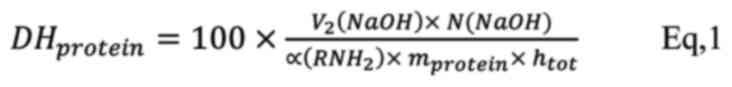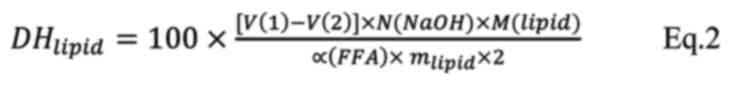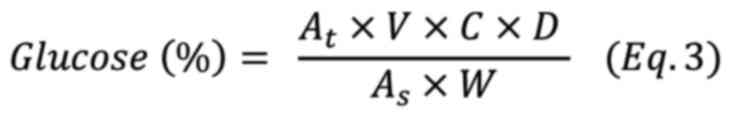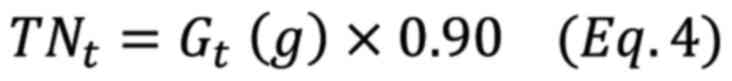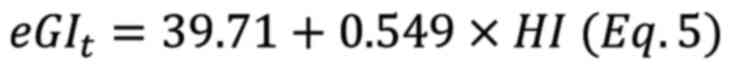Introduction
According to epidemiological and clinical studies
reviewed in the report by the FAO and WHO, dietary preferences and
physical activity exert critical effects on the risk of developing
certain chronic diseases and cancers. By considering the energy
density of foods in the diet, reducing the intake of added sugar,
high fat/saturated fats and sodium, and increasing the intake of
dietary fiber and bioactive compounds, can prevent the risk of
developing chronic and degenerative diseases. This can aid
individuals in the maintenance of their body weight, and can help
protect cognitive and physiological health (1). However, according to statistical data
including 46 European countries, 1 in 3 children between the ages
of 6 and 9 years is either overweight [body mass index (BMI),
25-30] or obese (BMI ≥30). The number of obese children in Europe
was 41 million in 2016 and is expected to reach 70 million by
2025(2). In the childhood and
adolescence period, snacks with high-calorie and unbalanced
nutrients are creating dietary issues. The majority of dietary
guidelines describe all high-calorie foods and beverages consumed
between main meals as ‘snacks’. In some recommendations, ‘snack
foods’ are defined as foods with a low nutrient density, but high
calories, saturated fats and added sugar (3-6).
Studies indicate a positive association between snack food
consumption and the risk of developing chronic diseases, such as
cardiovascular diseases, obesity, type-2 diabetes and insulin
resistance (7). Nutrition in
childhood plays a critical role in the prevention of obesity in
both childhood and adulthood. The United States Department of
Agriculture (USDA) has issued A Guide to Smart Snacks in School; in
this standard, there are limits to the ingredients, nutrients and
energy value of snacks (8). As a
result of this new standard, it has become increasingly popular to
employ snack designs that incorporate healthier recipes (containing
added proteins, fibers, unsaturated fats, vitamins and mineral
sources), as opposed to solely oil and/or sugar. Whey protein
concentrate (WPC), natural sweeteners, dietary fibers, such as
inulin and pectin, omega-3 fatty acids, resistant starch or certain
bioactive compounds, such as gallic acid and ellagic acid are used
in new product designs or enrichment studies (9-12).
However, the nutritional quality of food does not only depend on
its nutrient profile, but the matrix and physicochemical properties
of food are also important for the fate properties and metabolic
responses of nutrients and bioactive compounds in the digestive
system. Research has indicated that foods of the same composition
have differences in their digestion and responses, such as on the
glycemic index (GI) or satiety, depending on its structural
properties and applied technological processes (13). Another study proposed a method to
evaluate the impact of complex food matrix structures on digestion
kinetics (14). This method
combines the pH-stat technique with a static in vitro
digestion protocol based on the INFOGEST guidelines (15). That method was used to investigate
the digestion behavior of two emulsion-type food matrices that have
the same compositions (10 wt% fat, 15 wt% whey proteins), but have
different structures at the macroscopic and microscopic scales.
However, studies on the structural effects of real food matrices on
both lipolysis and proteolysis kinetics and hydrolysis degrees are
required (14).
The present study aimed to develop healthier snacks
and evaluate the in vitro hydrolysis of macronutrients
within a real food matrix, utilizing a combined approach of pH-stat
titration technique and the INFOGEST in vitro digestion
protocol. A total of five snack prototypes were formulated
following the Smart Snack criteria (8). These prototypes were then assessed
for their compliance with nutrient content claims permitted by
Regulation (EC) No. 1924/2006 (http://data.europa.eu/eli/reg/2006/1924/2014-12-13).
Various foods (dry nuts, dry fruits, kefir, eggs, butter and
sunflower oil) or ingredients rich in bioactive compounds and
nutrients (whey protein, chickpea flour, green tea, inulin,
cinnamon and honey) were used in the formulations. Of note, three
of the snacks were baked and two of them were prepared as
ready-to-eat. In all the snacks, the sensory evaluation, the
approximate and fatty acid compositions, the degree of protein and
lipid hydrolysis in vitro using pH-stat and in vitro
starch hydrolysis were determined.
Materials and methods
Foods, ingredients and chemicals
All foods and ingredients were purchased from local
suppliers. Goat kefir was purchased from Baltali Food Company. The
80% purity WPC (WPC80) was provided by Dr. Oetker Türkiye Food
Company (https://www.droetker.com.tr/kunye). The vacuum dried
vegetables; red pepper, beet roots, spinach, leeks, zucchini and
carrot were a kind gift from Eregli AgroSan Company. Pullulanase
(EC-3.2.1.41), pepsin (≥2500U/mg protein, EC 232-629-3), pancreatin
(EC 232-468-9), α-amylase (EC 232-565-6), α-chymotrypsin (C 4129),
trypsin (T 030) and bile salt (B 8638) were purchased from
MilliporeSigma. The D-glucose measurement kit was purchased from
Megazyme.
Lyophilized goat kefir, resistant
starch flour and brewed green tea
Goat kefir was freeze-dried using a freeze-dryer
(Model-FT33, Armfield) and the dry matter ratio of goat kefir was
increased from 12 to 96%, while the total fat ratio was increased
from 4 to 28%.
Chickpea flour was diluted at a ratio of 1:8 with
water and pre-gelatinized for 10 min. The autoclave was then held
for 15 min at 121˚C. Subsequently, pullulanase (80 U/g sample) was
added followed by incubation in a water bath (60˚C, 6 h). The
retrogradation process was then repeated and the samples were dried
at 55˚C for 7 h. The dried samples were kept at 4˚C in powder form
until the resistant starch content was analyzed (16).
