Introduction
Pseudomonas aeruginosa (P.
aeruginosa), a Gram-negative, aerobic pathogen, infects both
immunocompetent and immunocompromised hosts. It can lead to a
variety of community-acquired illnesses, including pneumonia,
osteomyelitis-causing puncture wounds, folliculitis, urinary tract
infections, septicemia, endocarditis and otitis externa (swimmer's
ear) (1). Exopolysaccharide, lytic
enzymes, such as protease and elastase, pyocyanin pigment formation
and motility are all necessary for the pathogenic activity of P.
aeruginosa (2-4).
Quorum sensing (QS) depends on protein systems that
synthesize auto inducer (AI). P. aeruginosa uses four
primary QS systems, each of which is linked with its unique
autoinducer: 3-oxododecanoyl-L-homoserine lactone (3-oxo-C12-HSL),
N-butanoyl homoserine lactone (C4-HSL),
2-heptyl-3-hydroxy-4-quinolone (Pseudomonas quinolone
signal) and 2-(2-hydroxy-phenyl)-thiazole-4-carbaldehyde
(integrated quorum sensing signal) (5). When these acyl-homoserine lactone
(AHL) molecules bind to their respective receptors, LasR and RhlR
induce the expression of pathogenic traits (2,6). The
RhlI/RhlR system regulates rhamnolipid expression, while the
LasI/LasR system also significantly contributes to virulence
factors (7). The synchronized
expression of these factors in response to QS enables bacteria to
adapt to shifts in population density and environmental conditions.
Targeting and disrupting these QS systems with antimicrobial
compounds may be a promising approach with which to combat P.
aeruginosa infections. The production of virulence factors
frequently reduces the efficacy of a number of antibiotics,
resulting in increased bacterial pathogenicity and a mortality rate
of 18 to 61% in patients (8).
Natural products have long been a source of novel
pharmacological compounds, and there is a renewed interest in
examining them as potential candidates for drugs, particularly in
the fight against antimicrobial resistance (9). Plant-based bioactive compounds can
suppress disease pathogenesis-related genes by disrupting
QS-associated virulence factors and preventing biofilm formation.
Research findings indicate that natural products exert synergistic
effects when combined with antibiotics against microbial pathogens
(10,11). A wide range of natural products
with medicinal properties, including flavonoids (e.g., curcumin and
quercetin), quinones (e.g., plumbagin), alkaloids (e.g., piperine),
triterpenoids and essential oil phenols (e.g., eugenol and thymol),
have been shown to be effective against certain bacteria (9). Some of these products have been
demonstrated to be potent antibacterial and antibiofilm agents;
they can also inhibit cell attachment and adhesion, and suppress
the production of virulence factors, inhibit polymer matrix
formation, and thus disrupt the QS network (12,13).
Therefore, determining potent QS inhibitors is essential, ideally
sourced from natural sources.
Artocarpus heterophyllus Lam. (A.
heterophyllus), belonging to the family Moraceae, produces the
largest edible fruit among evergreen trees, commonly known as
jackfruit. This species yields more fruit than any other fruit tree
species. However, despite its abundance, the leaves of the
jackfruit tree (A. heterophyllus) are often regarded as
agro-industrial waste. Only a small proportion of the total biomass
is utilized, primarily as cattle fodder, and occasionally, for
managing asthma, diarrhea and dermatitis. Traditional medicine has
extensively utilized the fruits, leaves and bark of the jackfruit
tree due to their various medicinal properties. These include
anticarcinogenic, antibacterial, antifungal, anti-inflammatory,
wound-healing and hypoglycemic qualities (14). According to research, A.
heterophyllus contains bioactive compounds, such as alkaloids,
flavonoids, phenolic acids and terpenoids, which are known for
their antimicrobial properties. These compounds have the potential
to limit the growth and survival of pathogenic microbes, either
individually or in combination (15). It has been previously demonstrated
that flavonoids, particularly artonin and artocapones, exhibit
anti-plasmodial activity (16).
Additionally, the methanolic extract of dried A.
heterophyllus leaf powder has been shown to exhibit potent
antibacterial properties against a variety of microbes, including
Escherichia coli (E. coli) and Salmonella
enterica (S. enterica) (17). Furthermore, ethyl acetate extracts
from unutilized parts of the jackfruit have been found to exhibit
optimal antibacterial activity against Xanthomonas
axonopodis, with the peels exhibiting the most potent
inhibitory effects, followed by the fiber and the core exhibiting
the least potent effects (18).
Additionally, it has been reported that A. heterophyllus
exhibits antimicrobial activity against certain foodborne pathogens
(19). In a previous study, it was
found that the seed powder extract of A. heterophyllus,
which is used in the green synthesis of silver nanoparticles
(AgNPs) from an aqueous solution of silver nitrate (AgNO3),
contains jacalin (20). This
lectin constitutes more than half of the proteins in the jackfruit
crude seed extract and has a variety of biological activities. The
resulting AgNPs demonstrate potent antibacterial activity against
both Gram-positive and -negative bacteria, suggesting potential
applications in nanomedicine (20). In the study conducted by Sato et
al (21), it was found that
artocarpin, extracted from A. heterophyllus, demonstrated
potent antibacterial activity against cariogenic bacteria, with
minimum inhibitory concentration (MIC) values ranging from 3.13 to
12.5 µg/ml. At this MIC, the compound effectively inhibited the
growth of cariogenic bacteria (21). Furthermore, Sun et al
(22) found that artocarpin
exerted selective cytotoxic effects on human colon cancer cells. It
attenuated anchorage-independent growth, inhibited colon cancer
cell growth and caused G1 phase cell cycle arrest, followed by
apoptotic and autophagic death (22).
It has also been shown that isolated bioactive
compounds from A. heterophyllus fruits exert
anti-inflammatory effects. Jackfruit contains flavonoids, which can
inhibit the production of inflammatory chemicals from mast cells,
neutrophils and macrophages (23).
Prakash et al (24)
documented in their study that A. heterophyllus contains
compounds, such as morin, dihydromorin, cynomacurin, artocarpin,
isoartocarpin, cycloartocarpin, artocarpesin,
oxydihydroartocarpesin, artocarpetin, betulinic acid, artocarpanone
and heterophylol. These compounds have been found to be beneficial
in treating fever, boils, wounds, skin diseases, convulsions,
diuretic conditions, constipation, ophthalmic disorders and snake
bites (24). Fernando et al
(25) found that a hot water
extract of A. heterophyllus leaves significantly improved
glucose tolerance in both normal and diabetic subjects when taken
orally in doses of 20 g/kg.
