Introduction
Autophagy is a physiological process that is vital
to homeostasis, which is responsible for the elimination of damaged
or aged organelles from the cell (1). Autophagy can be stimulated by various
stressors, including starvation and oxidative stress, or by
treatment with some pharmacological agents (2). In addition to its roles in
maintaining normal cellular homeostasis, autophagy has an important
role in the occurrence of cancer, as well as infectious,
inflammatory, neurodegenerative and metabolic diseases (3–7). It
has previously been reported that autophagy acts as a 'double-edged
sword' in tumor therapy; namely, it enables tumor cell survival in
response to stress, thus promoting tumorigenesis; however, it also
induces tumor cell death, thus preventing tumor occurrence
(8). Autophagy-induced cell death
is classified as type II programmed cell death (PCD), which differs
from apoptosis, which is classified as type I PCD (9,10).
In this setting, autophagy is considered an important underlying
mechanism of tumor suppression via the induction of non-apoptotic
cell death. During autophagy, the presence of autophagosomes is
considered the typical morphological feature, the outer membrane of
which fuses with lysosomes to form autolysosomes in which luminal
materials, including the internal membrane, are degraded (11). Microtubule-associated protein light
chain 3 (LC3)-II, which is involved in autophagosome biogenesis and
is localized to the autophagosome membrane, is a marker of
autophagosomes. In addition, sequestosome 1 (SQSTM1)/p62 is another
protein marker that can be used to monitor autophagic flux
(12).
Chemotherapy is currently considered a valuable
therapeutic strategy for the treatment of tumors.
Chemotherapy-induced apoptosis, which is associated with the
release of cytochrome c from the mitochondria via extrinsic
or intrinsic pathways, is the predominant mechanism underlying
cancer cell growth inhibition by chemotherapeutic drugs such as
fluorouracil, cisplatin, cyclophosphamide and paclitaxel (13–15).
Furthermore, autophagy-induced cell death has an important role in
tumor cell death under certain conditions. Mono-Pt, which is a
novel cytoprotective monofunctional platinum (II) complex, inhibits
cell growth and proliferation via autophagic cell death in
apoptosis-resistant ovarian cancer. Treatment of Caov-3 ovarian
carcinoma cells with Mono-Pt resulted in punctate distribution of
LC3, an increased ratio of LC3-II to LC3-I, and accumulation of
autophagic vacuoles in the cytoplasm (16). In addition, Q6, gefitinib, vitexin
6, and other compounds or plant extracts exert potent
antiproliferative effects via the autophagy-dependent degradation
pathway (17–19). Autophagic cell death induced by
chemical drugs or plant extracts may shed new light on therapeutic
strategies used to suppress tumor growth.
Cabazitaxel was approved by the US Food and Drug
Administration in 2010 for use in treating paclitaxel resistance or
advanced prostate cancer. In addition, it has been reported that
cabazitaxel possesses broad-spectrum antitumor activity against the
proliferation of colon cancer cells, as well as pancreatic cancer,
gastric cancer, head and neck cancer, breast cancer, etc. (20,21).
Taxane agents, including paclitaxel, have been demonstrated to
induce autophagy in various cancer cells (22). As a broad-spectrum antitumor and
novel taxane agent, it remains unclear whether cabazitaxel may
induce cytotoxic effects via the autophagic pathway in A549 cells.
In order to extend the future clinical application of cabazitaxel,
the present study aimed to investigate the cytotoxic effects
induced by cabazitaxel and to illustrate its underlying mechanism
in A549 cells. The results demonstrated that cabazitaxel may induce
autophagic cell death in a phosphoinositide 3-kinase
(PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway-dependent
manner.
Materials and methods
Cell culture
The A549 human lung adenocarcinoma epithelial cell
line was purchased from Type Culture Collection of Chinese Academy
of Sciences (Shanghai, China). The cells were cultured in RPMI-1640
supplemented with 10% fetal bovine serum (FBS; Sijiqion, Hangzhou,
China) and 100 U of penicillin G with 100 µg of streptomycin
per ml, cells were grown in a humidified atmosphere containing 5%
CO2 at 37°C.
Chemicals, reagents and antibodies
Cabazitaxel was kindly provided by Professor
Shengyong Zhang (Department of Medicinal Chemistry, Fourth Military
Medical University, Xi'an, China). Cabazitaxel was dissolved at a
concentration of 50 mg/ml in absolute ethyl alcohol as a stock
solution, which was stored at 4°C, and was diluted with medium
prior to each experiment. z-VAD-FMK and Annexin V/propidium iodide
(PI) apoptosis detection kit was obtained from Beyotime Institute
of Biotechnology (Hangzhou, China). Anti-LC3 (cat. no. L8918;
1:800), anti-beclin1 (cat. no. SAB5300513-100UL; 1:1,000) and
anti-p62 (cat. no. P0067; 1:1,000) antibodies, 5-fluorouracil
(5-FU) and 3-methyladenine (3-MA) were purchased from Sigma-Aldrich
(Merck Millipore, Darmstadt, Germany). Anti-mTOR (cat no 653401;
1:1,000), anti-phosphorylated (p)-mTOR (Ser2481) (ca. no 651701;
1:500), anti-Akt (cat. no. 603401; 1:500) and anti-p-Akt (Ser473)
(cat. no. 649001; 1:500) antibodies were purchased from BioLegend
(San Diego, CA, USA). Anti-human β-actin monoclonal antibody was
provided by Cwbiotech (Beijing, China; cat. no. CW0096M;
1:2,000).
Apoptosis assay
Apoptosis assay was conducted with Annexin V/PI
detection kit according to manufacturer's protocol. Briefly,
1×105 A549 cells/well were treated with 5 µg/ml
cabazitaxel and 20 µM 5-FU for 30 h. Next, the treated cells
were harvested and washed 3 times with cold PBS. Cells were
subsequently stained with 5 µl FITC Annexin V and 10
µl PI for 15 min at room temperature and subjected to flow
cytometry for analysis apoptosis.