Dried green tea leaves (0.8 g) were added to 250 ml
of boiling water and kept for 4 min for infusion. The leaves were
filtered, and the tea was stored at 4˚C until use.
Snack preparation
The classic homemade shortbread (R) was used as a
control and its recipe was used as the basis for the five snacks.
The ingredient list of the prepared snacks is presented in Table I. For snacks 1-3, the dry
ingredients were mixed and green tea was then added. The dough was
kneaded (7 min) by hand and shaped 5.5 cm width, 15 cm length and
2.5 cm in height before the pre-baking process in the convection
oven (9629 CMS, Beko) for 40 min at 150˚C, without a fan.
Subsequently, the pre-baked dough was removed from the oven and cut
into rectangular shapes, which were 1-cm-thick, and baked again for
10 min at 150˚C, with a fan. For snacks 4 and 5, dried nuts were
ground in the kitchen grinder (BH259CG, Blue House) for 10 sec and
transferred to a bowl. The dried fruits were then mixed with WPC80
for snack 5 and with inulin for snack 4. All ingredients in the
bowl were then kneaded by hand for 180 sec with the addition of
honey, cinnamon, glycerol or sunflower lecithin. Baking was not
applied to these snacks and they were prepared as ready-to-eat. For
this, the dough was formed into a bar and stored at 4˚C for 2 h in
the refrigerator, to obtain its firmness. All snacks were stored at
-18˚C until analyses were performed.
 | Table IList of ingredients of the snacks (100
g samples). |
Table I
List of ingredients of the snacks (100
g samples).
| | Snack sample |
|---|
| Ingredients | R | 1 | 2 | 3 | 4 | 5 |
|---|
| Whole wheat
flour | 49.75 | 35.56 | 30.10 | 30.10 | - | - |
| Chickpea flour
(RS) | - | 8.47 | 6.02 | 4.32 | - | - |
| Butter (82%
fat) | 15.40 | 7.62 | 10.16 | 10.16 | - | - |
| Sunflower oil | - | 2.54 | - | - | - | - |
| Egg (whole) | 12.90 | 25.4 | 18.07 | 24.9 | - | - |
| Desiccated
coconut | - | - | - | - | 8.55 | 8.55 |
| Goat kefir
powder | - | 3.11 | 3.11 | 3.11 | - | - |
| Sugar (beet) | 12.15 | - | 4.81 | 3.00 | - | - |
| WPC 80 | - | 6.35 | 6.35 | 3.18 | - | 21.40 |
| Nut mix | 7.35 | - | 4.51 | 4.51 | 28.06 | 27.36 |
|
Hazelnuts | 2.45 | - | - | - | 14,03 | - |
|
Almonds | 4.90 | - | 4.51 | 4.51 | - | - |
|
Walnuts | - | - | - | - | 14.03 | 13.68 |
|
Peanuts | - | - | - | - | - | 13.68 |
| Dried fruits | 2.45 | - | 6.30 | 9.16 | 44.70 | 38.50 |
|
Raisin
(sultanas) | - | - | 3.15 | 4.58 | 29.80 | 25.65 |
|
Blueberry | - | - | - | - | 8.77 | - |
|
Cranberry | 2.45 | - | 3.15 | 4.58 | - | 8.55 |
|
Sour
cherry | - | - | - | - | 6.13 | 4.30 |
| Dried
vegetables | - | 3.56 | - | - | - | - |
| Inulin | - | 3.05 | 3.65 | 2.53 | 13.80 | - |
| Brewed green
tea | - | 4.23 | 6.82 | 4.23 | - | - |
| Cinnamon
(powder) | - | - | - | 0.70 | - | - |
| Salt (sea) | 0.10 | 0.10 | 0.10 | 0.10 | - | - |
| Honey (pine) | - | - | - | - | - | 1.24 |
| Other ingredients
(glycerol, sunflower lecithin) | - | - | - | - | 4.89 | 2.95 |
Proximate composition
Fat, total sugar, moisture and ash analyses were
determined according to AOAC (17). The water activities (aw)
of the snacks were determined with a precision humidity measuring
instrument (Testo 650, Testo SE & Co. KGaA). The total
carbohydrate amount (CHO) was determined using the difference
method. The energy values of the snacks were calculated using
Atwater calorie constants (18).
The fatty acid composition of the snacks was measured using gas
chromatography (Agilent Technologies, Inc.) with a FID detector at
235˚C.
Sensory evaluation
The standard hedonic scale (5-point scale; from 1,
dislike; to 5, like, ISO 11136:2014; https://www.iso.org/standard/50125.html) was used to
assess the sensory attributes and overall acceptance of the
developed snacks. A total of 80 panelists (aged between 18-55
years) were randomly selected and asked to evaluate the snacks
according to their taste preferences. Overall acceptability was
calculated as the mean of scores describing each attribute and
multiplied by the importance factor. Snacks >70% points were
considered successful.