Numerous studies have focused on A.
heterophyllus as an achievable source of starch. Tulyathan
et al (26) reported that
jackfruit seeds contain ~20% starch on a dry basis, the recovery
yield of starch extracted from jackfruit seeds was ~77%, indicating
that it could be a valuable source of starch for the food and
pharmaceutical industries (26).
These properties highlight the potential of A.
heterophyllus. The primary aim of the present study was to
examine the effects of A. heterophyllus on P.
aeruginosa (PAO1). To the best of our knowledge, the
anti-quorum sensing (anti-QS) properties of A. heterophyllus
are unexplored in relation to P. aeruginosa.
The present study aimed to investigate the
antimicrobial and antibiofilm properties of A. heterophyllus
against PAO1. The objective was to evaluate the effectiveness of
A. heterophyllus in preventing and disrupting biofilm
formation by this common pathogen.
Materials and methods
Sample collection
In the present study, which was conducted from
January to October, 2023, A. heterophyllus was obtained from
an indigenous botanical garden in Chennai, Tamil Nadu, India. A
qualified botanist examined the plant and confirmed its
authenticity. The leaves were cleaned with water and allowed to dry
naturally for 1 week. To prepare the extract, 10 g A.
heterophyllus leaf powder were mixed with 50 ml methanol and
distributed across two maceration containers for 48 h, with
occasional shaking using a shaker. Following the extraction
process, the resulting suspension was filtered through No. 1 filter
paper (Whatman, HiMedia Laboratories, LLC) and placed over a funnel
lined with a white muslin cloth. The methanol solvent was then
evaporated from the filtrate using a hot water bath set precisely
at 50˚C. The dehydrated filtrate was measured, and the dried
substance was weighed before being stored at 4˚C for later use.
Bacterial strain and growth
condition
The culture of Pseudomonas aeruginosa (PAO1)
samples utilized in the present study was generously provided by Dr
Busi Siddhardha from Pondicherry University, Puducherry, Tamil
Nadu, India. The samples were sub-cultured in Luria Bertani (LB)
broth (HiMedia Laboratories, LLC). PAO1 cultures were then
incubated at 37˚C in a shaking incubator set at 100 rpm for 24 h.
Characteristic growth patterns were observed on LB agar and
Nutrient agar. To verify the identity of PAO1, a preliminary
identification was conducted by laboratory personnel at Saveetha
Dental College and Hospital in Chennai, Tamil Nadu, India,
utilizing the VITEK 2 automated system. As previously described by
David H. Pincus (BioMérieux, Inc.), the biochemical reactions of
the bacterial isolate were compared to a comprehensive database to
provide accurate identification (27). In addition, various phenotypic
tests, including Gram staining, catalase, oxidase, motility and
citrate utilization, were conducted and documented based on
standard microbiological investigations, as previously described by
others (28). The bacterial
cultures underwent routine sub-culturing for experimental use.
Antimicrobial activity and antibiotic
susceptibility testing (AST)
The agar well-diffusion method, an established
technique, was employed to evaluate the antibacterial activity of
A. heterophyllus (29). The
bacterial culture of PAO1 was spread onto Mueller Hinton agar (MHA)
(HiMedia Laboratories, LLC) using a swab moistened with the
bacterial suspension. Subsequently, a well with a diameter of 8 mm
was punched into the MHA medium using a sterile cork borer, and a
well was filled with 50 µl A. heterophyllus extract, while
water served as a control in another well. The plate was then
incubated upright at 37˚C for a 24 h. Following incubation, the
zone of inhibition around the wells was measured using a Vernier
caliper on a mm scale to detect the antibacterial activity of the
A. heterophyllus extract.
Subsequently, the antibiotic susceptibility of PAO1
was assessed using the Kirby-Bauer disk diffusion method (30). Using this standard technique, the
authors were able to determine the susceptibility of PAO1 to
various antibiotics. Initially, the culture of PAO1 was evenly
distributed onto MHA plates using a sterile swab saturated with the
bacterial suspension. Subsequently, these plates were subjected to
testing with a comprehensive panel of conventional antibiotics,
including colistin, tobramycin, ciprofloxacin, azlocillin,
aminoglycosides, netilmicin, piperacillin and carbenicillin
(HiMedia Laboratories, LLC).
Evaluation of MIC
A 2-fold broth dilution method was used to determine
the MIC of A. heterophyllus extract against PAO1. The
assessment was carried out at concentrations ranging from 10 to
0.01 mg/ml. The MIC for the methanol extract was determined using
established protocols (31,32).
In brief, 20 µl PAO1 broth culture with the cell mass equivalent of
0.5 McFarland turbidity standard (1.5x108 CFU/ml) was
filled in tubes containing LB broth. Following the successive
dilution with A. heterophyllus extract, each of the tubes
underwent incubation at 37˚C for 24 h. Following incubation, 40 µl
2,3,5-triphenyl tetrazolium chloride (HiMedia Laboratories, LLC)
was added to each tube to check for color changes and confirm the
results. The minimum concentration that caused no growth (no color
change) was recorded as the MIC. Additional antibiofilm studies
were carried out in accordance with these findings.
Crystal violet biofilm inhibition
assay
To determine the effects of A. heterophyllus
extract on PAO1 biofilm formation, the crystal violet staining
assay was used (32). An overnight
culture of PAO1 (20 µl) was added to a microtiter plate containing
180 µl fresh LB medium, and the A. heterophyllus extract was
added in a concentration-dependent manner (ranging from 5 to 0.009
mg/ml). The mixture was incubated for 48 h at 37˚C. Following
incubation, the surface-adherent biofilm was stained with a 0.1%
crystal violet solution (HiMedia Laboratories, LLC) at room
temperature for 2 min, and the planktonic cells were washed away
with sterile distilled water. After 10 min, the CV-bound biofilm
was eluted in 200 µl 70% ethanol. A UV-Vis spectrophotometer (JASCO
UV/Vis, India) was used to determine the concentration of the
eluted CV by measuring the crystal violet intensity at 520 nm. The
percentage of inhibition was then calculated using the following
equation: Control optical density (OD) 520 nm-treated OD 520
nm/control OD 520 nm x100.