Cell death assay
Cell death was assessed by trypan blue exclusion
assay. Following treatment with various concentration of
Cabazitaxel (1, 2, 5, 1 and 20 µg/ml) at different times,
suspended and adherent cells were collected and stained with 0.4%
trypan blue dye for 3 min at room temperature. Cells were
subsequently counted using a hemocytometer under a light
microscope. Pharmaceutic inhibitors, z-VAD-FMK and 3-MA, were used
to determine the apoptotic and autophagic cell death. Cells were
pretreated with 20 µM z-VAD-FMK or 10 mM 3-MA for 1 h at
37°C and then incubated with cabazitaxel. Next, cells were stained
with 0.4% trypan blue in order to detect cell death.
Cell cycle analysis
Cell cycle analysis was performed as described
previously (23). Briefly, cells
were synchronized by culturing in RPMI-1640 medium containing 0.5%
FBS overnight. The cells were then incubated in fresh RPMI-1640
medium supplemented with 10% FBS and serially diluted cabazitaxel
at 37°C. The treated cells (5 µg/ml cabazitaxel) were
harvested after a 24 h incubation and were washed with cold
phosphate-buffered saline (PBS) before being fixed with 70% ethanol
at 4°C overnight. After thoroughly washing with PBS, the fixed
cells were stained in the dark with 50 µg/ml propidium
iodile (PI) containing 100 µg/ml RNase A and 1% Triton X-100
for 45 min at room temperature.. The cells were finally subjected
to flow cytometric analysis.
Transmission electron microscopy
(TEM)
A549 cells were seeded in a 6-well plate and treated
with 5 µg/ml cabazitaxel for 24 h. Cells were digested and
fixed with 3% glutaraldehyde in PBS (pH 7.8) for 3 h at room
temperature, and were then incubated with 0.1% osmium tetroxide for
1 h at 4°C. Cells were subsequently dehydrated in increasing
concentrations of ethanol (50, 60, 80 and 100%) and were embedded
in Epon resin. Ultrathin sections were loaded onto copper grids and
were stained with uranyl acetate and lead citrate, prior to being
observed under a transmission electron microscope (JEM-1230; JEOL
Ltd., Tokyo, Japan).
Western blotting
A549 cells (seeded at 2×105) were treated
with various concentrations (1, 2, 5, 10 and 20 µg/ml) of
cabazitaxel for 24 h at 37°C and were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Proteins were quantified using a bicinchoninic acid
assay kit (Cwbiotech Co., Ltd., Beijing, China) then 30 µg
proteins per well were subjected to 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and were transferred to
polyvinylidene fluoride membranes. After blocking with 5% bovine
serum albumin for 1 h at room temperature, the membranes were
sequentially incubated with the corresponding primary antibodies
overnight at 4°C, and with horseradish peroxidase-conjugated
anti-rabbit immunoglobulin (Ig)G (cat. no. CW0103S; 1:1,000;
Cwbiotech) or anti-mouse IgG (cat. no. 0102S, 1:1,000; Cwbiotech)
for 1 h at room temperature. Finally, blots were developed and
visualized using the SuperSignal West Dura chemiluminescence kit
(Pierce; Thermo Fisher Scientific, Inc., Rockford, IL, USA) and an
ImageQuant LAS 4000 mini system (GE Healthcare Life Sciences,
Pittsburgh, PA, USA). Image J software (version 1.38; National
Institutes of Health, Bethesda, MD, USA) was used to quantify
protein bands. β-actin was used as a protein loading control.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
TUNEL staining was conducted to assess the levels of
apoptosis induced by cabazitaxel or 5-FU (positive control)
treatment according to the manufacturer's protocol (Beyotime
Institute of Biotechnology). Briefly, A549 cells were fixed in 4%
paraformaldehyde for 1 h at room temperature following treatment
with cabazitaxel or 5-FU. Subsequently, the cells were
permeabilized with 0.1% Triton X-100 and were incubated with 50
µl TUNEL reaction buffer at 37°C for 1 h in the dark. After
staining with 4′,6-diamidino-2-phenylindole, slides were visualized
by fluorescence microscopy.
Caspase 3 activity assay
Caspase 3 protease activity was measured using a
Caspase-3 activity detection kit according to the manufacturer's
protocol (Beyotime Institute of Biotechnology). Briefly, A549 cells
were lysed at various time points following treatment with
cabazitaxel or 5-FU. Cell lysates were centrifuged at 12,000 × g
for 15 min at 4°C prior to incubation with Ac-DEVD-pNA for
60 min at 37°C. Absorbance was measured at 405 nm using a Biotek
Eon microplate spectrophotometer (Biotek, Winooski, VT, USA) and
caspase 3 activity was calculated according to a pNA
standard curve.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total mRNA was extracted using TRIzol reagent from
the A549 cells treated with various concentrations of cabazitaxel
(1, 2, 5, 10 and 20 µg/ml) for 24 h at 37°C. Subsequently,
cDNA was synthesized using the RevertAid First Strand cDNA
synthesis kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Briefly, the total volume of 20 µl reverse transcription
mixer (500 ng RNA, 1 µl oligo(dT)18, 1µl RevertAid
RT) was incubated for 60 min at 42°C, then the reaction was
terminated by heating at 70°C for 5 min. qPCR was performed on an
ABI StepOnePlus™ thermocycler (Applied Biosystems; Thermo Fisher
Scientific, Inc., Foster City, CA, USA) using a SYBR®
Green PCR kit (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Each sample was run in triplicate in a final volume of 25 µl
containing 1 µl first-strand cDNA template, 10 pmol of each
primer (Table I; obtained from
Sangon Biotech Co., Ltd, Shanghai, China), 12.5 µl of
SYBR® Green PCR master mix and 11.5 µl distilled
water. Cycling conditions of the qPCR were as follows: 1 cycle at
95°C for 30 sec, followed by 40 cycles at 95°C for 20 sec, 60°C for
30 sec and extension at 72°C for 15 sec.. Data analyses were
performed according to the 2−ΔΔ Cq method (24).
 | Table IPrimer sequences of autophagy-related
genes. |
Table I
Primer sequences of autophagy-related
genes.
