:40816–40829. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Václav T: Surgical treatment of
hepatocellular carcinoma. Klin Onkol. 33 (Suppl 3):S30–S33.
2020.
|
|
41
|
Tsoulfas G, Agorastou P, Tooulias A and
Marakis GN: Current and future challenges in the surgical treatment
of hepatocellular carcinoma: A review. Int Surg. 99:779–786. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Delis SG and Dervenis C: Selection
criteria for liver resection in patients with hepatocellular
carcinoma and chronic liver disease. World J Gastroenterol.
14:3452–3460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ohkubo T, Yamamoto J, Sugawara Y, Shimada
K, Yamasaki S, Makuuchi M and Kosuge T: Surgical results for
hepatocellular carcinoma with macroscopic portal vein tumor
thrombosis. J Am Coll Surg. 191:657–660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiao T, Tang H, Zhang W, Hu B, Wan T, Cao
Y, Zhang Z, Wang Y, Cao J, Cui M and Lu S: Long-term survival and
portal vein patency with novel PVTT surgery approach in advanced
HCC patients with Vp3/4 PVTT following combination therapy of TKIs
and PD-1 inhibitors. BMC Surg. 23:3842023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang J, Yan L and Wang W: Current status
of multimodal & combination therapy for hepatocellular
carcinoma. Indian J Med Res. 136:391–403. 2012.PubMed/NCBI
|
|
46
|
Lau WY, Ho SKW, Yu SC, Lai ECH, Liew CT
and Leung TWT: Salvage surgery following downstaging of
unresectable hepatocellular carcinoma. Ann Surg. 240:299–305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fimognari FL and Violi F: Portal vein
thrombosis in liver cirrhosis. Intern Emerg Med. 3:213–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu JI and Park HC: Radiotherapy as valid
modality for hepatocellular carcinoma with portal vein tumor
thrombosis. World J Gastroenterol. 22:6851–6863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yoon HI, Song KJ, Lee IJ, Kim DY, Han KH
and Seong J: Clinical benefit of hepatic arterial infusion
concurrent chemoradiotherapy in locally advanced hepatocellular
carcinoma: A propensity score matching analysis. Cancer Res Treat.
48:190–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nakazawa T, Hidaka H, Shibuya A, Okuwaki
Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S and Koizumi
W: Overall survival in response to sorafenib versus radiotherapy in
unresectable hepatocellular carcinoma with major portal vein tumor
thrombosis: Propensity score analysis. BMC Gastroenterol.
14:842014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shiozawa K, Watanabe M, Ikehara T,
Matsukiyo Y, Kogame M, Kishimoto Y, Okubo Y, Makino H, Tsukamoto N,
Igarashi Y and Sumino Y: Comparison of percutaneous radiofrequency
ablation and CyberKnife(®) for initial solitary
hepatocellular carcinoma: A pilot study. World J Gastroenterol.
21:13490–13499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hamaoka M, Kobayashi T, Kuroda S, Iwako H,
Okimoto S, Kimura T, Aikata H, Nagata Y, Chayama K and Ohdan H:
Hepatectomy after down-staging of hepatocellular carcinoma with
portal vein tumor thrombus using chemoradiotherapy: A retrospective
cohort study. Int J Surg. 44:223–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu F, Chen B, Dong D, Rong W, Wang H, Wang
L, Wang S, Jin J, Song Y, Liu Y, et al: Phase 2 evaluation of
neoadjuvant intensity-modulated radiotherapy in centrally located
hepatocellular carcinoma: A nonrandomized controlled trial. JAMA
Surg. 157:1089–1096. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chang Y, Jeong SW, Young Jang J and Jae
Kim Y: Recent updates of transarterial chemoembolilzation in
hepatocellular carcinoma. Int J Mol Sci. 21:81652020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen C, Qiu H, Yao Y, Zhang Z, Ma C, Ma Y,
Zhao C, Xiang H, Zhao H, Zheng C, et al: Comprehensive predictive
factors for CalliSpheres® microspheres (CSM)
drug-eluting bead-transarterial chemoembolization and conventional
transarterial chemoembolization on treatment response and survival
in hepatocellular carcinoma patients. Clin Res Hepatol
Gastroenterol. 45:1014602021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, Li
ZK, Qi F and Zhao C: Transarterial chemoembolization compared with
conservative treatment for advanced hepatocellular carcinoma with
portal vein tumor thrombus: Using a new classification. Med Oncol.