In vitro hydrolysis of protein and
lipids using pH-stat
The in vitro digestion of snacks was
performed according to the INFOGEST standardized static digestion
protocol (15). The preparation of
digestive solutions; simulated saliva fluid (SSF), simulated
gastric fluid (SGF) and simulated intestinal fluid (SIF) and enzyme
activity assays were performed according to this protocol. The oral
and gastric phases of the protocol were applied the same as in
INFOGEST protocol (15). According
to this protocol, samples containing starch are mixed with saliva
containing α-amylase enzymes and treated for 2 min. At this stage,
starch digestion begins and dextrin form is formed. The digestion
continues with the stomach and intestinal phases. In the present
study, after performing the mouth and stomach stages according to
this protocol, the method of Mat et al (14) was used to monitor fat and protein
hydrolysis with pH-stat (14). For
oral digestion, 3 g of the sample was mixed with 4 ml SSF and 0.025
ml of 0.3 M CaCl2 and the volume was adjusted to 10 ml
using ultrapure water. Subsequently, the mixture was incubated in a
shaking incubator operated at 200 rpm and 37˚C for 2 min. Following
mouth digestion, 8 ml SGF and 0.005 ml CaCl2 were added
to the bolus. The pH was then adjusted to 3 using 6 M HCl and 1 ml
pepsin enzyme (2,000 U/ml) was added to the mixture. The gastric
digestion was performed in a shaking incubator operated at 200 rpm
and 37˚C for 2 h. Following the gastric phase, some modifications
were made in the SIF formulation (with NaCl instead of
NaHCO3) to prevent buffering effect during intestinal
digestion (14). The volume of
gastric bolus was completed to 20 ml with SIF and pancreatin added
with pancreatic lipase (100 U/ml trypsin activity and 2,000 U/ml
lipase activity in the final mixture). For the intestinal phase, an
automatic titrator (Kyoto KEM AT-510, Kyoto Electronics
Manufacturing Co., Ltd.) with a pH-stat program was then used to
record the reaction kinetics by maintaining the pH at 7.0 with
addition of 0.1 N NaOH as a titrant and determined with the action
of enzymes release protons, and subsequently a decrease in pH. The
analysis was performed in two steps. In the first step, the
determination of the total degree of hydrolysis
(DHtotal) (proteolysis + lipolysis), intestinal
digestion was performed with the action of pancreatin (100 U/ml
final solution based on trypsin activity), which can hydrolyze both
proteins (peptides and remaining proteins after gastric phase) and
lipids. The volume of NaOH consumed during the titration was
recorded (V1). In the second step, for the determination of only
the degree of proteolysis, intestinal digestion was conducted with
trypsin (100 U/ml final mixture) and chymotrypsin (25 U/ml final
mixture). The volume of NaOH consumed during the titration was
recorded (V2). Following 2 h of digestion of the small intestine,
the degree of proteolysis was calculated using the following
equation (Eq. 1):
where V1 (NaOH) is the total volume of
NaOH consumed (mL), N (NaOH) is the normality of NaOH (Eq/L),
mprotein is the protein content of the sample (g),
α(RNH2) is the mean degree of dissociation of α-amino
groups, htot is the number of peptide bonds with respect
to the origin of the protein (meqv/g protein). Since the recorded
data from the pH-stat device in the intestinal phase is the result
of all physical and enzymatic actions throughout the digestion, it
is assumed that both total degree of hydrolysis and degree of
proteolysis calculations represent the entire digestion procedure,
consisting of oral, gastric, and intestinal phases (14). For the in vitro degree of
lipolysis calculation, the following equation was used (Eq. 2):
where V2 (NaOH) is the total consumption
of NaOH in the presence of pancreatin enzymes at the intestinal
phase. V1 (NaOH) is the total amount of NaOH consumed
(ml) during the intestinal phase of in vitro proteolysis, N
(NaOH) is the normality of NaOH, m(lipid) is the oil
mass of the sample (g), Mlipid is the molecular weight
of the triglycerides in the oil fraction of the sample (g/mol) and
α(FFA) is the mean degree of dissociation of the carboxylic groups
of FFA.
In vitro starch hydrolysis and
estimated GI (eGI)
All stages, namely oral, gastric and intestinal were
applied according to the method suggested in the study by Minekus
et al (15). Starch
digestion begins in the mouth with α-amylase and dextrin is formed.
The glucose measurements were taken by sampling at the beginning of
the oral phase (t0) and every 30 min of the intestinal
phase for each snack. The end of the oral stage (t0) was accepted
as the initial moment for glucose measurement, to subtract the
naturally available glucose (if accessible) derived from raw
materials in the recipe, such as dry fruits. Additionally, these
data would be used to subtract from the further glucose
measurements during digestion to calculate starch hydrolysis. With
this approach, it is expected to prevent a potential error in the
calculation of starch hydrolysis stemming from the available
glucose in the recipe. Enzymatic reactions in the samples were then
terminated by the addition of 1 M HCl and centrifuged at 6,500 x g,
20˚, 20 min. The samples were diluted with water then treated using
GOPOD reactive solution and incubated in a water bath for 20 min at
45˚C. The D-glucose analysis kit (cat. no 700004297, Megazyme) was
used for the determination of the absorbance of sample at a 510 nm
wavelength in UV visible spectrophotometer (Cary 50 Scan, Varian).
Glucose was calculated according to following equation (Eq 3)
(19):
where At is the absorbance of the sample,
ΔAs is the absorbance of the glucose standard solution,
V is the volume of the measured sample (ml), C is the concentration
of the glucose standard solution (mg/ml), D is the dilution factor,
W is the weight of the sample (mg).
The rate of released glucose from sample starch
digestion was expressed at different times and the hydrolysis index
(HI) was obtained by dividing the area under the curve of the
sample to the area of control (white bread). The eGI was calculated
using equations 3, 4 and 5 respectively (20). The HI of white bread was accepted
as 100. Calculations using equations 4 and 5 are the best
correlated formulas for in vitro measurements when they are
compared with in vivo glycemic responses according to
reference study (21).
where HI is the percentage of total starch
hydrolysis, TNt is the total amount of hydrolyzed starch
at the time of t (g), and Gt is the amount of glucose in
the sample at the time of t (g).
Statistical analysis
All analyses were performed in triplicate. Data were
analyzed using one-way analysis of variance (ANOVA) with Tukey's
post hoc test using SPSS Statistics 20 software (IBM Corp.). The
confidence interval was selected at 95%. All results are presented
as the mean ± standard deviation. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results and Discussion
Proximate composition
The chemical composition and energy value of the
snacks are presented in Table II.
The percentages of cooking loss of R (recipe), and snacks 1, 2 and
3 were 16, 28, 18 and 17%, respectively. The final percentage
calculations of chemical compositions and caloric values for the
snacks were adjusted to account for the mass loss incurred during
the cooking process. The energy values of all snacks apart from R
(control) were <135 kcal when they were evaluated on a serving
size (30 g) basis. While the amounts of total CHO in the snacks
varied between 14.4 and 16.56 g, the reference was found as 18.3 g
per serving (Table II). The
highest total sugar content was determined at 17.4% for the control
formula (R). The main fatty acids were palmitic (16:0), oleic
(18:1) and linoleic acid (18:2) in all snacks (Table III). The values of water activity
(aw) of all snacks were measured <0.6 (Table II), which provides the minimum
limit to prevent microbial growth and plays a crucial role in
biochemical reactions during storage. The water activities of the
baked snacks (snacks 1-3) were found to be lower than those of the
ready-to-eat snacks (snacks 4 and 5) (Table II), resulting from the heat
treatment applied to snacks1-3 and the glycerol and lecithin
involved in the ready-to-eat snacks.
 | Table IIChemical composition and energy value
of the snacks. |
Table II
Chemical composition and energy value
of the snacks.
| Sample | Moisture (%) | Ash (%) | Protein (%) | Fat (%) | Water activity
(aw) | Total sugar
(%) | Total CHO (%) | Energy (kcal/30
g) |
|---|
| R | 3.60±1.01 | 1.22±0.04 | 8.7±0.26 | 21.90±0.34 | 0.354±0.08 | 17.42±2.48 | 62.7±0.74 | 148 |
| 1 | 3.10±1.01 | 2.70±0.06 | 20.5±0.31 | 18.30±0.60 | 0.308±0.07 | 2.20±0.93 | 55.2±0.34 | 134 |
| 2 | 3.29±1.12 | 2.18±0.04 | 17.7±0.68 | 16.05±0.11 | 0.309±0.09 | 10.95±2.72 | 59.5±0.22 | 127 |
| 3 | 3.34±0.43 | 1.97±0.15 | 16.5±0.26 | 17.10±0.34 | 0.323±0.06 | 8.66±5.95 | 61.0±0.22 | 129 |
| 4 | 7.30±0.29 | 2.20±0.08 | 5.7±0.69 | 24.30±0.51 | 0.520±0.04 | 17.08±3.45 | 60.2±0.71 | 119 |
| 5 | 7.70±0.26 | 2.20±0.04 | 22.6±0.21 | 19.00±2.01 | 0.509±0.03 | 15.40±2.32 | 48.3±2.06 | 110 |
 | Table IIIFatty acid composition of the samples
(%). |
Table III
Fatty acid composition of the samples
(%).