| Gene | Forward primer | Reverse primer |
|---|
| Atg5 |
atgacagatgacaaagatg |
caacgtcaaataacttactc |
| Atg7 |
tgacgatcggatgaatgagcc |
gctcatgtcccagattttggaag |
| Atg12 |
ctgtgtaattgcgtccccct |
gaagctgcaacacagactgc |
| LC3-II |
ccgcaccttcgaacaaagag |
aagctgcttctcacccttgt |
| β-actin |
tgacggggtcacccacactg |
aagctgtagccgcgctcggt |
Plasmid and small interfering (si)RNA
transfection
Beclin1 siRNA (sense
5′-GGAGCCAUUUAUUGAAACUTT-3′ and antisense
5′-AGUUUCAAUAAAUGGCUCCTT-3′) was synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China). Transfection of cells with green
fluorescent protein (GFP)-LC3 plasmid (provided by Dr. Ye Wei) or
Beclin1 siRNA was carried out using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according the manufacturer's protocol. Briefly, 4 µg GFP-LC3
plasmid, 100 pmol Beclin1 siRNA and 5 µl
Lipofectamine 2000 were diluted in 50 µl Opti-MEM I medium
(Invitrogen; Thermo Fisher Scientific, Inc.), respectively, and
were incubated for 5 min at room temperature. The diluted GFP-LC3
plasmid and Beclin1 siRNA were mixed with Lipofectamine
2000, separately, and were incubated for 20 min at room
temperature. The complexes were then added to 6-well plates,
2×105 cells/well for transfection. Cabazitaxel was added
to the cells at 5 µg/ml/well 48 h post-transfection, and the
cells were incubated for a further 24 h at 37°C. LC3 puncta was
visualized by fluorescence microscopy, and the protein expression
levels of Beclin1 were determined by western blotting.
Statistical analysis
Data are presented as the mean ± standard deviation.
Student's t-test and one-way analysis of variance were used to
analyze data. Differences between experimental groups were assessed
by non-parametric analysis using SPSS software, version 12 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cabazitaxel treatment induces autophagy
in A549 cells
An increasing body of evidence has suggested that
manipulation of autophagy may provides a useful way to prevent
cancer development (25,26); therefore, the present study aimed
to investigate whether cabazitaxel could induce autophagy in A549
lung cancer cells. Autophagosomal formation and the expression of
autophagy-associated proteins were used to evaluate
cabazitaxel-induced autophagy. Following exposure to various
concentrations of cabazitaxel for 24 h, RT-qPCR was conducted to
determine the mRNA expression of autophagy related (Atg) genes; the
results demonstrated that the expression levels of LC3, Atg5, Atg7
and Atg12 were increased (Fig.
1A), with Atg7 exhibiting the highest expression levels.
Furthermore, immunoblotting indicated that LC3-II protein
expression and the conversion of LC3-I to LC3-II were significantly
upregulated in cabazitaxel-treated cells compared with the control
group (Fig. 1B). Conversely,
SQSTM1/p62 expression was decreased following exposure to
cabazitaxel (Fig. 1C).
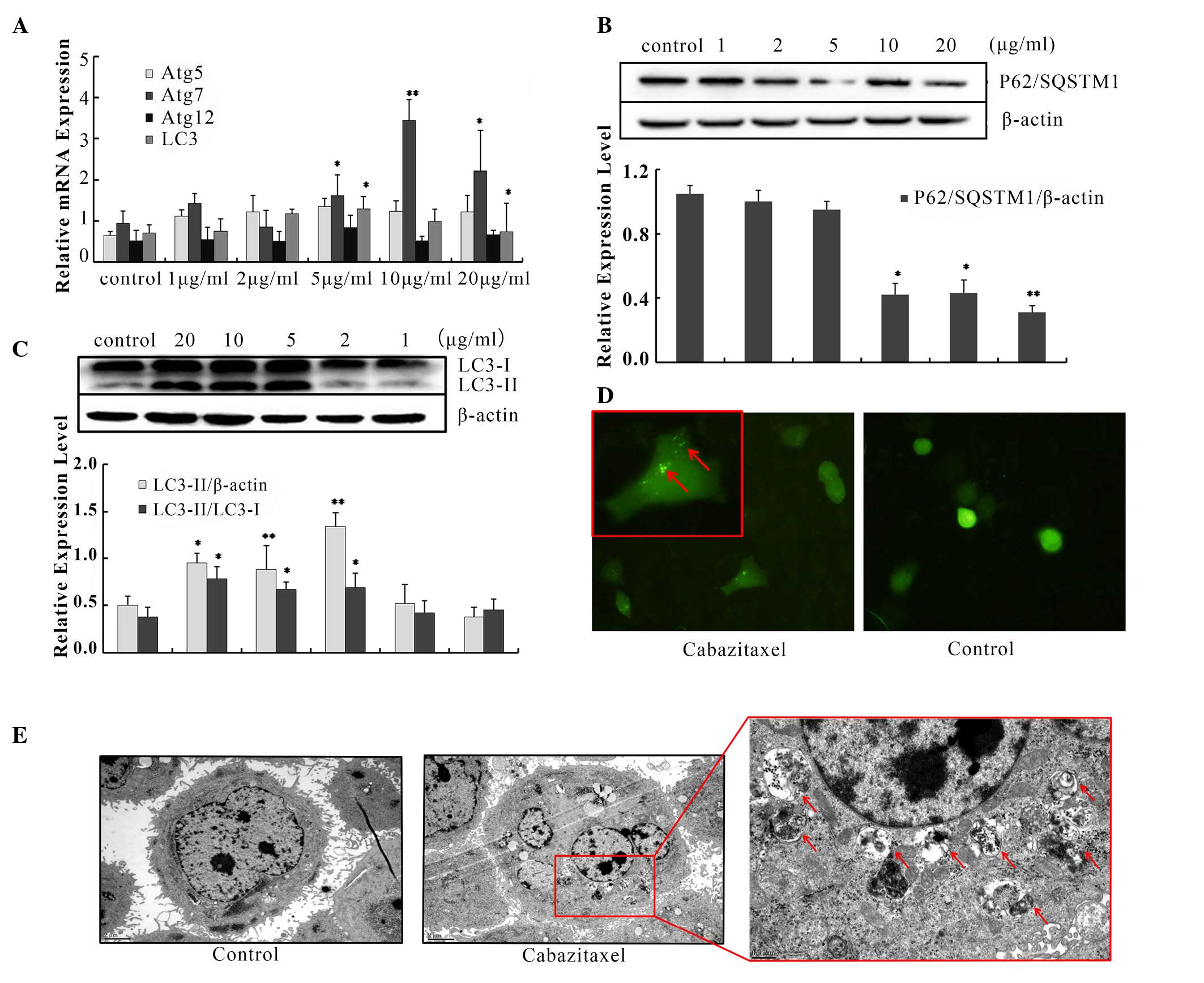 | Figure 1Cabazitaxel induces autophagy in A549
cells. (A) A549 cells were treated with various concentrations of
cabazitaxel for 24 h; total RNA was extracted by TRIzol, and
autophagy related (Atg) mRNA expression levels were analyzed by
quantitative polymerase chain reaction using specific primers. (B
and C) Proteins were extracted by radioimmunoprecipitation assay
buffer following treatment with cabazitaxel. Samples underwent
western blotting to determine protein expression levels. (D) A549
cells were transfected with green fluorescent
protein-microtubule-associated protein light chain 3 (LC3) plasmids
for 48 h, and were then treated with 5 µg/ml cabazitaxel for
another 24 h. LC3 puncta were observed by fluorescence microscope.
