Introduction
Table olives have been known since antiquity to have
beneficial effects on human health. Edible table olives contain
substances which potentially can protect the body from oxidative
stress and cardiovascular diseases. These substances are phenolic
compounds, which are found in extra virgin olive oil, but not in
any other edible fruit. While a number of studies have been
conducted on the phenolic compounds contained in extra virgin olive
oil (1-5),
the number of studies conducting in-depth investigations concerning
table olives and the phenols contained therein with regards to
potential the biological effects on human health are limited
(6-8).
In previous research, the health benefits associated
with tyrosol and hydroxytyrosol, prominent bioactive compounds
present in table olives, were extensively explored. Hydroxytyrosol,
a compound found in olive oil and table olives, has shown promising
results in mitigating cardiovascular risk factors. Notable studies
include a randomized double-blind, placebo-controlled parallel
study by Knaub et al (9),
which investigated the low-density lipoprotein (LDL)-cholesterol
lowering effect of hydroxytyrosol (HTEssence®).
Additionally, others studies (10,11)
have provided insight into the hypocholesterolemic effects of
hydroxytyrosol, demonstrating its potential to lower cholesterol
levels and to function as an antioxidant. The study by Tabernero
et al (12) further
contributed to the understanding of the metabolic effects of
hydroxytyrosol, emphasizing its role in ameliorating
hypercholesterolemia.
By directly targeting the mitochondria in inflamed
endothelial cells, hydroxytyrosol reduces mitochondrial peroxide
production, enhances superoxide dismutase activity and suppresses
inflammatory angiogenesis, thereby mitigating oxidative stress and
inflammation at the cellular level (13,14).
Additionally, hydroxytyrosol prevents lipid peroxidation, a key
process in protecting LDLs from oxidative damage, which is critical
for reducing the cardiovascular risk (15,16).
Experimental studies have also highlighted the ability of
hydroxytyrosol to address liver-related metabolic dysregulation. By
alleviating oxidative stress and liver inflammation, hydroxytyrosol
prevents early-stage insulin resistance and non-alcoholic fatty
liver disease (NAFLD), restoring glucose homeostasis. This
protective effect was exemplified in animal studies, where
hydroxytyrosol administration led to marked reductions in
cholesterol levels and improved insulin sensitivity and glucose
tolerance (17,18). Taken together, these findings
expand the understanding of the hypolipidemic and metabolic effects
of hydroxytyrosol, aligning with its observed benefits in clinical
trials.
Similarly, studies on tyrosol, another phenolic
compound found in Olea europaea, have highlighted its
antihyperlipidemic effects. Chandramohan and Pari (19) conducted research showcasing the
antihyperlipidemic effects of tyrosol in rats with
streptozotocin-induced diabetes. Furthermore, Boronat et al
(20) explored the cardiovascular
benefits of tyrosol, indicating its potential to contribute to
cardiovascular health and its conversion into hydroxytyrosol in
humans. Tyrosol exhibits notable cardiometabolic protective effects
through diverse biochemical mechanisms. Its anti-inflammatory
properties are attributed to its ability to modulate the
upregulation of cluster of differentiation 14 (CD14) and suppress
inflammatory processes, which play pivotal roles in hypertension,
coronary artery disease and insulin resistance (21). Moreover, tyrosol enhances
high-density lipoprotein (HDL) functionality by reducing oxidative
modifications and promoting cholesterol efflux via the ATP-binding
cassette transporter A1 (ABCA1) pathway, a critical step in
maintaining cholesterol homeostasis (22). It also demonstrates
anti-atherogenic activity by inhibiting leukotriene B4 production,
thereby preserving endothelial function and reducing vascular
inflammation (23). The
hepatoprotective effects of tyrosol have been highlighted in
studies demonstrating its capacity to reduce lipid synthesis in
primary rat hepatocytes and mitigate NAFLD through the upregulation
of cystathionine β-synthase and cystathionine γ-lyase expression,
leading to increased hepatic hydrogen sulfide (H2S)
synthesis (24,25). Taken together, these findings
underscore the potential health-promoting properties of both
tyrosol and hydroxytyrosol, emphasizing their key role in the
context of cardiovascular well-being and metabolic health.