| | Snack samples |
|---|
| Fatty acids | 1 | 2 | 3 | 4 | 5 |
|---|
| C10:0 | 1.99 | 4.13 | 2.44 | 0.20 | 0.95 |
| C12:0 | 2.84 | 4.23 | ND | 5.67 | 8.31 |
| C14:0 | 9.73 | 13.20 | 8.62 | 2.01 | 3.71 |
| C16:0 | 29.90 | 32.44 | 28.32 | 9.49 | 6.09 |
| C18:0 | 8.04 | 8.66 | 9.34 | ND | ND |
| C18:1 | 33.89 | 23.72 | 35.41 | 41.97 | 42.39 |
| C18:2 | 10.18 | 5.62 | 10.59 | 32.83 | 30.41 |
| C18:3 | ND | ND | ND | 6.17 | 5.51 |
Sensory evaluation
The results of sensory analysis on the hedonic scale
are expressed with a score >100. For snacks 1, 2, 3, 4 and 5,
the scores were 70.9, 80.8, 81.8, 71.5 and 77.1, respectively. The
snack preferences were in the following order:
3>2>5>4>1. The consumer preference for snacks 3 and 2
may be attributed to the presence of dried fruits and a higher
butter content. Additionally, the lower utilization of retrograded
chickpea flour in these snack samples compared to snack 1 may
contribute to a more pleasant aroma. Conversely, the lower
preference for snacks 4 and 5 is likely due to their higher content
of dietary fiber (13.80% in sample 4) and WPC (21.40% in sample 5)
compared to the preferred samples (2 and 3). These ingredients may
have resulted in a less favorable sensory perception. Finally,
snack 1, containing retrograded chickpea flour with no added sugar
or dried fruits, may have been perceived as the least favorable due
to its relative dryness and potentially strong salty taste.
Degree of proteolysis
Proteolysis was evaluated by decreasing the pH value
of the protons of the carboxyl ends of amino acids and peptides,
which are released following GI digestion. According to the data
obtained from pH-stat during the intestinal digestion of the
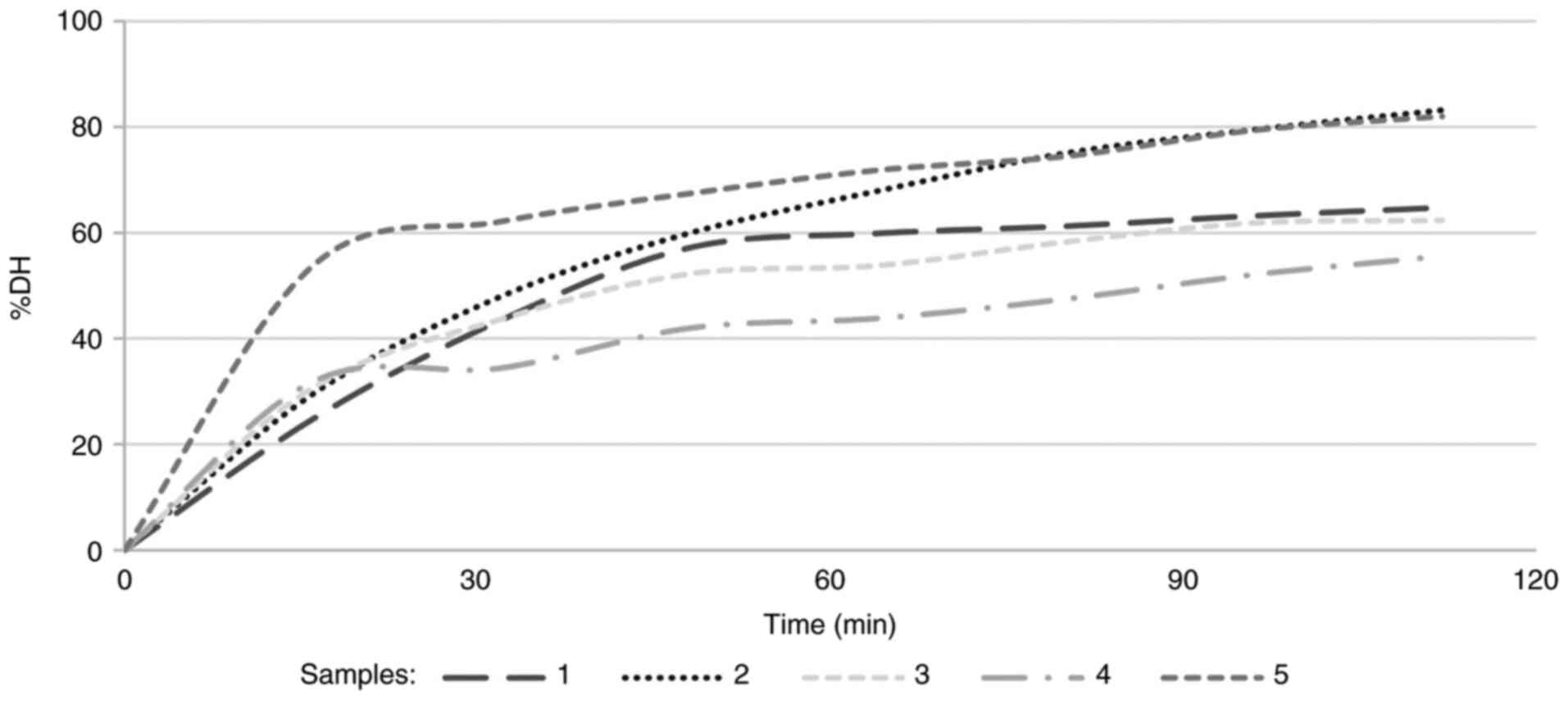
snacks, the kinetics of all snacks, apart from snack 2 were similar
in shape, exhibiting a rapid rising rate at the beginning (first 45
min) followed by a gradual attenuation, leading to a plateau. Snack
2 reached the highest degree of hydrolysis with an increasing speed
up to 115 min, but did not exhibit a tendency to plateau; probably
the reaction was continuing at this moment (Fig. 1). Apart from snack 4, all snacks
had >40% proteolysis within 30 min. At the end of the intestinal
digestion, the degree of hydrolysis was estimated >80% for
snacks 2 and 5. The degree of hydrolysis of all the snacks at the
end of the intestinal phase is presented in Table IV. There was no significant
difference between snacks 1 (65.17%) and 3 (63.12%) (P>0.05),
while snack 4 had the lowest degree of hydrolysis (57.37%), and
snacks 2 and 5 had high degrees of hydrolysis (84.84 and 84.46%,
respectively; P<0.05). In the recipe for snack 4, a high amount
(13.8%) of inulin was used; thus, it could be a distinct difference
compared to snacks 1, 2 and 3 (inulin contents: 3.05, 3.65 and
2.53%, respectively). In snack 5 which had the highest degree of
hydrolysis, inulin was not used. On the other hand, it could be
said that resistant starch had no effect on protein hydrolysis in
snacks 1, 2 and 3 (P<0.05). Sciarini et al (22) examined the in vitro
digestibility of gluten-free bread enriched with inulin. They noted
that the protein hydrolysis of bread decreased when inulin was
added at 10% to the bread and increased at the level of 5%. The
addition of fiber can cause a different structure between protein
and starch in bread, which could make starch and protein less
accessible to enzyme hydrolysis and influence protein aggregation
(13,23). Inulin as a viscous soluble dietary
fiber increases digesta viscosity and decreases the kinetics of
enzyme reactions due to reducing the rate of mixing with
proteolytic enzymes released in the stomach or small intestine
during digestion (23,24). However, this physicochemical
barrier for enzyme reactions can be evaluated with the view that it
provides a low GI due to lower carbohydrate digestion. This effect
will be explained in further detail below in the subsection
entitled ‘Degree of starch hydrolysis and eGI’.