Magnification × 20, close-up image magnification, ×40. (E)
Cabazitaxel-treated A549 cells were fixed and dehydrated, and
subjected to transmission electron microscopy after staining with
uranyl acetate and lead citrate. Bottom panel, magnification
×8,000. Top panel, ×30,000) Data are presented as the mean ±
standard deviation of three independent experiments
*P<0.05, **P<0.01 vs. control group.
SQSTM1, sequestosome 1. |
Since LC3 is a specific marker for autophagosomal
formation, GFP-tagged LC3 was used to measure autophagic flux. As
shown in Fig. 1D, the green puncta
were distributed in the cytoplasm post-transfection, and the
percentage of cells expressing LC3 was much higher in the treated
group compared with in the control group. In addition, the presence
of autophagic compartments was detected in cabazitaxel-treated
cells by TEM. Following exposure to cabazitaxel for 24 h,
accumulation of massive autophagic vacuoles (autophagosomes) was
detected in the cytoplasm under TEM, and these autophagosomes
consisted of double membrane structural compartments, which contain
recognizable cellular organelles and a high electron density
substance. Conversely, fewer autophagosomes were observed in the
control group (Fig. 1E).
Furthermore, typical apoptotic morphology was not observed under
TEM, including chromatin condensation and apoptotic bodies; the
nuclear membrane integrity and chromatin distribution of
cabazitaxel-treated cells were normal. These results indicate that
cabazitaxel could not induce apoptosis of A549 cells. Taken
together, these findings support the hypothesis that cabazitaxel
induces autophagy in A549 cells.
Cabazitaxel promotes cell death by
inducing cell cycle arrest at G2/M phase
The present study subsequently assessed whether
cabazitaxel-induced autophagy promoted cell survival or cell death.
When A549 cells were exposed to various concentrations of
cabazitaxel, cells detached from the bottom of the flask and were
observed to be round-shaped. Therefore, it was hypothesized that
cabazitaxel exhibited cytotoxicity in A549 cells. Cell death was
observed by trypan blue assay following treatment with various
doses of cabazitaxel (1–20 µg/ml) for 12, 24 or 48 h. As
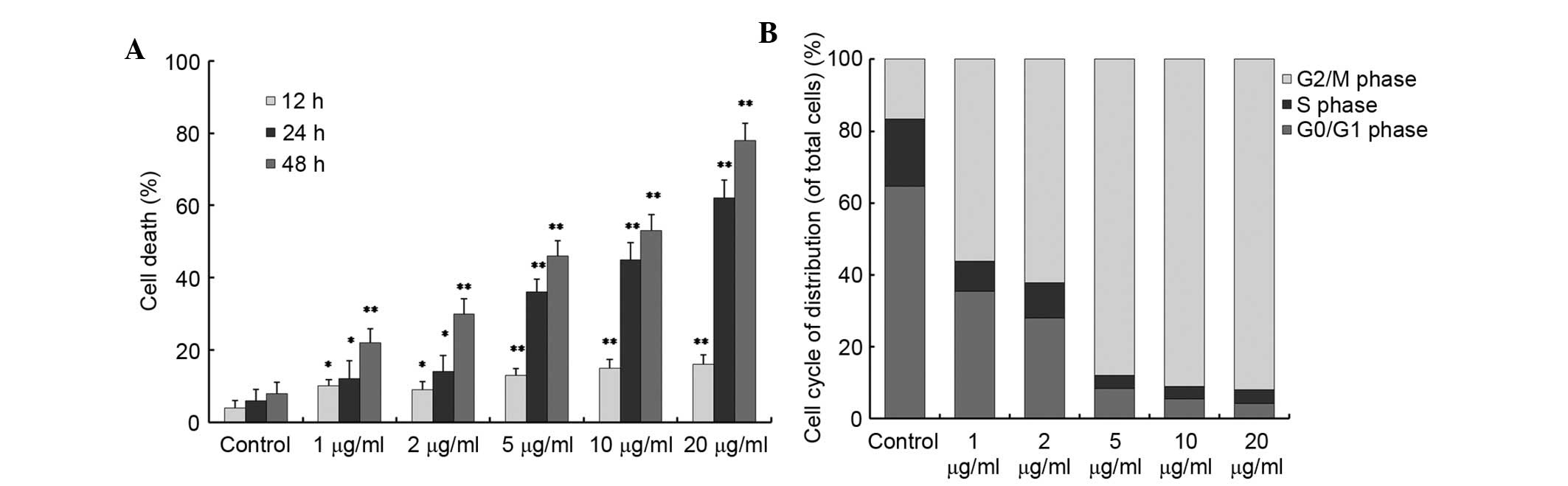
shown in Fig. 2A, cabazitaxel
induced a time- and dose-dependent increase in A549 cell death; 1
µg/ml cabazitaxel treatment for 12 h induced slight cell
death of A549 cells compared with the control group; subsequently,
cell death was significantly increased in a dose- and
time-dependent manner.