Bacterial growth curve
The growth curve analysis was conducted following
previously established protocols (33). The concentration of 2.5 mg/ml A.
heterophyllus was selected based on the crystal violet biofilm
inhibition assay, which indicated that this concentration inhibited
PAO1 biofilm formation by 55.12%. Subsequently, the present study
wished to confirm its influence on bacterial growth at the same
concentration through growth curve experiments to determine whether
it inhibited growth as well. To encapsulate, the present study
delved into the growth dynamics of PAO1 bacteria under dual
conditions, with or without the presence of A.
heterophyllus, at a concentration of 2.5 mg/ml. The cultures
were meticulously incubated at 37˚C, with hourly recordings of OD
at 600 nm spanning a duration of up to 24 h.
Statistical evaluation
Each experiment was carried out three times, with
statistical significance rigorously demonstrated for both the
growth curve analysis and biofilm quantification. The Student's
t-test with GraphPad prism 10.1.0 software (Dotmatics) served as
the tool for statistical analysis. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Bacterial identification of PAO1
The VITEK 2 system accurately confirmed the identity
of the bacterial strain as PAO1 with high confidence. The
biochemical profile aligned with the expected characteristics for
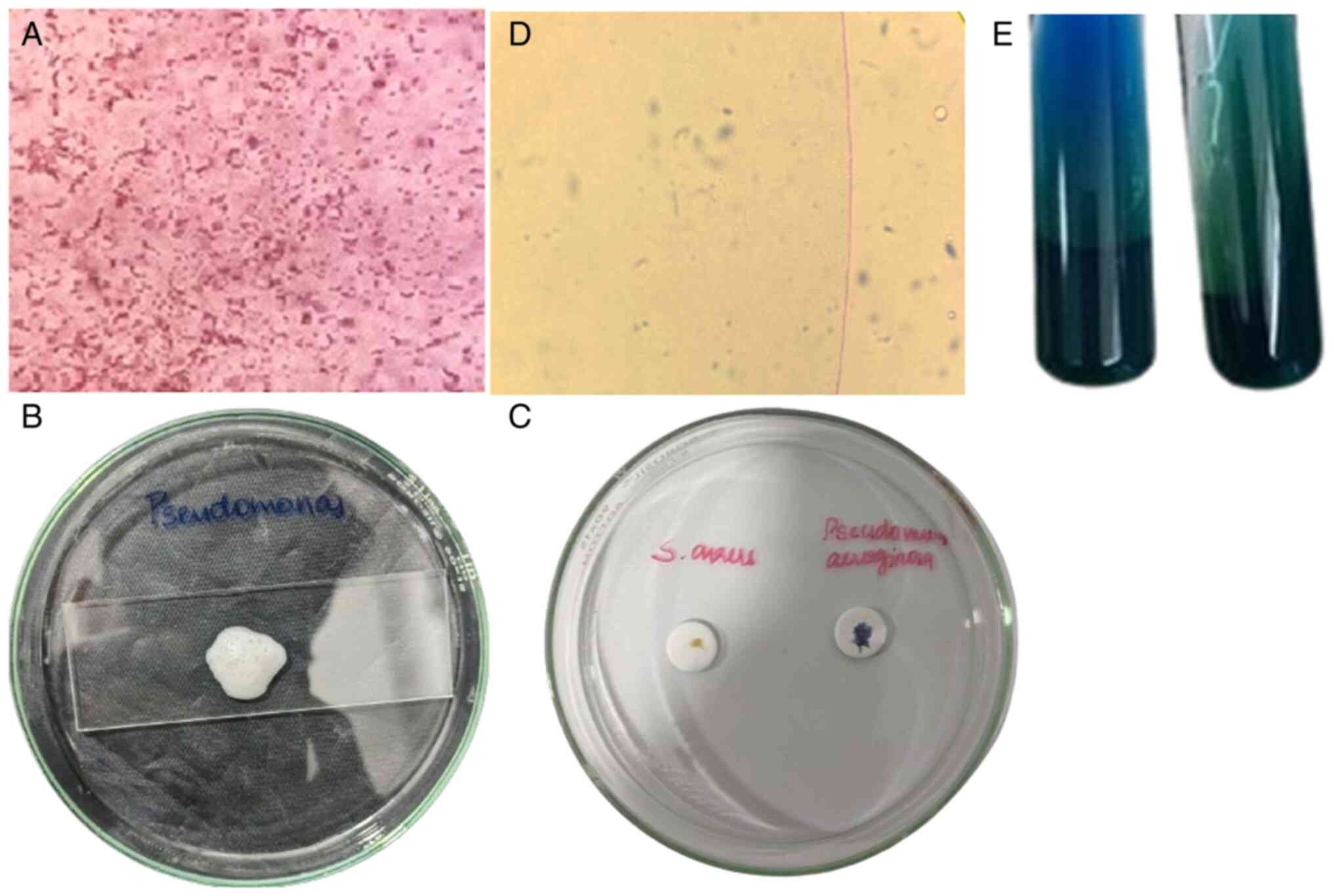
PAO1, thus validating the initial identification methods (34). The morphological profiling of the
bacterial isolates further supported the findings, revealing
distinct morphotypes consistent with PAO1. Notably, Gram staining
revealed the characteristic Gram-negative, rod-shaped morphology of
PAO1 (Fig. 1A). Additionally, the
identity of PAO1 was further confirmed by positive results in
catalase (Fig. 1B), oxidase
(Fig. 1C), motility (Fig. 1D) and citrate utilization (Fig. 1E) tests.
Antimicrobial susceptibility and
AST
The present study initially examined the
antimicrobial activity of A. heterophyllus extract against
PAO1, indicated by a measured zone of inhibition with a recorded
diameter of 8 mm, highlighting its potent efficacy (Fig. 2). Subsequent AST, conducted in
accordance with the Clinical and Laboratory Standards Institute
(CLSI) Guidelines 2022(35),
unveiled PAO1 resistance to a majority of tested drugs, including
colistin, ciprofloxacin, piperacillin and azlocillin (Table I).
 | Table IAntibiogram of PAO1 against several
antibiotics. |
Table I
Antibiogram of PAO1 against several
antibiotics.
| Serial no. | Antibiotics | P.
aeruginosa (PAO1) |
|---|
| 1 | Tobramycin | 22.3±2.9 |
| 2 | Azlocillin | R |
| 3 |
Aminoglycosides | 15±0.9 |
| 4 | Netilmicin | 18±1.1 |
| 5 | Piperacillin | R |
| 6 | Colistin | R |
| 7 | Ciprofloxacin | R |
| 8 | Carbenicillin | 16±1.1 |
Antibacterial activity of A.
heterophyllus at the MIC level
By employing a 2-fold serial dilution method, the
present study explored the antibacterial potential of the A.
heterophyllus extract across a spectrum ranging from 10 to
0.019 mg/ml. Notably, at the end point concentration of 10 mg/ml,
the inhibition of PAO1 growth was observed (Table II), suggestive of the promising
antimicrobial efficacy of A. heterophyllus against PAO1.