 | Table IVIn vitro protein, lipid
hydrolysis, released glucose, HI and eGI values of the snack
samples. |
Table IV
In vitro protein, lipid
hydrolysis, released glucose, HI and eGI values of the snack
samples.
| | Snack sample |
|---|
| Hydrolysis (%) | White bread | R | 1 | 2 | 3 | 4 | 5 |
|---|
|
DHproteolysis | - | - |
65.17±3.91a |
84.84±5.39b |
63.12±10.95a |
57.37±3.44c |
84.46±7.96b |
|
DHlipolysis | - | - |
76.85±1.76d |
62.63±12.68d |
74.65±7.07d |
80.94±1.54e |
91.92±5.07f |
| RG% | 77.83 | 64.02 | 50.84 | 65.46 | 64.13 | 90.13 | 79.93 |
| HI | 100 | 105.57g |
61.54±1.76h |
105.12±12.68i |
107.19±7.07i | NM | NM |
| eGI | 70 | 73.04j | 56.21k | 69.03l | 68.33l | NM | NM |
Starch hydrolysis
Another reason for the highest hydrolysis degree
found in snack 5 may be the presence of WPC in the recipe; snack 5
had the highest amount of WPC (21.40%) among the snacks. This
result is consistent with the findings in the literature (13,25),
reporting that the denatured form of the whey protein structure is
easily hydrolyzed with intestinal protease enzymes. However, a
previous study found that the proteolysis results of biscuits
enriched with 20% whey protein and pea protein is was not altered
compared with the control (23).
Furthermore, different proteolysis rates have been reported when
heat-induced whey protein gel structures are subjected to simulated
intestinal digestion. It has been observed that the gel structure
may affect the reaction rate and hydrolysis mechanisms, and the
differences in the diffusion of the enzyme into the chyme during
proteolysis may result from the steric barrier created by the gel
structure (26,27). In the present study, the use of
enriched whey protein without the use of heat treatment may
correspond to high levels of enzyme activity.
Degree of lipolysis
Lipolysis was calculated using data obtained by the
subtraction of the DH values obtained in the presence of
proteolytic enzymes, from DH values obtained in the presence of
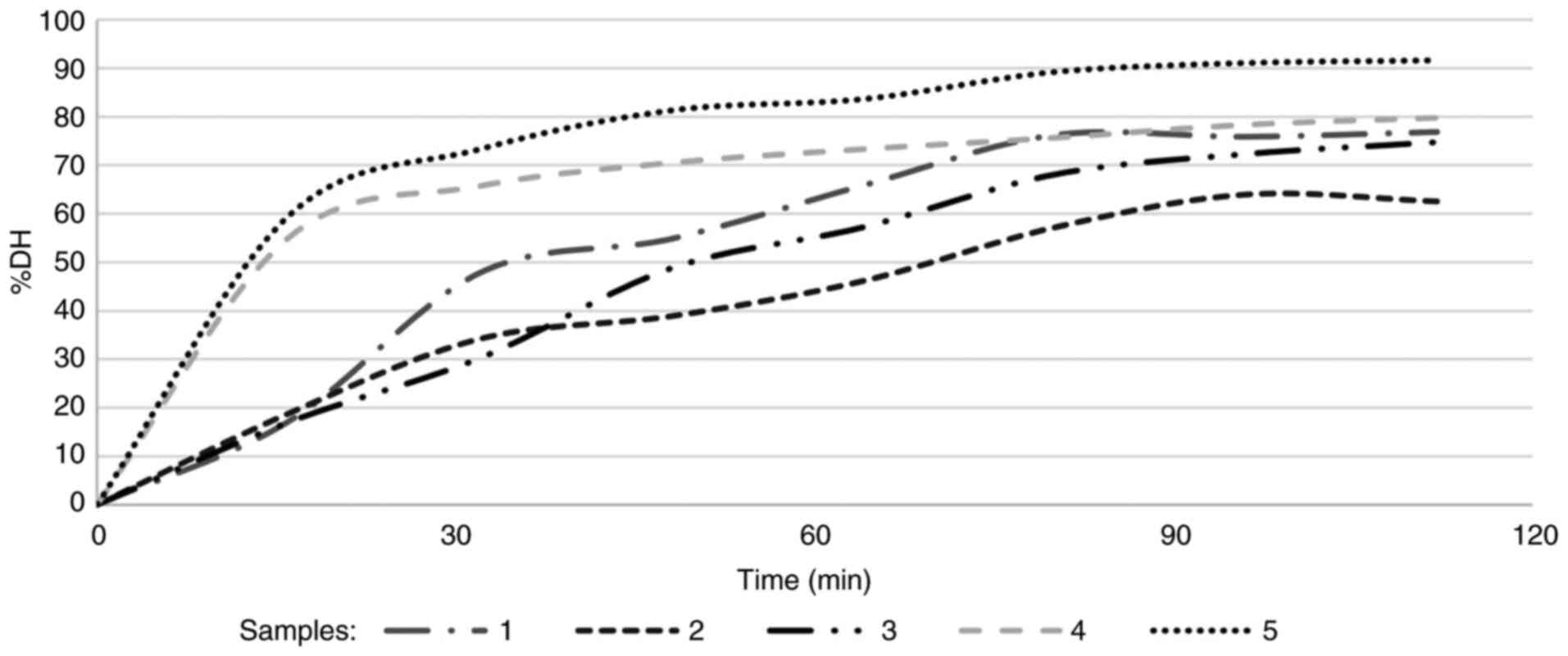
pancreatin enzymes (Fig. 2). In
snacks 4 and 5 (ready-to-eat samples), a lipolysis >80% was
observed, which was higher than that of the baked snacks (snacks 1,
2 and 3) (P<0.05; Table IV);
snacks 4 and 5 exhibited rapid digestion at the beginning; however,
the release rate of fatty acids then gradually decreased, and the
lipolysis rate remained relatively constant after 75 min, until the
end of intestinal digestion. However, in the baked snacks, the rate
of lipolysis was slower, with hydrolysis occurring in the first 25
min at ~30%; this value was >65% in snacks 4 and 5. There were
no marked differences between snacks 1, 2 and 3 (P>0.05);
however, the lipolysis values of snacks 4 and 5 differed
significantly (P<0.05) (Table
IV). The difference between snacks 4 and 5 can be explained by
the degree of lipolysis being negatively affected due to the
interaction of dietary fibers with molecules, such as bile salts,