To gain insight into the mechanism underlying the
antiproliferative effects of cabazitaxel, the present study
investigated the effects of cabazitaxel treatment on the cell
cycle. The number of A549 cells at G2/M phase was
significantly increased to 80–90% following incubation with various
doses (5, 10, 20 µg/ml) of cabazitaxel for 24 h (Fig. 2B). Taken together, these results
suggest that cabazitaxel exerts a cytotoxic cell death-promoting
effect via the induction of G2/M phase arrest.
Cabazitaxel induces non-apoptotic cell
death
Apoptosis is one of the major cell death pathways.
To determine whether cabazitaxel-mediated cell death was induced
via the apoptotic pathway, cabazitaxel-treated cells were stained
with Annexin V/PI and TUNEL-fluorescein isothiocyanate (FITC).
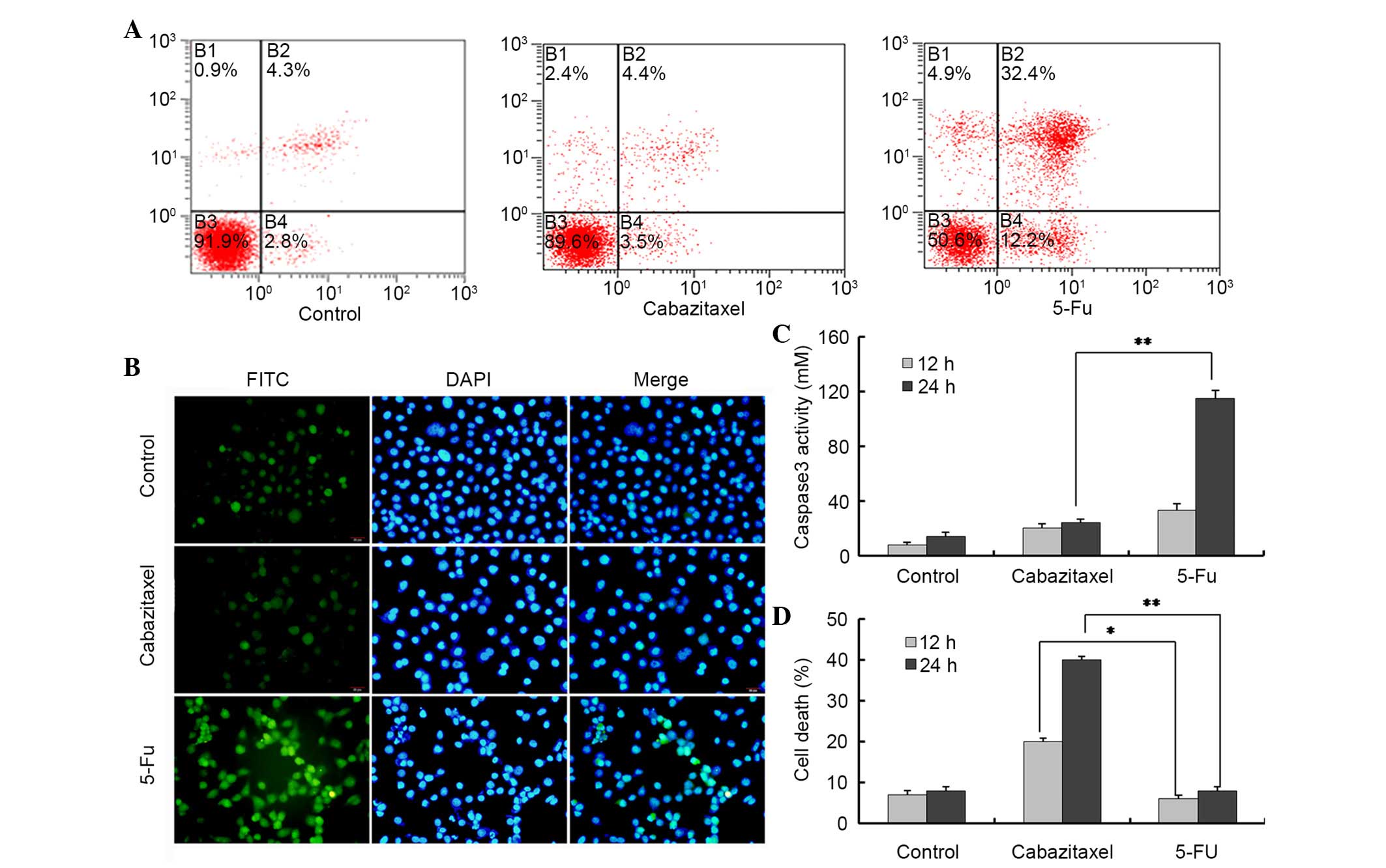
Following exposure to 5 µg/ml cabazitaxel for 24 h, the
percentage of apoptotic cells was not significantly altered
compared with in the control group, as determined by Annexin/PI
staining and flow cytometry. Conversely, the majority of
5-FU-treated A549 cells were positive for apoptosis staining
(Fig. 3A). To further confirm
these results, a TUNEL-FITC assay was used to detect the apoptosis
of cabazitaxel-treated A549 cells. Only weak fluorescence was
detected in a very small number of cabazitaxel-treated and control
A549 cells; however, strong fluorescence was detected in
5-FU-treated A549 cells (Fig.
3B).