In a previous study by the authors, biochemical
tests were performed in a small group of normal control volunteers
after consuming table olives of ‘Kalamon’ variety with a high
phenolic content (unpublished data, derived from the Master's
thesis of Ms. Maria Vlachakou, performed by the Research Group of
Clinical Pharmacology and Pharmacogenomics, National and
Kapodistrian University of Athens, 2017). The material of the study
was selected due to the high content in tyrosol and hydroxytyrosol,
measured using quantitative nuclear magnetic resonance (qNMR)
protocols, established by Mousouri et al (26) in 2014. The results of the
biochemical blood measurements following a period of a 30 days of
the daily consumption of five olives suggested that there was an
improvement in the levels of specific critical markers,
characterized as risk factors of metabolic syndrome. Based on the
initial results, herein, the 30-day study was repeated with
volunteers with mild dyslipidemia consuming a nutritional
supplement that was developed in a daily dosage corresponding to
five table olives of high-phenolic Kalamon variety. In the initial
study, volunteers consumed table olives that were standardized for
their content in tyrosol and hydroxytyrosol. In the present
follow-up study, these bioactive compounds were extracted from the
olive matrix to confirm whether the extract itself is responsible
for the observed effects. By using this approach, the present study
also aimed to explore the potential of utilizing olive by-products
to create a dietary supplement, thus providing access to the health
benefits of olives to individuals who may not have regular access
to table olives.
Subjects and methods
Supplement production
The selected Kalamon table olives, provided by
Sakellaropoulos Organic Farms (Laconia, Greece), were initially
processed into a paste using a mill, which served as the starting
material for the extraction of bioactive compounds. The olive paste
in this stage consisted of ~15% olive oil, solid materials of the
olive flesh and an aqueous phase, containing the bioactive
compounds implemented in the supplement. The first step of the
extraction of bioactive compounds was the olive oil removal, using
an OlioMio decanter (Pieralisi). Tyrosol, hydroxytyrosol and lactic
acid were then extracted from the paste by the addition of purified
water at a 1:1 w/w ratio and stirring continuously for 40 min. The
aqueous phase was obtained by filtration and evaporated under
vacuum using a rotary evaporator. This procedure yielded 85% of the
bioactive compounds present in the original material. The dry
material obtained from this procedure contained significant amounts
of salt, that was removed using a 3:1 v/w dilution in ethanol,
filtration and evaporation. Finally, the extracted bioactive
compounds were formulated into capsules using microcrystalline
cellulose as a carrier. To validate the concentration of bioactive
compounds in the developed supplement, a re-extraction of the
compounds from the carrier was performed. This process involved the
addition of syringaldehyde (MilliporeSigma), as an internal
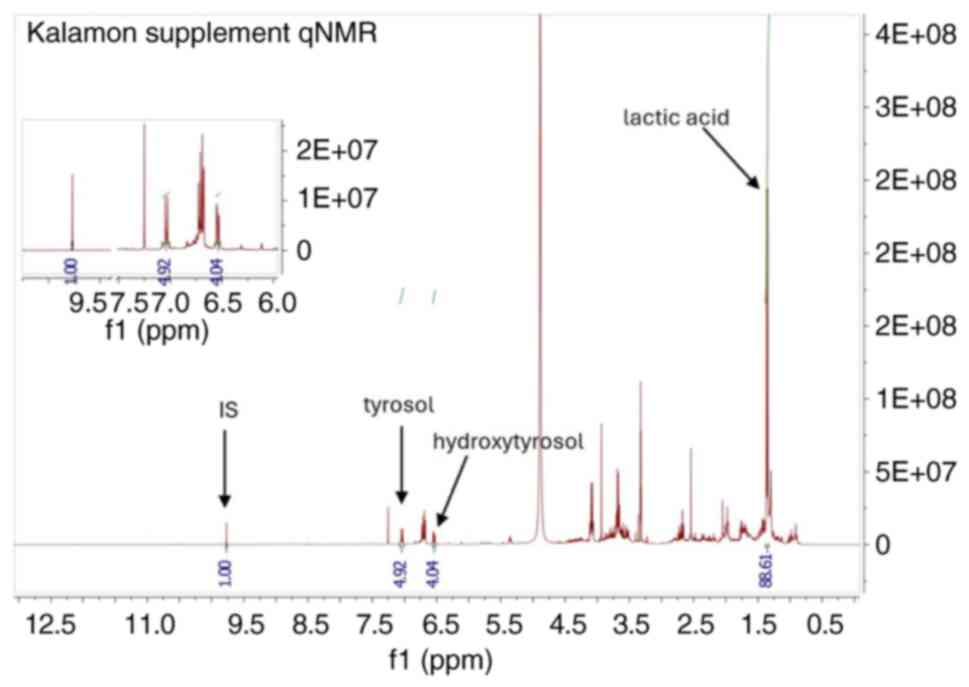
standard to the re-extracted solution, followed by qNMR analysis
(Fig. 1). The proton NMR signals
were integrated and quantified relative to the internal standard,
allowing for the accurate determination of the concentrations of
tyrosol, hydroxytyrosol and lactic acid. This method was adapted
from a previously published study by the authors (27), which has been successfully applied
to quantify bioactive compounds in thousands of table olive and
olive oil samples annually. Using this approach, it was determined