fatty acids and calcium during gastro-intestinal digestion
(28). Furthermore, the lower
lipolysis values of the baked samples compared with the
ready-to-eat snacks may be based on the presence of starch that
negatively affects lipolysis. A previous study investigated the
influence of intestinal conditions, non-lipid components and food
matrix on lipolysis with a selection of 52 real foods. The results
were then statistically processed using factors such as energy,
protein, total lipid, starch, saturated fatty acid, monounsaturated
fatty acid, polyunsaturated fatty acid, fiber, iron, calcium,
sodium content of the foods; and lipid structures (complex solid,
continuous aqueous phase and continuous lipid phase) in the food
matrix (28). The authors of that
study pointed out that lipid-protein and lipid-starch interactions
negatively affected lipolysis in real foods when using the INFOGEST
(15) static in vitro
digestion method. Notably, high-fat foods were not affected by
these macronutrient interactions (29). In the present study, snacks 4 and 5
that had a total lipid content of 24 and 19%, respectively, the
lipolysis values were higher than those of the baked snacks
(P<0.05). It was found that snack 5, which had the highest
protein content (22.6%), had the highest lipolysis value (91.9%;
P<0.05; Table IV) compared
with the other snacks. Additionally, the high unsaturated fatty
acid content in the snacks positively affected the lipolysis
values, resulting in the uncooked snacks (ready-to-eat) having
higher lipolysis values than the baked snacks (P<0.05; Table IV). On the contrary, the content
of mono or polyunsaturated fatty acids has been reported to have no
effect on lipolysis in real foods (29).
Degree of starch hydrolysis and
eGI
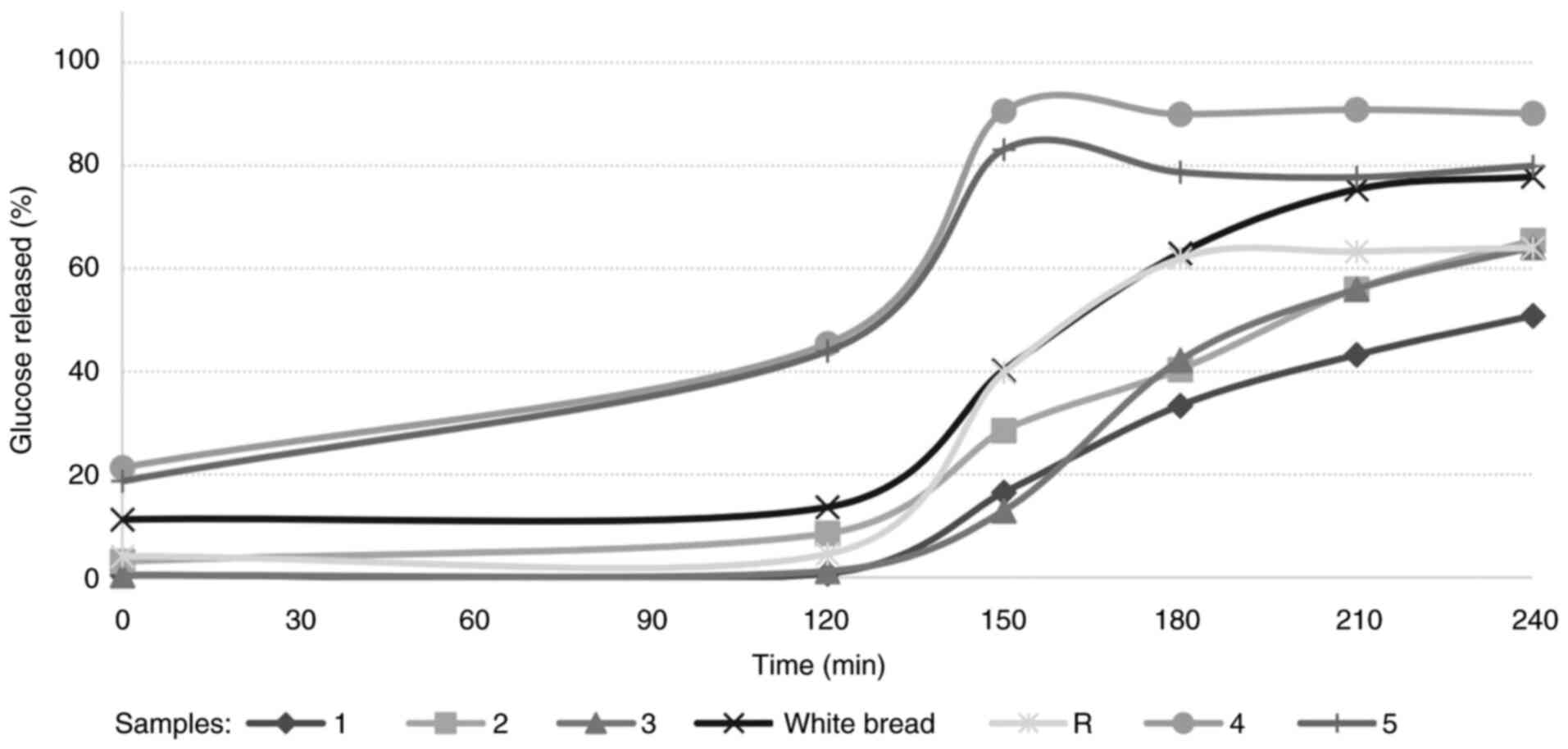
The glucose released during the hydrolysis of the
sample during simulated gastrointestinal digestion is illustrated
in Fig. 3. Starch hydrolysis was
examined in R (control recipe), white bread and baked snacks
(numbered as 1, 2 and 3) that contain a certain amount of complex
carbohydrates (Fig. 3). The
calculated HI and eGI values of the samples are presented in
Table IV. The eGI values were
correlated to 70 (white bread) (≤55: Low; 56<eGI<69:
Moderate; 70≥eGI: High) and although the control (R) was on the
border, it was found to have a high GI, while snacks 1, 2 and 3
were evaluated as having a moderate GI. The hydrolysis rate (HI) of
snack 1 was significantly lower than that of the other snacks
(P<0.05). According to the eGI calculations, all baked snacks
differed significantly compared with R (P<0.05). This is
particularly crucial for assisting the regulation of the blood
glucose level for insulin resistance, type-2 diabetes and obesity
(20). The glucose released during
in vitro digestion of snacks was measured by sampling at the
beginning of the oral phase (t0) and every 30 min of the intestinal
phase. According to the results (Fig.