Caspase 3 is an apoptosis-associated cysteine
peptidase, which has a central role in the execution phase of cell
apoptosis. Therefore, the present study investigated caspase 3
activity in A549 cells, and demonstrated that 5-FU, rather than
cabazitaxel, activated caspase 3 in A549 cells (Fig. 3C). z-VAD-FMK is a pan-caspase
inhibitor, which can be used to block all features of apoptosis,
including apoptotic cell death. Notably, z-VAD-FMK was not able to
block cabazitaxel-induced cell death; however, it could inhibit
5-FU-induced cell death, thus providing evidence for
caspase-independent cell death in A549 cells (Fig. 3D). These results indicate that
cabazitaxel-induced cytotoxicity is caused by alternative caspase
3-independent non-apoptotic cell death.
Cabazitaxel-induced autophagy contributes
to cell death
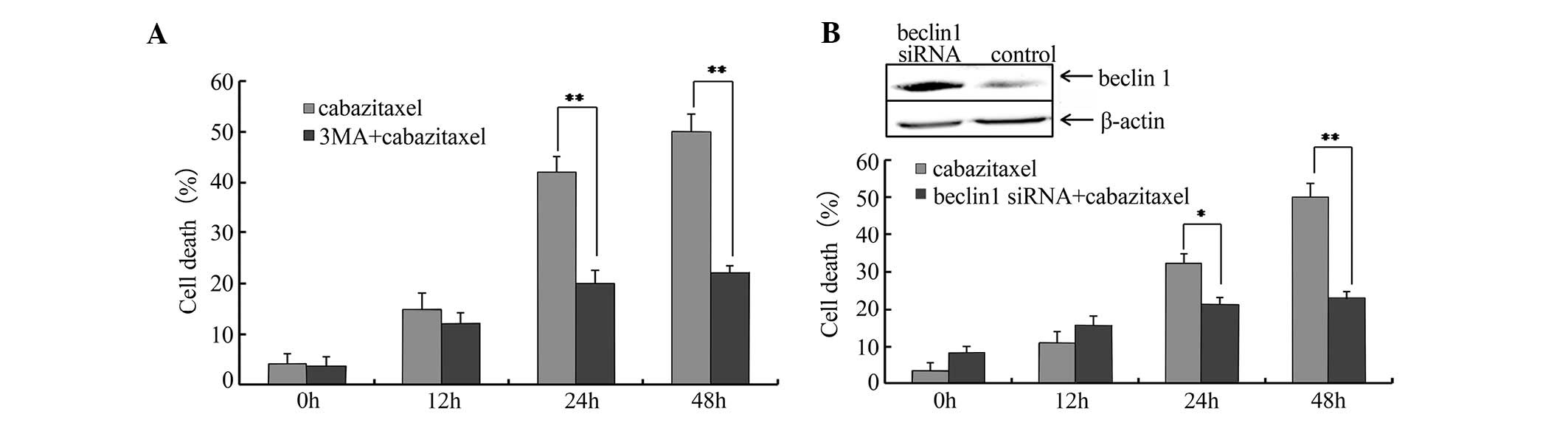
As aforementioned, cabazitaxel treatment was able to
induce autophagy in A549 cells. Subsequently, the present study
used 3-MA, a well-known inhibitor of autophagy, to examine whether
cell death was triggered by autophagy. A549 cells were pretreated
with 10 mM 3-MA for 1 h, and cabazitaxel was then added to the
pretreated cells for an additional 24 h. As shown in Fig. 4A, 3-MA resulted in a partial but
significant inhibition of cabazitaxel-induced cell death.
Subsequently, cells were transfected with siRNA specific for
Beclin1, which is required for autophagy, in order to
elucidate the association between autophagy and cabazitaxel-induced
cell death. Beclin1-siRNA suppressed Beclin1
expression and decreased cabazitaxel-induced cell death
post-transfection (Fig. 4B). These
findings indicate that autophagy contributes to the
cabazitaxel-induced death of A549 cells.
PI3K/Akt/mTOR signaling pathway
inhibition activates cabazitaxel-induced autophagy
The PI3K/Akt/mTOR pathway is an intracellular
signaling pathway that is involved in autophagy regulation;
inhibition of the PI3K/Akt/mTOR pathway promotes autophagy. To
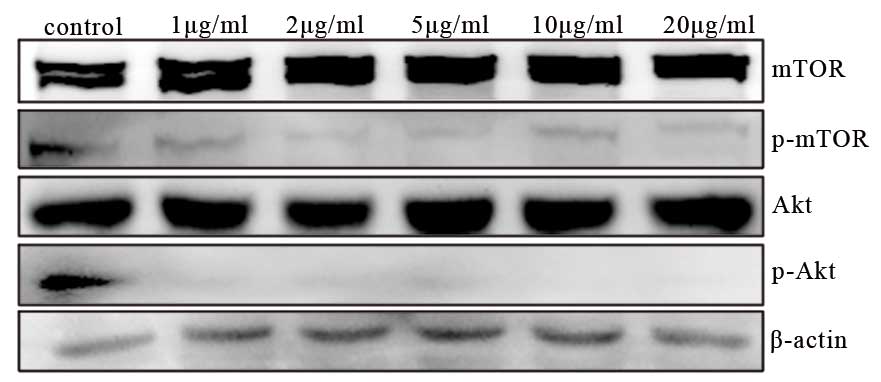
determine the role of this pathway, two critical proteins, Akt and
mTOR, were examined. Following treatment with cabazitaxel for 24 h,
the levels of p-mTOR and p-Akt were markedly decreased compared
with the control (Fig. 5). These
results indicate that cabazitaxel targets the PI3K/Akt/mTOR pathway
to induce autophagic cell death via the inhibition of Akt and mTOR
phosphorylation.
Discussion
Cabazitaxel is a novel agent for the treatment of
metastatic hormone-refractory prostate cancer. A previous study
reported that cabazitaxel acts as a broad-spectrum chemotherapy
agent that may prevent cancer progression (20). In addition, our pilot studies
confirmed that cabazitaxel inhibited the proliferation of various
types of human cancer cell, including A549 lung cancer cells; SW480
and HT-29 colon cancer cells; LNCaP, DU145 and PC-3 prostate cancer
cells; as well as murine M16 melanoma cancer cells. We also
demonstrated that morphological alterations of the
cabazitaxel-treated A549 cells were more obvious than in the
prostate cancer cell lines; the cabazitaxel-treated cells exhibited
detachment, a round shape and shrinkage. Furthermore, cell death
induced by cabazitaxel was increased in A549 cells compared with in
other human tissue-derived cancer cell lines (data not published).