that two capsules of the supplement, hereby referred to as
CHOLESTOLIVE, contained 28 mg hydroxytyrosol, 12 mg tyrosol and 81
mg lactic acid, an amount corresponding to five Kalamon table
olives of high-phenolic content. Following the described procedure,
180 kg Kalamon table olives yielded a total of 3,000 daily doses of
the supplement, with an extraction efficiancy of 85% achieved
during the upscale process.
Pilot observational study
In collaboration with three major hospitals of
Greece (Hippokration General Hospital of Athens, Elpis General
Hospital of Athens, both in Athens Greece, and Achilopoulio, Volos
General Hospital, Volos, Greece), 48 volunteers (19 males and 29
females; mean age, 54 years; range, 26-76 years) exhibiting mild
dyslipidemia were enrolled in a 30-day pilot study. All
participants were extensively briefed, before signing an informed
consent form. All collected samples were anonymized before further
processing. The research protocol was reviewed and approved by the
55th Scientific Council of Hippokration General Hospital during its
meeting on March 22, 2021 (Reference no. ΕΣ. 55Ο/22-6-2021) and
expanded without any modifications, in accordance with
institutional guidelines. The inclusion and exclusion criteria,
referring to total cholesterol levels <290 mg/dl, and a
low-to-medium cardiovascular risk, as assessed using HeartScore
(<5%), are summarized in Table
I. HeartScore is a risk assessment tool developed by the
European Society of Cardiology to estimate the 10-year risk of an
individual of fatal cardiovascular disease based on factors such as
age, sex, blood pressure, cholesterol levels and smoking status.
Volunteers were instructed not to alter anything regarding their
lifestyle, to consume the daily dosage of CHOLESTOLIVE and to
record their diet for 30 days. The first volunteer was enrolled on
January 31, 2022, and the final lipid profile examination of the
last enrolled participant was conducted on July 20, 2023. No
control group was included, as this was a pilot observational study
for the daily consumption of a naturally derived supplement. The
design did not involve blinding or randomization, as the primary
aim was to assess feasibility and collect preliminary data for
future studies. Additionally, this pilot study was specifically
designed to focus on lipid parameters, and therefore did not assess
other parameters, such as blood pressure or kidney and liver
function.
 | Table IInclusion and elimination criteria for
the study participants. |
Table I
Inclusion and elimination criteria for
the study participants.
| Inclusion
criteria | Elimination
criteria |
|---|
| Age between 18 and 70
years | Currently treated for
hyperlipidemia |
| No pharmaceutical
treatment | Known olive
allergies |
| Low or medium heart
condition risk (HEARTSCORE <5%) | High/very high
cardiovascular disease risk |
| Cognitive
competence | Non-compliant to the
study |
Biomarker evaluation
Blood samples were collected from the participants
at baseline and after the 30-day supplementation period.
Triglyceride, HDL and LDL cholesterol levels were measured using
enzymatic colorimetric assays at the clinical laboratories of the
collaborating hospitals. These biomarkers were evaluated against
established clinical guidelines for normal ranges: LDL cholesterol
<130 mg/dl, triglycerides <150 mg/dl, and HDL cholesterol ≥40
mg/dl for males and ≥45 mg/dl for females These parameters were
selected as they are clinically significant and widely recognized
biomarkers for evaluating dyslipidemia and cardiovascular risk
(28).
Statistical analysis
Statistical analyses were performed using R Studio.