This prompted further investigation into the anti-biofilm
properties of the A. heterophyllus extract at sub-MIC
concentrations.
 | Table IIMinimum inhibitory concentration. |
Table II
Minimum inhibitory concentration.
| Serial no. | Two-fold dilution
concentration (mg/ml) | Growth
measureda |
|---|
| 1 | 10 | - |
| 2 | 5 | + |
| 3 | 2.5 | + |
| 4 | 1.25 | + |
| 5 | 0.62 | + |
| 6 | 0.312 | + |
| 7 | 0.156 | + |
| 8 | 0.078 | + |
| 9 | 0.039 | + |
| 10 | 0.019 | + |
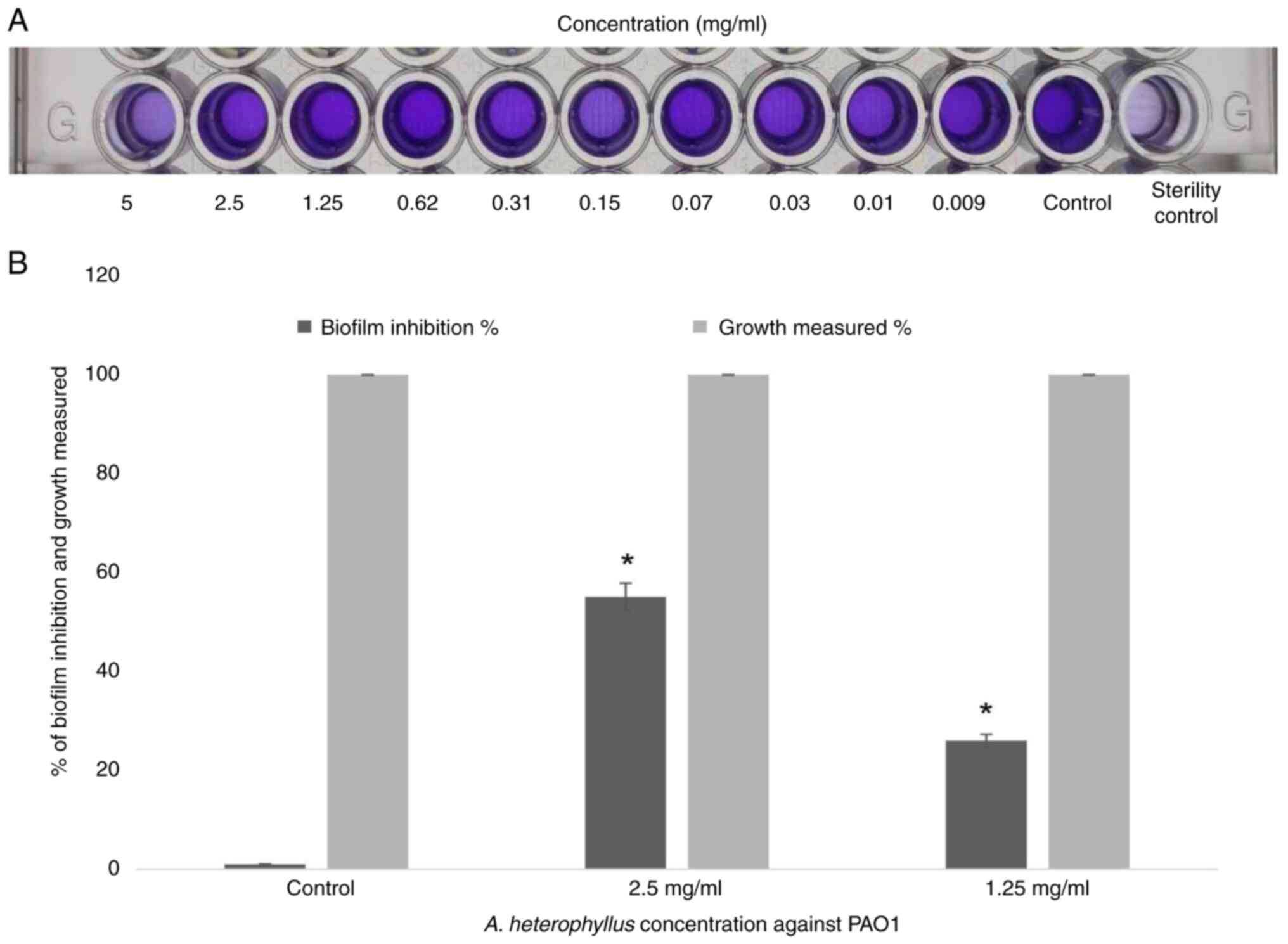
Effect on biofilm formation
The present study investigated the ability of A.
heterophyllus, at concentrations ranging from 5 to 0.009 mg/ml,
to inhibit PAO1 biofilm formation using 0.1% crystal violet dye on
a static microtiter plate. The findings indicated that the A.
heterophyllus extract significantly reduced biofilm formation
when PAO1 was exposed to concentrations of 2.5 and 1.25 mg/ml,
resulting in reductions of 55.12 and 26%, respectively. In the
control, no biofilm inhibition was observed (without A.
heterophyllus extract) (Fig.
3). Therefore, conducting a bacterial growth curve analysis
will further assess the efficacy of the A. heterophyllus
extract at these same concentrations.