Based on these findings, the present study used A549 cells as the
target to study the antitumor effects of cabazitaxel.
The present study revealed a novel molecular
mechanism underlying the antitumor activity of cabazitaxel in A549
cells. When A549 cells were treated with cabazitaxel, the number of
autophagosomes, LC3-II expression and autophagic flux were
increased. Concurrently, cabazitaxel was able to promote A549 cell
death independent of the apoptotic pathway. Further evidence
indicated that cabazitaxel-induced autophagy contributed to A549
cell death via the PI3K/Akt/mTOR pathway. Therefore, it may be
concluded that cabazitaxel-induced autophagic cell death is the
predominant mechanism underlying A549 cell proliferation
inhibition. These data may help to provide the theoretical basis
for the clinical application of cabazitaxel.
PCD is a crucial process for the organized
destruction of cells, which is essential for the development and
maintenance of cellular homeostasis. PCD can be divided into three
categories: Apoptotic, autophagic and necrotic cell death, and
apoptotic cell death is considered a vital defense mechanism to
eliminate cancer cells (27). In
the present study, cabazitaxel-treated A549 cells did not exhibit
typical apoptotic morphology and increased caspase 3 activity. In
addition, z-VAD-FMK was not able to inhibit cell death, thus
suggesting that cabazitaxel may induce A549 cell death independent
of the apoptotic pathway. Subsequently, the present study
investigated whether cabazitaxel was able to induce autophagic cell
death. Following exposure to cabazitaxel, typical features of
autophagy were observed, including high LC3-II expression, Atg gene
upregulation, p62 downregulation and autophagic vacuole formation;
however, features associated with apoptosis were not detected in
A549 cells. In addition, siRNA Beclin1 gene silencing and
treatment with an autophagy inhibitor rescued cabazitaxel-induced
cell death. There are three criteria to certify autophagic cell
death: i) Cell death occurs without the involvement of apoptosis;
ii) there is an increase in autophagic flux, alongside an increase
in autophagic markers, in the dying cells; and iii) suppression of
autophagy via pharmacological inhibitors or genetic approaches is
able to rescue or prevent cell death (28). The results of the present study
fully coincided with the aforementioned criteria of autophagic cell
death. Therefore, the present study concluded that autophagy
contributes to cabazitaxel-induced A549 cell death, which serves as
a protective mechanism to facilitate the elimination of cancer
cells.
Previous studies have demonstrated that autophagic
cell death is triggered by numerous signaling pathways, such as the
adenosine monophosphate-activated protein kinase pathway, the
PI3K/AKT/mTOR pathway, and the mitogen-activated protein kinases
(extracellular signal-regulated kinases, p38 and c-Jun N-terminal
kinases) pathway (29–32). The PI3K/AKT/mTOR signaling pathway
represents one of the major survival pathways that is dysregulated
in various types of human cancer, and contributes to cancer
pathogenesis and therapy resistance. It has previously been
demonstrated that inhibition of the PI3K/Akt/mTOR pathway induces
G2/M arrest, whereas Akt promotes cell cycle progression
through the G2/M transition via suppression of the
cyclin-dependent kinase cell division control (Cdc)2 and Cdc25c
pathway (33). In the present
study, A549 cells were arrested at G2/M phase following
treatment with cabazitaxel, thus suggesting that
cabazitaxel-mediated G2/M arrest may be induced via
inhibition of the PI3K/Akt/mTOR pathway. Mounting evidence has
indicated that the PI3K/AKT/mTOR signaling cascade regulates
autophagy. As a member of the PI3K-related kinase family, mTOR is
associated with cell proliferation and cell metabolism. Previous
studies have reported that inhibition of Akt and its downstream
target mTOR contributes to the initiation of autophagy (34,35).
Activation of the PI3K/Akt/mTOR pathway has also been associated
with pathogenesis of non-small cell lung cancer (NSCLC), and
inhibition of the PI3K/AKT/mTOR signaling pathway has been
suggested as a potential therapeutic target in NSCLC (36). The present study demonstrated that
Akt and mTOR phosphorylation were significantly decreased in the
cabazitaxel-treated A549 cells, thus indicating that cabazitaxel
may be considered a candidate agent for the treatment of lung
cancer through inhibition of the PI3K/AKT/mTOR signaling
pathway.
In conclusion, the results of the present study
suggested that cabazitaxel may induce autophagic cell death in
human adenocarcinoma lung cancer cells via PI3K/Akt/mTOR signaling
inhibition. These findings represent a novel anticancer mechanism
of cabazitaxel, and may help expand its application in the
chemotherapeutic treatment of various cancers other than those of
prostate gland origin.
Acknowledgments
The present study was supported by the Science and
Technolgy Innovation Project Plan of Shaanxi Province (grant nos.
2015KTCL03-01 and 2014FWPT-11).
References
|
1
|
McLeod IX, Jia W and He YW: The
contribution of autophagy to lymphocyte survival and homeostasis.
Immunol Rev. 249:195–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y and Qin ZH: Coordination of
autophagy with other cellular activities. Acta Pharmacol Sin.
34:585–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deretic V: Autophagy in infection. Curr
Opin Cell Biol. 22:252–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang S, Dupont N, Castillo EF and Deretic
V: Secretory versus degradative autophagy: Unconventional secretion
of inflammatory mediators. J Innate Immun. 5:471–479. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janda E, Isidoro C, Carresi C and Mollace
V: Defective autophagy in Parkinson's disease: Role of oxidative
stress. Mol Neurobiol. 46:639–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren SY and Xu X: Role of autophagy in
metabolic syndrome-associated heart disease. Biochim Biophys Acta.