The Kolmogorov-Smirnov test was used to assess the normality of the
data. For normally distributed variables, the paired sample t-test
was applied to compare measurements before and after the
intervention. For non-normally distributed variables, the Wilcoxon
signed-rank test was used as a non-parametric alternative. A
two-sided P-value <0.05 was considered to indicate a
statistically significant difference. A power analysis was
conducted prior to the study, targeting 80% power with an alpha
level of 0.05. This analysis suggested the required sample size to
be 80 participants. Despite the final sample size being 48
participants, the data generated statistically significant findings
that align with the objectives of this pilot observational
study.
Results
Total cholesterol
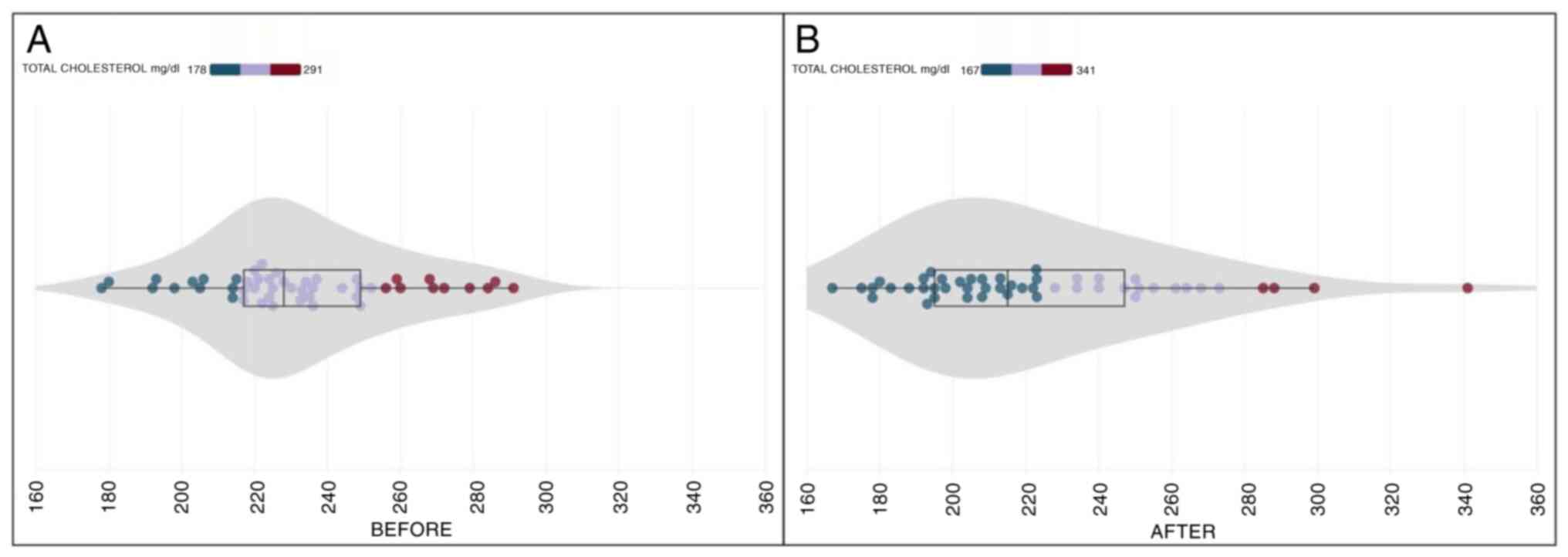
In the initial examination, the mean total
cholesterol levels were recorded at 232.54±26.88 (SD) mg/dl.
Following a 30-day daily intake of CHOLESTOLIVE, the final
examination revealed a mean total cholesterol level of 222.85±36.68
(SD) mg/dl. This represents a notable mean reduction of 4.16%
(Table II). Statistical analysis,
employing the Wilcoxon test, demonstrated a significant difference
between the mean total cholesterol levels before and after
CHOLESTOLIVE supplementation (p.s.=5%, Z=-2.518, P=0.012). The
reduction observed in the total cholesterol levels was
statistically significant. It is noteworthy that the initial
measurement of total cholesterol levels ranged from 178 to 291
mg/dl, while the final measurement exhibited a range of 167 to 341
mg/dl (Fig. 2).