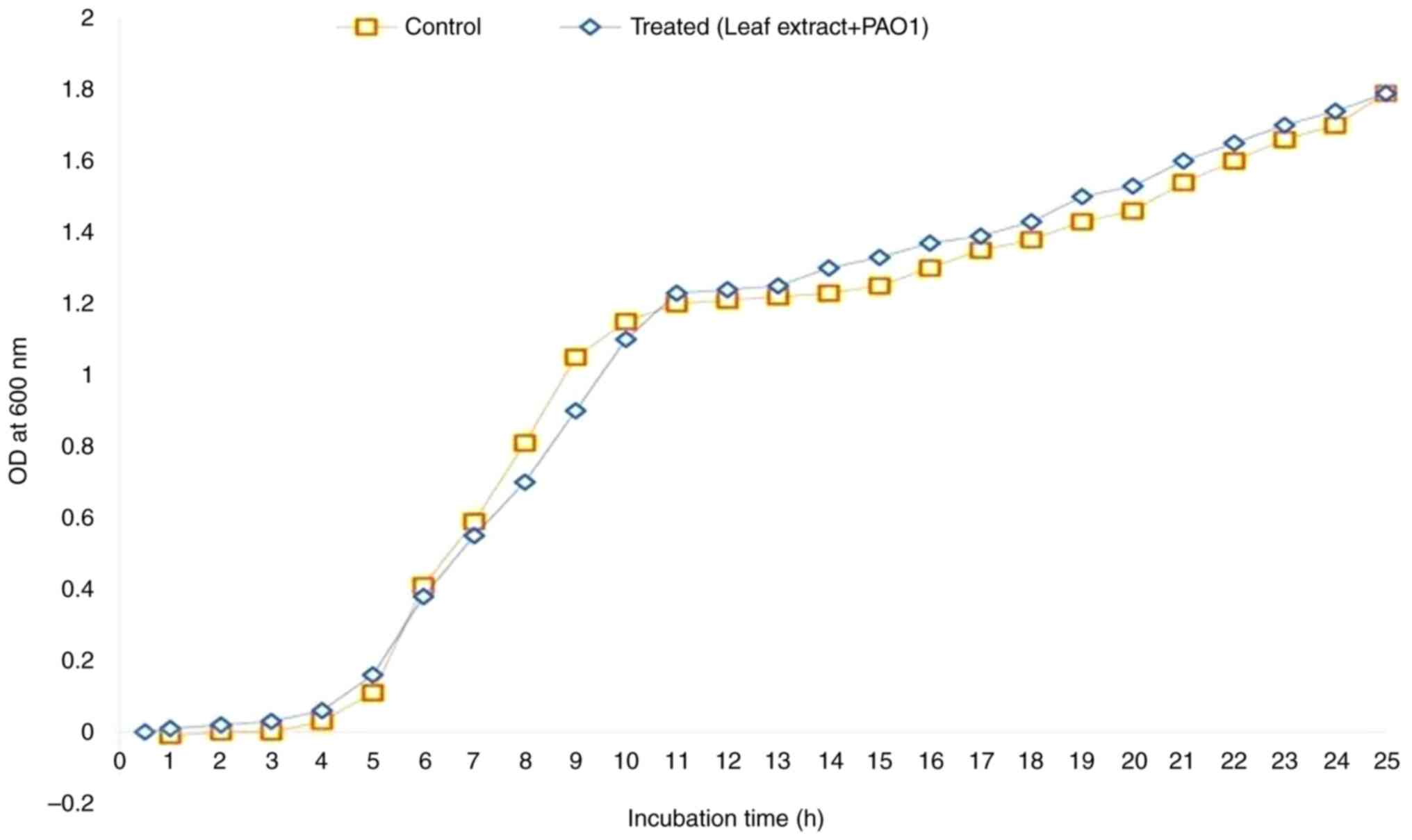
Bacterial growth curve analysis
The growth curve was analyzed both with and without
A. heterophyllus. The results revealed that A.
heterophyllus at a concentration of 2.5 mg/ml did not inhibit
bacterial growth (Fig. 4). The
spectrophotometric analysis revealed no noticeable disparity
between the control and treated bacterial cells at 600 nm. These
findings suggest that A. heterophyllus does not exert
inhibitory effects on bacterial growth at a concentration of 2.5
mg/ml under the tested conditions. However, it does exhibit
inhibitory effects specifically on biofilm formation.
Discussion
Gram-negative bacteria primarily cause infections
and form biofilms due to the activity of QS signaling molecules.
However, treating infections caused by biofilm-forming P.
aeruginosa poses a considerable challenge due to bacterial
resistance to traditional antibiotics (36). The AHL molecule plays a crucial
role in the pathogenesis of P. aeruginosa, and inhibiting
its activity holds promise for mitigating the pathogenicity of this
pathogen.
The present study evaluated the potency of A.
heterophyllus, which contains bioactive compounds, such as
alkaloids, flavonoids, phenolic acids and terpenoids, and examined
whether it has the ability to reduce AHL-dependent factors
production in PAO1. In the study by Khan et al (17), A. heterophyllus, which
contains flavonoids, was shown to exert an inhibitory effect on
several bacteria, including E. coli and S. enterica
(17). Similarly, a recent study
by Alam et al (37)
demonstrated that the plant-derived extract, Berginia
ciliata, which also contains flavonoids, inhibited the
pathogenic bacteria PAO1(37).
The present study evaluated the antibiofilm
activities of A. heterophyllus extract against PAO1. The
preliminary findings indicated that A. heterophyllus
effectively inhibited biofilm formation at the lowest concentration
of 10 mg/ml. The current data support the findings of the study by
Sivagnanasundaram and Karunanayake (38), which reported that A.
heterophyllus had potent bactericidal activity against E.
coli at 3 mg/ml, and the presence of phytosterols and
terpenoids was reported (38). In
another study, it was found that artocarpin, extracted from A.
heterophyllus, inhibited the growth of Streptococcus
mutans at an MIC of 1.95 µg/ml by altering cell membrane
permeability, leading to the release of intracellular proteins
(39). The study by Sato et
al (21) demonstrated that
artocarpin exhibited potent antibacterial activity against
cariogenic bacteria, with MIC values ranging from 3.13 to 12.5
µg/ml. At these MIC levels, artocarpin was able to inhibit the
growth of cariogenic bacteria (21).
In the present study, A. heterophyllus
extract inhibited QS-dependent biofilm formation in PAO1 at
concentrations below the MIC. The crystal violet biofilm inhibition
assay revealed that at a concentration of 2.5 mg/ml, the A.
heterophyllus extract significantly reduced biofilm formation
(Fig. 3). Vijayaraghavan et
al (40) reported that peel
waste from jackfruit was identified as a source for anaerobic
biohydrogen production and exhibited potential for removing toxic
dyes and chemicals from wastewater released by the textile and
pharmaceutical industries. Moreover, Majik et al (41) reported that natural pyrrolidine
alkaloid (R)-Bgugaine extracted from Arisarum vulgare, which
decreased the biofilm density by 83%, also inhibited the pyocyanin
pigmentation, LasA protease and rhamnolipid production of
PAO1(41). Similarly, Vijayakumar
and Ramanathan (42) reported that
the tropical plant Musa acuminata contains flavonoids and
5-hydroxymethylfurfural, which inhibited P. aeruginosa
biofilm formation at a concentration of 400 µg/ml. Furthermore,
methanolic extract from the leaves and stems of A.
heterophyllus contains chromones and flavonoids that have
anti-proliferative activity. These compounds have been shown to
exert significant inhibitory effects against various human cancer
cells, with IC50 values ranging from 0.36±0.02 to
22.09±0.16 µM (43). A previous
study found that flavonoids act to inhibit bacterial movement and
reduce biofilm formation in P. aeruginosa, while also
inhibiting the production of bacterial toxins in Staphylococcus
aureus (44).