29:2992–2997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Iwamoto H, Shimose S, Shirono T, Niizeki T
and Kawaguchi T: Hepatic arterial infusion chemotherapy for
advanced hepatocellular carcinoma in the era of chemo-diversity.
Clin Mol Hepatol. 29:593–604. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hatooka M, Kawaoka T, Aikata H, Inagaki Y,
Morio K, Nakahara T, Murakami E, Tsuge M, Hiramatsu A, Imamura M,
et al: Hepatic arterial infusion chemotherapy followed by sorafenib
in patients with advanced hepatocellular carcinoma (HICS 55): An
open label, non-comparative, phase II trial. BMC Cancer.
18:6332018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen XP and Huang ZY: Surgical treatment
of hepatocellular carcinoma in China: Surgical techniques,
indications, and outcomes. Langenbecks Arch Surg. 390:259–265.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen XP, Qiu FZ, Wu ZD and Zhang BX:
Chinese experience with hepatectomy for huge hepatocellular
carcinoma. Br J Surg. 91:322–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Clark TWI: Complications of hepatic
chemoembolization. Semin Intervent Radiol. 23:119–125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou WP, Lai ECH, Li AJ, Fu SY, Zhou JP,
Pan ZY and Lau WYand Wu MC: A prospective, randomized, controlled
trial of preoperative transarterial chemoembolization for
resectable large hepatocellular carcinoma. Ann Surg. 249:195–202.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wu X, Wang Y, Wang S, Chen Y, Han J, Wang
C, Zhang M, Hu X, Song B, Wan X, et al: Neoadjuvant targeted
immunotherapy followed by surgical resection versus upfront surgery
for hepatocellular carcinoma with macrovascular invasion: A
multicenter study. J Cancer. 15:3024–3033. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu QQ, Chen YX, Zheng TT, Dai MT, Ye T, Xu
YH, Hu Y, Du SS and Zeng ZC: Which is the best combination of
surgery for hepatocellular carcinoma with hepatic/portal vein
thrombosis in China: A network meta-analysis of randomized
controlled trials. J BUON. 26:889–896. 2021.PubMed/NCBI
|
|
66
|
Wu QQ, Gao H, Du SS, Chen YX, Hu Y, Yang
P, Hou JZ and Zeng ZC: Comparing the efficacy and safety of
local-regional treatments for hepatocellular carcinoma with
portal/hepatic vein tumor thrombosis in China: A network
meta-analysis of randomized controlled trials. J BUON.
26:1950–1957. 2021.PubMed/NCBI
|
|
67
|
Leung JH, Wang SY, Leung HWC and Chan ALF:
Comparative efficacy and safety of multimodality treatment for
advanced hepatocellular carcinoma with portal vein tumor thrombus:
Patient-level network meta-analysis. Front Oncol. 14:13447982024.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lee HA, Seo YS, Shin IS, Yoon WS, Lee HY
and Rim CH: Efficacy and feasibility of surgery and external
radiotherapy for hepatocellular carcinoma with portal invasion: A
meta-analysis. Int J Surg. 104:1067532022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chan SL, Chong CCN, Chan AWH, Poon DMC and
Chok KSH: Management of hepatocellular carcinoma with portal vein
tumor thrombosis: Review and update at 2016. World J Gastroenterol.
22:7289–7300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lu J, Zhang XP, Zhong BY, Lau WY, Madoff
DC, Davidson JC, Qi X, Cheng SQ and Teng GJ: Management of patients
with hepatocellular carcinoma and portal vein tumour thrombosis:
Comparing east and west. Lancet Gastroenterol Hepatol. 4:721–730.
2019. View Article : Google Scholar : PubMed/NCBI
|