 | Table IIOverall changes in lipid parameters
following intervention with the supplement. |
Table II
Overall changes in lipid parameters
following intervention with the supplement.
| Before/after
supplementation | Cholesterol | HDL | LDL | TG |
|---|
| Prior to
supplementation | 232.54±26.88 | 56.37±14.53 | 150.95±22.33 | 125.93±64.50 |
| After
supplementation | 222.85±36.68 | 56.06±13.20 | 142.39±36.13 | 118.37±46.26 |
| Difference | -4.16% | -0.54% | -5.67% | -6.01% |
| P-value. | 0.012 | 0.783 | 0.048 | 0.430 |
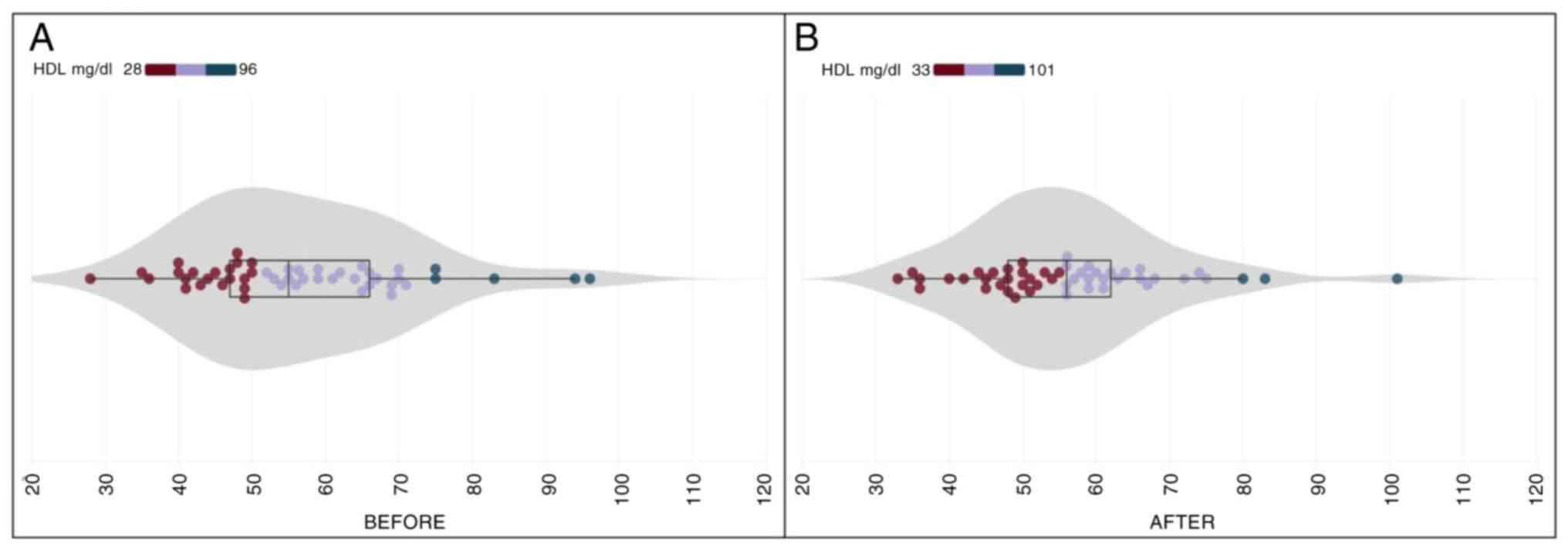
HDL
At the commencement of the study, the mean HDL
cholesterol level was 56.37±14.53 (SD) mg/dl; and following the
30-day CHOLESTOLIVE supplementation, the final examination reported
a marginal decrease to 56.06±13.20 (SD) mg/dl. On average, there
was a minor change of -0.54% in the HDL cholesterol levels
(Table II), which was not
substantial (p.s.=5%, t=0.277, P=0.783). This suggested that
CHOLESTOLIVE intake did not lead to any modification in HDL
cholesterol. The initial measurement of HDL cholesterol levels
spanned from 28 to 96 mg/dl, while the final measurements showcased
a broader range, fluctuating between 33 and 101 mg/dl (Fig. 3). This variance emphasizes the
individualized response observed in HDL cholesterol levels among
the participants.