In the present study, growth curve analysis
conducted in the presence of A. heterophyllus extract at a
concentration of 2.5 mg/ml, as depicted in Fig. 4, indicated that A.
heterophyllus did not impede bacterial growth. These results
suggest that, under the conditions tested, A. heterophyllus
does not exert inhibitory effects on bacterial growth at the
specified concentration. However, it is noteworthy that A.
heterophyllus does demonstrate inhibitory effects specifically
on biofilm formation. Rashmi et al (45) conducted a growth curve experiment
using antibiofilm concentrations. The results of their study
revealed that the growth pattern of P. aeruginosa with and
without treatment of Alternaria alternata extract at three
concentrations did not impede bacterial growth. However, the
extract exhibited inhibitory effects specifically on biofilm
formation (45).
Taken together, the results of the present study
suggest that the methanol extract of A. heterophyllus
inhibits the QS system in PAO1 by targeting the LasI/LasR and
RhlI/RhlR systems, possibly due to the presence of bioactive
compounds. However, additional research is warranted to identify
active components within A. heterophyllus extract that may
harbor anti-QS and anti-biofilm producing properties.
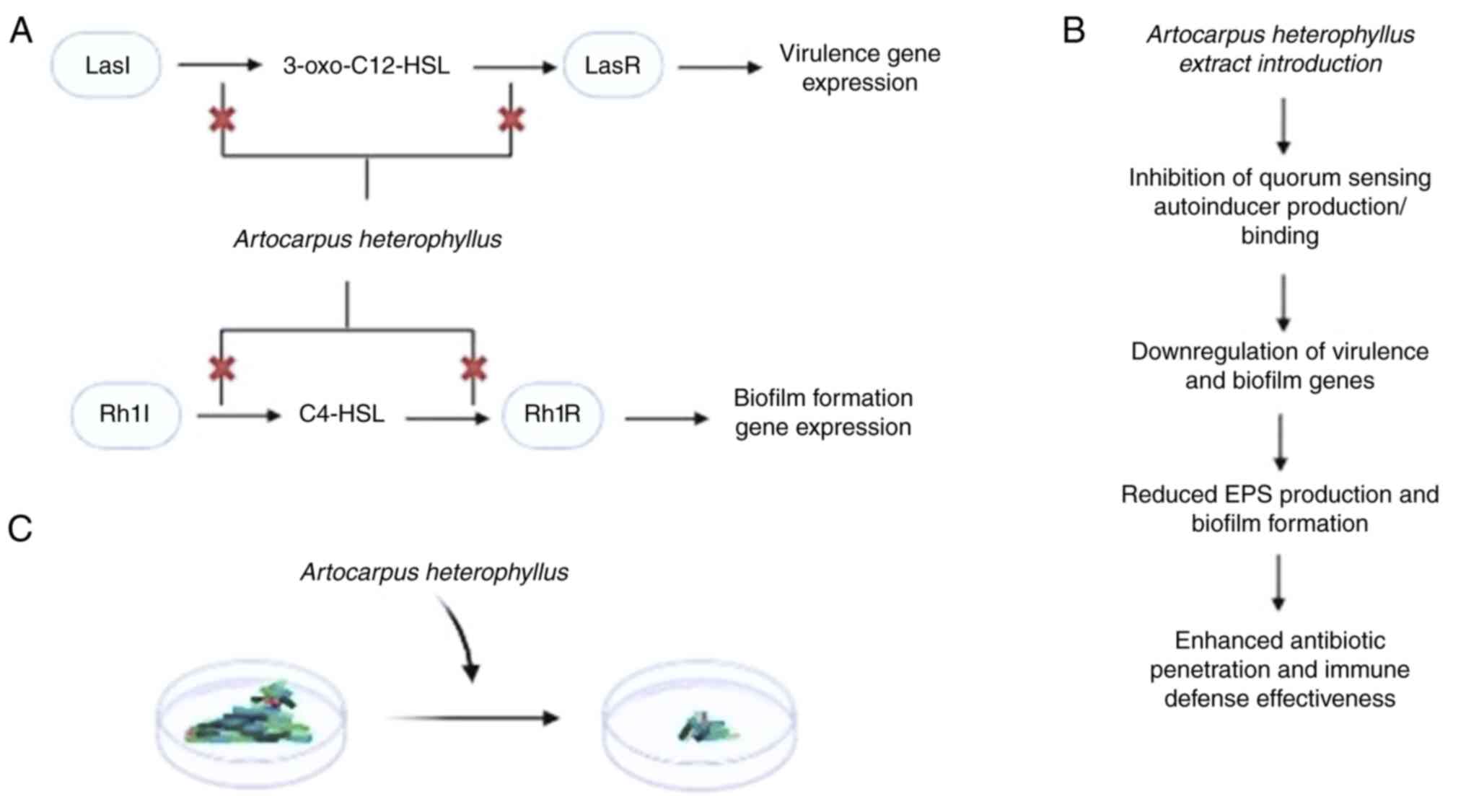
In conclusion, A. heterophyllus was found to
exhibit notable potential in disrupting the QS pathways in PAO1.
Specifically, it targets the LasI/LasR and RhlI/RhlR systems. By
inhibiting the production of autoinducer molecules, such as
3-oxo-C12-HSL and C4-HSL, or by preventing their binding to the
LasR and RhlR receptors, A. heterophyllus downregulates the
expression of genes critical for virulence and biofilm formation
(Fig. 5A). This interference leads
to a reduction in extracellular polymeric substance production and
impaired biofilm development (Fig.
5B). Additionally, the extract introduction enhances antibiotic
penetration and immune defense effectiveness (Fig. 5C).