1852:225–231. 2015. View Article : Google Scholar
|
|
8
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galluzzi L, Vicencio JM, Kepp O, Tasdemir
E, Maiuri MC and Kroemer G: To die or not to die: That is the
autophagic question. Curr Mol Med. 8:78–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen S, Kepp O and Kroemer G: The end of
autophagic cell death? Autophagy. 8:1–3. 2012. View Article : Google Scholar
|
|
11
|
Kaminskyy V, Abdi A and Zhivotovsky B: A
quantitative assay for the monitoring of autophagosome accumulation
in different phases of the cell cycle. Autophagy. 7:83–90. 2011.
View Article : Google Scholar
|
|
12
|
Jiang P and Mizushima N: LC3- and
p62-based biochemical methods for the analysis of autophagy
progression in mammalian cells. Methods. 75:13–18. 2015. View Article : Google Scholar
|
|
13
|
Torrisi R, Balduzzi A, Ghisini R, Rocca A,
Bottiglieri L, Giovanardi F, Veronesi P, Luini A, Orlando L, Viale
G, et al: Tailored preoperative treatment of locally advanced
triple negative (hormone receptor negative and HER2 negative)
breast cancer with epirubicin, cisplatin, and infusional
fluorouracil followed by weekly paclitaxel. Cancer Chemother
Pharmacol. 62:667–672. 2008. View Article : Google Scholar
|
|
14
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: Therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo WJ, Zhang YM, Zhang L, Huang B, Tao
FF, Chen W, Guo ZJ, Xu Q and Sun Y: Novel monofunctional platinum
(II) complex Mono-Pt induces apoptosis-independent autophagic cell
death in human ovarian carcinoma cells, distinct from cisplatin.
Autophagy. 9:996–1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu XW, Cai TY, Zhu H, Cao J, Su Y, Hu YZ,
He QJ and Yang B: Q6, a novel hypoxia-targeted drug, regulates
hypoxia-inducible factor signaling via an autophagy-dependent
mechanism in hepatocellular carcinoma. Autophagy. 10:111–122. 2014.
View Article : Google Scholar
|
|
18
|
Dragowska WH, Weppler SA, Wang JC, Wong
LY, Kapanen AI, Rawji JS, Warburton C, Qadir MA, Donohue E, Roberge
M, et al: Induction of autophagy is an early response to gefitinib
and a potential therapeutic target in breast cancer. PLoS One.
8:e765032013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou J, Hu H, Long J, Wan F, Li L, Zhang
S, Shi YE and Chen Y: Vitexin 6, a novel lignan, induces autophagy
and apoptosis by activating the Jun N-terminal kinase pathway.
Anticancer Drugs. 24:928–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vrignaud P, Sémiond D, Lejeune P, Bouchard
H, Calvet L, Combeau C, Riou JF, Commerçon A, Lavelle F and Bissery
MC: Preclinical antitumor activity of cabazitaxel, a semisynthetic
taxane active in taxane-resistant tumors. Clin Cancer Res.
19:2973–2983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azarenko O, Smiyun G, Mah J, Wilson L and
Jordan MA: Antiproliferative mechanism of action of the novel
taxane cabazitaxel as compared with the parent compound docetaxel
in MCF7 breast cancer cells. Mol Cancer Ther. 13:2092–2103. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu F, Liu D, Yang Y and Zhao S: Effect of
autophagy inhibition on chemotherapy-induced apoptosis in A549 lung
cancer cells. Oncol Lett. 5:1261–1265. 2013.PubMed/NCBI
|
|
23
|
Yu D, Makkar G, Dong T, Strickland DK,
Sarkar R and Monahan TS: MARCKS signaling differentially regulates
vascular smooth muscle and endothelial cell proliferation through a
KIS-, p27kip1- dependent mechanism. PLoS One. 10:e01413972015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Sharma K, Le N, Alotaibi M and Gewirtz DA:
Cytotoxic autophagy in cancer therapy. Int J Mol Sci.
15:10034–10051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kung CP, Budina A, Balaburski G,
Bergenstock MK and Murphy M: Autophagy in tumor suppression and
cancer therapy. Crit Rev Eukaryot Gene Expr. 21:71–100. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen HM and Codogno P: Autophagic cell
death: Loch Ness monster or endangered species? Autophagy.
7:457–465. 2011. View Article : Google Scholar
|
|
28
|
Choi KS: Autophagy and cancer. Exp Mol
Med. 44:109–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Høyer-Hansen M and Jäättelä M:
AMP-activated protein kinase: A universal regulator of autophagy?
Autophagy. 3:381–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi WY, Xiao D, Wang L, Dong LH, Yan ZX,
Shen ZX, Chen SJ, Chen Y and Zhao WL: Therapeutic metformin/AMPK
activation blocked lymphoma cell growth via inhibition of mTOR
pathway and induction of autophagy. Cell Death Dis. 3:e2752012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Corcelle E, Djerbi N, Mari M, Nebout M,
Fiorini C, Fénichel P, Hofman P, Poujeol P and Mograbi B: Control
of the autophagy maturation step by the MAPK ERK and p38: Lessons
from environmental carcinogens. Autophagy. 3:57–59. 2007.
View Article : Google Scholar
|
|
33
|
Li Y, Zhang P, Qiu F, Chen L, Miao C, Li
J, Xiao W and Ma E: Inactivation of PI3K/Akt signaling mediates
proliferation inhibition and G2/M phase arrest induced by
andrographolide in human glioblastoma cells. Life Sci. 90:962–967.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa
T, Aoki H, Mills GB and Kondo S: Synergistic augmentation of
rapamycin-induced autophagy in malignant glioma cells by
phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer
Res. 65:3336–3346. 2005.PubMed/NCBI
|
|
35
|
Paglin S, Lee NY, Nakar C, Fitzgerald M,
Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N and
Yahalom J: Rapamycin-sensitive pathway regulates mitochondrial
membrane potential, autophagy, and survival in irradiated MCF-7
cells. Cancer Res. 65:11061–11070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fumarola C, Bonelli MA, Petronini PG and
Alfieri RR: Targeting PI3K/AKT/mTOR pathway in non small cell lung
cancer. Biochem Pharmacol. 90:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|