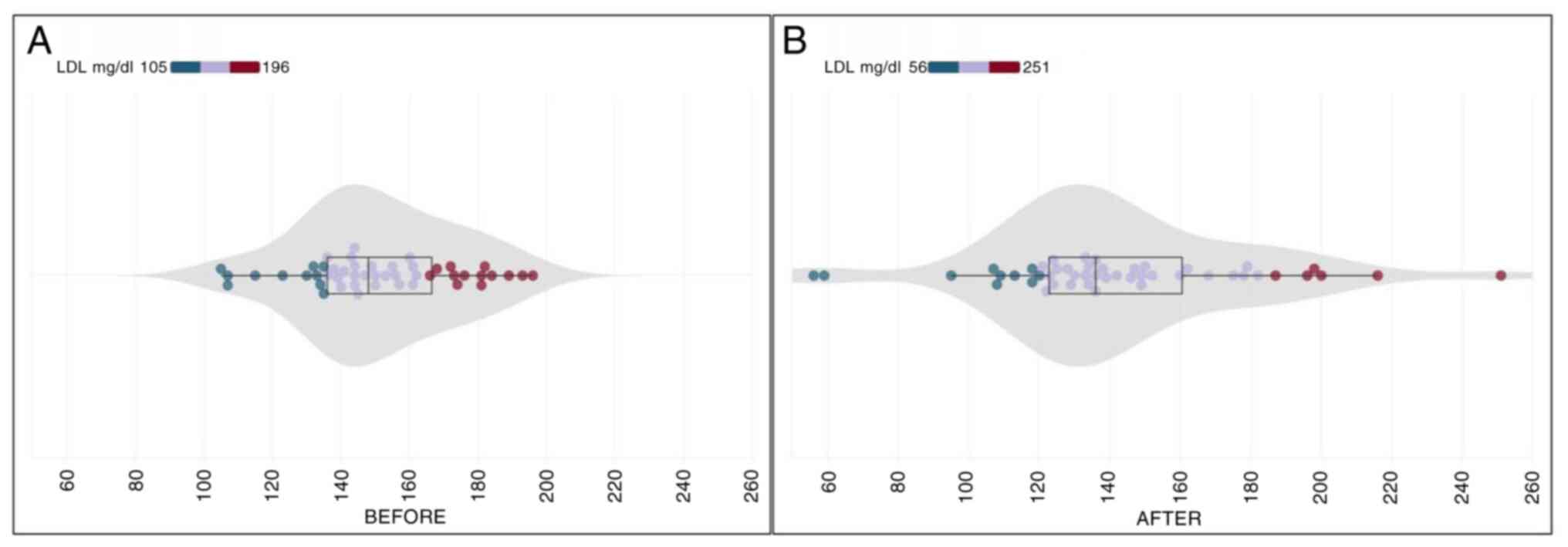
LDL
At the study onset, the mean LDL cholesterol level
was recorded at 150.95±22.33 (SD) mg/dl, with a noticeable
reduction in the final examination after 30 days of CHOLESTOLIVE
supplementation, registering at 142.39±36.13 (SD) mg/dl. The
calculated mean reduction in LDL cholesterol amounted to a
noteworthy 5.67% (Table II).
Utilizing the paired t-test for statistical analysis, a significant
difference emerged between the mean LDL cholesterol levels before
and after CHOLESTOLIVE intake (p.s.=5%, t=2.033, P=0.048). This
emphasizes the statistically significant impact of CHOLESTOLIVE on
reducing LDL cholesterol levels. The initial measurement range for
LDL cholesterol spanned from 105 to 196 mg/dl, showcasing
variability among participants. Notably, the final measurements
exhibited an even wider range, fluctuating between 56 and 251 mg/dl
(Fig. 4). This variance in
responses highlights the complex interplay of factors influencing
LDL cholesterol levels post-CHOLESTOLIVE supplementation.