3), glucose was not detected in snack 1 at the beginning (t120)
of the intestinal phase. The release of glucose molecules was
finished in unbaked snacks after the first 30 min (t150) of the
intestinal phase. However, glucose hydrolysis continued in baked
snacks until the time interval of t210 and t240. This difference
may have been caused by inhibitory and delaying effects of
ingredients such as whole wheat, resistant starch, and inulin
(30). Besides, the food matrix
could affect starch digestibility by creating interactions such as
starch-protein, starch-fiber and starch-lipid (31). In snacks 1, 2 and 3, brewed green
tea was used in ratios 4.23, 6.8 and 4.23%, respectively. In
another study, gallic acid and epigallocatechin gallate in green
tea extract may have inhibitory effects on enzymes that hydrolyze
starch (32). However, that
inhibitory effect was not observed in our study. Using brewed tea
instead of extract could be the reason for this result.
Evaluation of snacks as ‘Smart Snacks,
SS’ and nutritional claims
According to the Smart Snacks (SS) standard
(8), SS must meet certain
criteria. There must be a grain product with whole grains as the
main ingredient (≥50% by weight) or contain fruit and vegetables,
milk, dairy products or protein as the main ingredient, or a
combination of foods that provides at least 0.25 cups of fruit
and/or vegetables by weight. Additionally, there are five more
nutritional requirements based on the snacks' composition (8). When comparing the data from the
present study with these requirements, all snacks met the mandatory
criteria outlined in the Smart Snacks standard. These criteria
include having ≤200 calories, with ≤35% of the calories derived
from fat, <10% of the calories derived from saturated fat, and
35% of the weight derived from total sugars. The sources of sugar
and fat in the snacks complied with the exceptions specified in the
standard, such as dried fruits, dried vegetables, nuts and
seeds.
Furthermore, according to the Annex of Regulation
(EC) No. 1924/2006 (http://data.europa.eu/eli/reg/2006/1924/2014-12-13).
on labeling standards, all snacks can be nutritionally claimed as a
source of omega-3, containing a high amount of monounsaturated
fatty acids, rich in polyunsaturated fatty acids, and as a source
of protein. For snacks 1, 4 and 5 as ‘no sugar added’,
‘high amount of protein’ was amended by the Regulation (EU)
No. 1047/2012 (https://eur-lex.europa.eu/eli/reg/2012/1047).
The calorie target of the snacks in the present
study was set at 135 calories per serving. Additionally,
investigating the bioaccessibility of macronutrients in the samples
was critical for understanding the impact of the food matrix.
Chriqui et al (33)
observed in their study that implementing Smart Snacks policies in
school food environments effectively influenced the consumption of
fruits, vegetables, and healthier choices among young individuals.
The snacks developed in the present study were well-liked by the
panelists, which is a crucial criterion for replacing fatty,
sugary, or salty snacks with healthier alternatives.
In the present study, sensory successful products
with Smart Snack criteria, which can be sold in school canteens,
were developed considering the portion size. In the future, a
better approach perhaps would be to develop these standards
considering the bioaccessibility/bioavailability of proteins,
lipids and carbohydrates, and understanding the effects of the food
matrix and interactions of these macronutrients in food.
The healthy eating recommendations strive to enhance
overall well-being by focusing on specific dietary improvements.
These include reducing saturated fat intake, while increasing the
consumption of complex carbohydrates, such as dietary fiber and
bioactive compounds found in legumes, cereals, vegetables and
fruits. To further promote health and nutrition, the introduction
of Smart Snack standards has revolutionized snack choices by
evaluating them based on their positive impact. These new criteria
ensure that snacks available in school canteens align with health
goals. To address the diverse needs of consumers, the present study
successfully developed sensory appealing products that meet the
Smart Snack criteria, taking into account appropriate portion
sizes. However, to enhance these standards further, it would be
beneficial to consider the bioaccessibility/bioavailability of
essential nutrients, such as protein, lipids and carbohydrates
along with further standard updates on vitamins, minerals and trace
elements. This would involve the understanding of how these
macronutrients interact within the food matrix and their effects on
the body. By incorporating bioaccessibility/bioavailability factors
and assessing the intricate interactions among macronutrients,
future standards can better optimize the nutritional value of food
products. This approach will ensure that the snacks offered in
school canteens not only meet the smart snack criteria but also
provide optimal nutrient absorption and utilization. Consequently,
individuals will benefit from improved health outcomes and overall
well-being.
Acknowledgements
The present study is a part of the thesis of CD for
a Master of Science degree on nutrition section of food
engineering, under the supervision of SNE from the Food Engineering
Department of Ege University, Bornova, Turkey.
Funding
Funding: The present study received financial support from Ege
University (Project no: 16MUH022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CD and SNE worked collectively on the study design.
CD conducted the literature review, all the experiments, the
statistical analysis and participated in the discussion of the
results. SNE participated in the discussion of the results and
contributed to the structure and grammar of the whole article. CD
and SNE confirm the authenticity of all the raw data in that study.
Both authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO):
Sustainable healthy diets: guiding principles. WHO, Rome, 2019.
https://www.who.int/publications/i/item/9789241516648.
|
|
2
|
United European Gastroenterology (UEG):
The Survey of Digestive Health Across Europe Highlighting changing
trends and healthcare. UEG, Wien, 2016.
|
|
3
|
Bellisle F: Meals and snacking, diet
quality and energy balance. Physiol Behav. 134:38–43.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grunert KG, Brock S, Brunsø K,
Christiansen T, Edelenbos M, Kastberg H, Krogager SGS, Mielby LH
and Povlsen KK: Cool snacks: A cross-disciplinary approach to
healthier snacks for adolescents. Trends Food Sci Technol.