The results of the present study suggest that the
significant inhibition of the QS system in PAO1 by A.
heterophyllus is likely due to its bioactive compounds, such as
alkaloids, flavonoids, phenolic acids and terpenoids. The methanol
extract of A. heterophyllus demonstrates considerable
potential in restoring the effectiveness of antibiotics by
facilitating their penetration through the compromised biofilm
structure of PAO1. By degrading the biofilm matrix, this extract
can render the infectious bacteria more accessible to immune
defense.
Considering the broad availability of jackfruit in
India and the versatile use of all of its parts, including wood and
latex, which are known to have therapeutic properties, there is
significant potential for scientific research into its medicinal
benefits. The present study highlights the importance of exploring
plant-based compounds for enhancing current antimicrobial therapies
and addressing the challenges posed by P. aeruginosa
biofilm-associated infections. Further research with various
formulations would be beneficial in improving the pharmacological
therapeutic applications of the identified compounds, particularly
their anti-QS and anti-biofilm properties. Despite being
historically neglected, jackfruit is gaining recognition for its
medicinal properties, and continued research is essential to unlock
its full potential as an antibiotic adjuvant. In conclusion, A.
heterophyllus could serve as a valuable antibiotic adjuvant,
offering promising strategies to prevent or treat chronic
infections caused by P. aeruginosa. This underscores the
importance of plant-based compounds in addressing the growing
challenge of antibiotic resistance and biofilm-associated
infections.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS collected, managed the data and participated in
the writing of the manuscript. AV and NNP participated in writing
the proposal, performing data collection and in the writing of the
manuscript. RVG and PSG were involved in data curation, data
analysis and in revising the manuscript. RVG and PSG confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kerr KG and Snelling AM: Pseudomonas
aeruginosa: A formidable and ever-present adversary. J Hosp
Infect. 73:338–344. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bjarnsholt T and Givskov M: Quorum-sensing
blockade as a strategy for enhancing host defences against
bacterial pathogens. Philos Trans R Soc Lond B Biol Sci.
362:1213–1222. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Adonizio A, Kong KF and Mathee K:
Inhibition of quorum sensing-controlled virulence factor production
in Pseudomonas aeruginosa by South Florida plant extracts.
Antimicrob Agents Chemother. 52:198–203. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
De Kievit TR: Quorum sensing in
Pseudomonas aeruginosa biofilms. Environ Microbiol.
11:279–288. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee J and Zhang L: The hierarchy quorum
sensing network in Pseudomonas aeruginosa. Protein Cell.
6:26–41. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pesci EC, Pearson JP, Seed PC and Iglewski
BH: Regulation of las and rhl quorum sensing in Pseudomonas
aeruginosa. J Bacteriol. 179:3127–3132. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mukherjee S, Moustafa D, Smith CD,
Goldberg JB and Bassler BL: The RhlR quorum-sensing receptor
controls Pseudomonas aeruginosa pathogenesis and biofilm
development independently of its canonical homoserine lactone
autoinducer. PLoS Pathog. 13(e1006504)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chimi LY, Bisso BN, Njateng GSS and Dzoyem
JP: Antibiotic-potentiating effect of some bioactive natural
products against planktonic cells9410609biofilms, and virulence
factors of Pseudomonas aeruginosa. Biomed Res Int.
2023(9410609)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Atanasov AG, Zotchev SB and Dirsch VM:
International Natural Product Sciences Taskforce. Supuran CT:
Natural products in drug discovery: Advances and opportunities. Nat
Rev Drug Discov. 20:200–216. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hemaiswarya S, Kruthiventi AK and Doble M:
Synergism between natural products and antibiotics against
infectious diseases. Phytomedicine. 15:639–652. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bisso Ndezo B, Tokam Kuaté CR and Dzoyem
JP: Synergistic antibiofilm efficacy of thymol and piperine in
combination with three aminoglycoside antibiotics against
Klebsiella pneumoniae biofilms. Can J Infect Dis Med
Microbiol. 2021(7029944)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guzzo F, Scognamiglio M, Fiorentino A,
Buommino E and D'Abrosca B: Plant derived natural products against
Pseudomonas aeruginosa and Staphylococcus aureus:
Antibiofilm activity and molecular mechanisms. Molecules.
25(5024)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cowan MM: Plant products as antimicrobial
agents. Clin Microbiol Rev. 12:564–582. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ranasinghe RASN, Maduwanthi SDT and
Marapana RAUJ: Nutritional and health benefits of jackfruit
(Artocarpus heterophyllus Lam.): A review. Int J Food Sci.
2019(4327183)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Saha RK, Jamiruddin M and Acharya S:
Comparative analysis of lectins isolated from seed and testa of
Artocarpus heterophyllus LAM. Int J Curr Res Chem Pharma
Sci. 2:65–75. 2015.
|
|
16
|
Vazhacharickal JP, Sajeshkumar NK, Mathew
JJ, Kuriakose AC, Abraham B, Mathew RJ, Albin AN, Thomson D, Thomas
RS, Varghese N and Jose S: Chemistry and medicinal properties of
jackfruit (Artocarpus heterophyllus): A review on current
status of knowledge. Int J Innov Res Rev. 3:83–95. 2015.
|
|
17
|
Khan MR, Omoloso AD and Kihara M:
Antibacterial activity of Artocarpus heterophyllus.
Fitoterapia. 74:501–505. 2003.
|
|
18
|
Adan AA, Ojwang RA, Muge EK, Mwanza BK and
Nyaboga EN: Phytochemical composition and essential mineral
profile, antioxidant and antimicrobial potential of unutilized
parts of jackfruit. Food Res. 4:1125–1134. 2020.
|
|
19
|
Loizzo MR, Tundis R, Chandrika UG,
Abeysekera AM, Menichini F and Frega NG: Antioxidant and
antibacterial activities on foodborne pathogens of Artocarpus
heterophyllus Lam. (moraceae) leaves extracts. J Food Sci.
75:M291–M295. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jagtap UB and Bapat VA: Green synthesis of
silver nanoparticles using Artocarpus heterophyllus Lam.
seed extract and its antibacterial activity. Ind Crops Prod.
46:132–137. 2013.
|
|
21
|
Sato M, Fujiwara S, Tsuchiya H, Fujii T,
Iinuma M, Tosa H and Ohkawa Y: Flavones with antibacterial activity
against cariogenic bacteria. J Ethnopharmacol. 54:171–176.