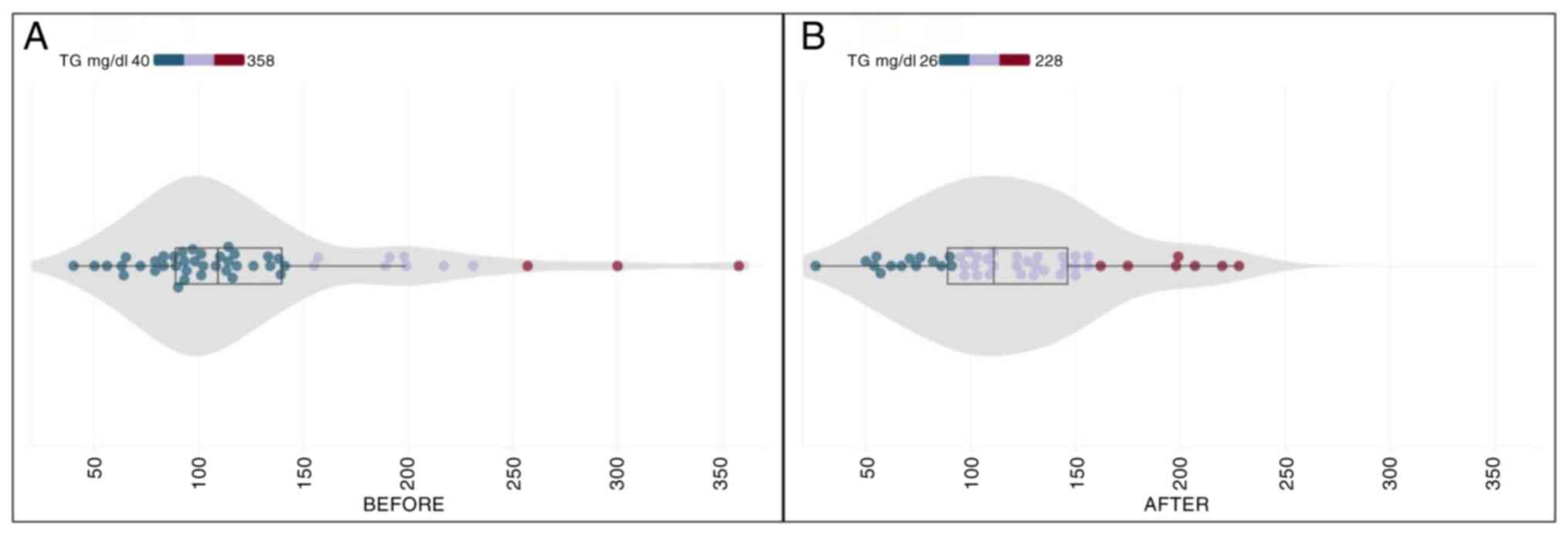
Triglycerides
In the initial examination, the participants
exhibited a mean triglyceride level of 125.93±64.50 (SD) mg/dl.
Following a 30-day daily intake of the dietary supplement, the
final examination reported a marginal decrease to 118.37±46.26 (SD)
mg/dl. The calculated mean reduction in triglyceride levels reached
6.01% (Table II). Contrary to the
observed trends in other lipid parameters, the Wilcoxon test
employed for statistical analysis indicated that mean triglyceride
levels before and after CHOLESTOLIVE intake did not exhibit a
significant difference (p.s.=5%, Z=-0.790, P=0.430). This suggests
that the reduction in the triglyceride levels, while notable, did
not reach statistical significance. The range of initial
triglyceride values was diverse, spanning from 40 to 358 mg/dl,
reflecting the heterogeneity among participants. Of note, the final
measurements showed a reduction in variability, ranging from 26 to
228 mg/dl (Fig. 5). This nuanced
pattern underscores the individualized response to CHOLESTOLIVE
supplementation in modulating triglyceride levels.
Discussion
The health-promoting properties of olives have been
recognized since antiquity, with olives and olive oil playing a
central role in the Mediterranean diet, which is widely regarded as
one of the healthiest diets globally (29,30).
Historical references to the cardioprotective benefits of olives
align with modern scientific findings that attribute these effects
to compounds, such as tyrosol and hydroxytyrosol (31). The use of Kalamon olives in
CHOLESTOLIVE connects these traditional uses with contemporary
science, demonstrating that the bioactive compounds in olives can
effectively reduce LDL cholesterol and total cholesterol, providing
natural protection against cardiovascular diseases. While the
present study was designed to evaluate CHOLESTOLIVE supplementation
independently, further research is required to further explore how
its bioactive compounds compare to direct Kalamon olive consumption
in dietary applications.