47:82–92. 2016.
|
|
5
|
Hess JM, Jonnalagadda SS and Slavin JL:
What Is a Snack, Why Do we snack, and how can we choose better
snacks? A review of the definitions of snacking, motivations to
snack, contributions to dietary intake, and recommendations for
improvement. Adv Nutr. 7:466–475. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zizza CA: Healthy snacking
recommendations: One size does not fit all. Physiol Behav.
134:32–37. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prapkree L, Uddin R, Jaafar JAA, Baghdadi
M, Coccia C, Huffman F and Palacios C: Snacking behavior is
associated with snack quality, overall diet quality, and body
weight among US college students. Nutr Res. 114:41–49.
2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
U.S. Department of Agriculture, Food and
Nutrition Service: A Guide to Smart Snacks in School. United States
Department of Agriculture, Washington, DC, 2016.
|
|
9
|
Mridula D, Singh KK and Barnwal P:
Development of omega-3 rich energy bar with flaxseed. J Food Sci
Technol. 50:950–957. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Aggarwal D, Sabikhi L and Sathish Kumar
MH: Formulation of reduced-calorie biscuits using artificial
sweeteners and fat replacer with dairy-multigrain approach. NFS J.
2:1–7. 2016.
|
|
11
|
Colantuono A, Ferracane R and Vitaglione
P: In vitro bioaccessibility and functional properties of
polyphenols from pomegranate peels and pomegranate peels-enriched
cookies. Food Funct. 7:4247–4258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stamataki NS, Nikolidaki EK, Yanni AE,
Stoupaki M, Konstantopoulos P, Tsigkas A and Karathanos VT:
Function Evaluation of a high nutritional quality snack based on
oat flakes and inulin : Effects on postprandial glucose, insulin
and ghrelin responses of healthy subjects. Food Funct. 7:3295–3303.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hiolle M, Lechevalier V, Floury J,
Boulier-Monthéan N, Prioul C, Dupont D and Nau F: In vitro
digestion of complex foods: How microstructure influences food
disintegration and micronutrient bioaccessibility. Food Res Int.
128(108817)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mat DJL, Le Feunteun S, Michon C and
Souchon I: In vitro digestion of foods using pH-stat and the
INFOGEST protocol: Impact of matrix structure on digestion kinetics
of macronutrients, proteins and lipids. Food Res Int. 88:226–233.
2016.
|
|
15
|
Minekus M, Alminger M, Alvito P, Balance
S, Bohn T, Bourlieu C, Carrière F, Boutrou R, Corredig M, Dupont D,
et al: A standardised static in vitro digestion method suitable for
food-an international consensus. Food Funct. 5:1113–1124.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Simsek S and El SN: Production of
resistant starch from taro (Colocasia esculenta L. Schott)
corm and determination of its effects on health by in vitro
methods. Carbohydr Polym. 90:1204–1209. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Association of Official Analytical
Chemists (AOAC): Official Methods of Analysis. 18th edition.
Association of Official Analytical Chemists, Gaithersburg,
2007.
|
|
18
|
Schakel SF, Jasthi B, Van Heel N and
Harnack L: Adjusting a nutrient database to improve calculation of
percent calories from macronutrients. J Food Comp Analysis. 22
(Suppl):S32–S36. 2009.
|
|
19
|
Englyst KN, Englyst HN, Hudson GJ, Cole TJ
and Cummings JH: Rapidly available glucose in foods: An in vitro
measurement that reflects the glycemic response. Am J Clin Nutr.
69:448–454. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Goñi I, Valdivieso L and Gudiel-Urbano M:
Capacity of edible seaweeds to modify in vitro starch digestibility
of wheat bread. Nahrung. 46:18–20. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Isabel G, Garcia-Alonso A and
Saura-Calixto F: A starch hydrolysis procedure to estimate glycemic
index. Nut Res. 17:427–437. 1997.
|
|
22
|
Sciarini LS, Bustos MC, Vignola MB,
Paesani C, Salinas CN and Pérez GT: A study on fibre addition to
gluten free bread: Its effects on bread quality and in vitro
digestibility. J Food Sci Technol. 54:244–252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Villemejane C, Denis S, Marsset-Baglieri
A, Alric M, Aymard P and Michon C: In vitro digestion of
short-dough biscuits enriched in proteins and/or fibres using a
multi-compartmental and dynamic system (2): Protein and starch
hydrolyses. Food Chem. 190:164–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jin Y, Yu Y, Qi Y, Wang F, Yan J and Zou
H: Peptide profiling and the bioactivity character of yogurt in the
simulated gastrointestinal digestion. J Proteomics. 141:24–46.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lorieau L, Halabi A, Ligneul A, Hazart E,
Dupont D and Floury J: Impact of the dairy product structure and
protein nature on the proteolysis and amino acid bioaccessiblity
during in vitro digestion. Food Hydrocoll. 82:399–411. 2018.
|
|
26
|
Dégen L, Halas V and Babinszky L: Effect
of dietary fibre on protein and fat digestibility and its
consequences on diet formulation for growing and fattening pigs: A
review. Acta Agric Scand A Anim Sci. 57:1–9. 2007.
|
|
27
|
Nyemb K, Guérin-Dubiard C, Pézennec S,
Jardin J, Briard-Bion V, Cauty C and Nau F: The structural
properties of egg white gels impact the extent of in vitro protein
digestion and the nature of peptides generated. Food Hydrocoll.
54:315–327. 2016.
|
|
28
|
Espinal-Ruiz M, Parada-Alfonso F,
Restrepo-Sánchez LP, Narváez-Cuenca CE and McClements DJ: Impact of
dietary fibers [methyl cellulose, chitosan, and pectin] on
digestion of lipids under simulated gastrointestinal conditions.
Food Funct. 5:3083–3095. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang H, Jiang Y, Pan J, Lv Y, Liu J,
Zhang S and Zhu Y: Effect of tea products on the in vitro enzymatic
digestibility of starch. Food Chem. 243:345–350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Calvo-Lerma J, Fornés-Ferrer V, Heredia A
and Andrés A: In vitro digestion of lipids in real foods: Influence
of lipid organization within the food matrix and interactions with
nonlipid components. J Food Sci. 83:2629–2637. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Singh J, Dartois A and Kaur L: Starch
digestibility in food matrix: A review. Trends Food Sci Technol.
21:168–180. 2010.
|
|
32
|
Vujić L, Vitali Čepo D and Vedrina
Dragojević I: Impact of dietetic tea biscuit formulation on starch
digestibility and selected nutritional and sensory characteristics.
LWT-Food Sci Technol. 62:647–653. 2015.
|
|
33
|
Chriqui JF, Leider J, Turner L,
Piekarz-Porter E and Schwartz MB: State wellness policy requirement
laws matter for district wellness policy comprehensiveness and
wellness policy implementation in the United States. Nutrients.
13(188)2021.PubMed/NCBI View Article : Google Scholar
|