1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun G, Zheng Z, Lee MH, Xu Y, Kang S, Dong
Z, Wang M, Gu Z, Li H and Chen W: Chemoprevention of colorectal
cancer by artocarpin, a dietary phytochemical from Artocarpus
heterophyllus. J Agric Food Chem. 65:3474–3480. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wei BL, Weng JR, Chiu PH, Hung CF, Wang JP
and Lin CN: Antiinflammatory Flavonoids from Artocarpus
heterophyllus and Artocarpus communis. J Agric Food
Chem. 53:3867–3871. 2005.
|
|
24
|
Prakash O, Gupta R and Banarasi B:
Artocarpus heterophyllus (Jackfruit): An overview. Phcog
Rev. 6:353–358. 2017.
|
|
25
|
Fernando MR, Wickramasinghe S, Thabrew MI,
Ariyananda PL and Karunanayake EH: Effect of Artocarpus
heterophyllus and Asteracanthus longifolia on glucose
tolerance in normal human subjects and in maturity-onset diabetic
patients. J Ethnopharmacol. 31:277–282. 1991.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tulyathan V, Tananuwong K, Songjinda P and
Jaiboon N: Some physicochemical properties of jackfruit
(Artocarpus heterophyllus Lam) seed flour and starch. Sci
Asia. 28:37–41. 2002.
|
|
27
|
Pincus DH: Microbial identification using
the bioMérieux Vitek® 2 system. Encyclopedia of Rapid
Microbiological Methods. Bethesda, MD: Parenteral Drug Association,
pp1-32, 2006.
|
|
28
|
Holt JG and Krieg NR: Bergey's Manual of
Systematic Bacteriology. Vol 2. Williams and Wilkins Publishers,
Baltimore, 2001.
|
|
29
|
Ganesh PS, Veena K, Senthil R, Iswamy K,
Ponmalar EM, Mariappan V, Girija ASS, Vadivelu J, Nagarajan S,
Challabathula D and Shankar EM: Biofilm-associated agr and sar
quorum sensing systems of Staphylococcus aureus are
inhibited by 3-hydroxybenzoic acid derived from Illicium
verum. ACS Omega. 7:14653–14665. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hudzicki J: Kirby-bauer disk diffusion
susceptibility test protocol. American Society for Microbiology,
2009.
|
|
31
|
Packiavathy IASV, Agilandeswari P,
Musthafa KS, Karutha Pandian S and Veera Ravi A: Antibiofilm and
quorum sensing inhibitory potential of Cuminum cyminum and
its secondary metabolite methyl eugenol against Gram negative
bacterial pathogens. Food Res Int. 45:85–92. 2012.
|
|
32
|
Venkatramanan M, Sankar Ganesh P, Senthil
R, Akshay J, Veera Ravi A, Langeswaran K, Vadivelu J, Nagarajan S,
Rajendran K and Shankar EM: Inhibition of quorum sensing and
biofilm formation in Chromobacterium violaceum by fruit
extracts of Passiflora edulis. ACS Omega. 5:25605–25616.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ganesh PS and Rai RV: Inhibition of
quorum-sensing-controlled virulence factors of Pseudomonas
aeruginosa by Murraya koenigii essential oil: a study in
a Caenorhabditis elegans infectious model. J Med Microbiol.
65:1528–1535. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Moehario LH, Tjoa E, Putranata H, Joon S,
Edbert D and Robertus T: Performance of TDR-300B and VITEK®2 for
the identification of Pseudomonas aeruginosa in comparison
with VITEK®-MS. J Int Med Res. 49(300060521989893)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
CLSI. Performance standards for
antimicrobial susceptibility testing, M100. 32nd edition. Clinical
and Laboratory Standards Institute, Wayne, PA, 2022.
|
|
36
|
Pang Z, Raudonis R, Glick BR, Lin TJ and
Cheng Z: Antibiotic resistance in Pseudomonas aeruginosa:
Mechanisms and alternative therapeutic strategies. Biotechnol Adv.
37:177–192. 2019.
|
|
37
|
Alam K, Farraj DAA, Mah-E-Fatima S, Yameen
MA, Elshikh MS, Alkufeidy RM, Mustafa AEMA, Bhasme P, Alshammari
MK, Alkubaisi NA, et al: Anti-biofilm activity of plant derived
extracts against infectious pathogen-Pseudomonas aeruginosa
PAO1. J Infect Public Health. 13:1734–1741. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sivagnanasundaram P and Karunanayake KOLC:
Phytochemical screening and antimicrobial activity of Artocarpus
heterophyllus and Artocarpus altilis leaf and stem bark
extracts. OUSL J. 9:1–17. 2015.
|
|
39
|
Daud NNNNM, Septama AW, Simbak N and Rahmi
EP: The phytochemical and pharmacological properties of artocarpin
from Artocarpus heterophyllus. Asian Pac J Trop Med. 13:1–7.
2020.
|
|
40
|
Vijayaraghavan K, Ahmad D and Ibrahim MKB:
Biohydrogen generation from jackfruit peel using anaerobic contact
filter. Int J Hydrogen Energy. 31:569–579. 2006.
|
|
41
|
Majik MS, Naik D, Bhat C, Tilve S, Tilvi S
and D'Souza L: Synthesis of (R)-norbgugaine and its potential as
quorum sensing inhibitor against Pseudomonas aeruginosa.
Bioorg Med Chem Lett. 23:2353–2356. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vijayakumar K and Ramanathan T: Musa
acuminata and its bioactive metabolite 5-hydroxymethylfurfural
mitigates quorum sensing (las and rhl) mediated biofilm and
virulence production of nosocomial pathogen Pseudomonas
aeruginosa in vitro. J Ethnopharmacol.
246(112242)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu YP, Yu XM, Zhang W, Wang T, Jiang B,
Tang HX, Su QT and Fu YH: Prenylated chromones and flavonoids from
Artocarpus heterophyllus with their potential
antiproliferative and anti-inflammatory activities. Bioorg Chem.
101(104030)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Biharee A, Sharma A, Kumar A and Jaitak V:
Antimicrobial flavonoids as a potential substitute for overcoming
antimicrobial resistance. Fitoterapia. 146(104720)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rashmi M, Meena H, Meena C, Kushveer JS,
Busi S, Murali A and Sarma VV: Anti-quorum sensing and antibiofilm
potential of Alternaria alternata, a foliar endophyte of
Carica papaya, evidenced by QS assays and in-silico analysis.
Fungal Biol. 122:998–1012. 2018.PubMed/NCBI View Article : Google Scholar
|