Before commencing the production of CHOLESTOLIVE,
multiple samples of different varieties of table olives were
screened using qNMR to identify those with the highest content of
tyrosol and hydroxytyrosol. This screening process ensured that the
selected Kalamon olives contained the optimal levels of bioactive
compounds necessary for the formulation of a supplement that
follows a patented production method (32). The innovative method involves a
clean, efficient separation process that maintains the integrity of
these phenolic compounds. To the best of our knowledge, the present
study is the first to evaluate the lipid-lowering and
cardioprotective effects of a dietary supplement produced using
this method, offering a novel approach to utilizing table olives
for health purposes.
In contrast to pharmaceutical interventions such as
statins, which are often prescribed to reduce LDL cholesterol and
prevent cardiovascular diseases, CHOLESTOLIVE provides a natural
alternative derived from table olives. Statins are well-known for
their effectiveness; however, they also carry a risk of
side-effects, including muscle pain, liver damage and an increased
risk of developing type 2 diabetes (33,34).
The use of natural supplements such as CHOLESTOLIVE, rich in
tyrosol, hydroxytyrosol and lactic acid, offers a safer approach
with the possibility of fewer side-effects, rendering it a
promising alternative for those with mild dyslipidemia who wish to
avoid statins. The absence of reported side-effects or adverse
events, as stated by the participants during the 30-day
supplementation period further supports the safety of CHOLESTOLIVE,
which is derived from natural, widely consumed table olives.
Moreover, the extraction process used for CHOLESTOLIVE preserves
the bioactive compounds found in Kalamon olives unaltered through
an environmentally responsible method that avoids harsh chemical
processing. The process achieves a high extraction yield of 85%
using purified water and ethanol, which are recyclable, non-toxic
and commonly employed in food and dietary supplement production.
This ensures that the beneficial phenolic compounds are retained,
maintaining their antioxidant properties, which are crucial for
protecting cardiovascular health and combating oxidative
stress.
The lipid-lowering effects observed with
CHOLESTOLIVE are comparable to those reported for other natural
supplements, such as those derived from plant sterols or fiber
supplements, such as psyllium husk (35,36).
Plant sterols, which are structurally similar to cholesterol,
reduce intestinal cholesterol absorption and are widely recognized
for their LDL-lowering efficacy, with studies reporting reductions
of 10-15%. Psyllium husk, a soluble fiber, also demonstrates
LDL-lowering effects by binding bile acids in the gut, with
reported reductions ranging from 5-10%. While both interventions
are effective at lowering LDL cholesterol, their effects on total
cholesterol or triglycerides are less pronounced. However, the
unique combination of phenolic compounds in Kalamon olives,
particularly hydroxytyrosol, provides additional antioxidant and
anti-inflammatory benefits, rendering CHOLESTOLIVE more than just a
cholesterol-lowering agent. Unlike plant sterols and psyllium husk,
which primarily function through intestinal mechanisms,
CHOLESTOLIVE offers a broader cardioprotective profile. The
reductions in total cholesterol (4.16%) and LDL cholesterol (5.67%)
observed in the present study are noteworthy, particularly in
comparison to other over-the-counter natural supplements (9). However, further studies are required
to explore the synergistic effects of combining CHOLESTOLIVE with
other lifestyle interventions or supplements to maximize its
potential.
The results of the present study demonstrate
significant reductions in total cholesterol and LDL cholesterol
following CHOLESTOLIVE supplementation, with some variability in
individual responses. Several factors could account for this
variability. Genetic differences among participants may influence
the metabolism of tyrosol and hydroxytyrosol, resulting in varying
degrees of lipid reduction. Additionally, the baseline lipid levels
of participants were diverse, which likely contributed to
differences in their response to the supplement. Lifestyle factors,
such as diet and physical activity, which were not controlled
during the study, may also have affected the outcomes. Furthermore,
individual differences in the absorption and bioavailability of the
bioactive compounds from the supplement could explain the range of
responses observed.
The present study has certain limitations, which
should be mentioned. The present observational pilot study was
designed as an initial exploration to assess the feasibility and
potential benefits of the first nutritional supplement derived only
from Kalamon table olives. As such, certain aspects, such as the
short follow-up period and the absence of randomization or a
control group, reflect the exploratory nature of the study. These
characteristics were necessary to focus on generating preliminary
data and evaluating the practicality of this approach. The authors
are confident that future studies with extended durations, larger
sample sizes, and more comprehensive designs will build upon these
findings and provide deeper insight into the long-term effects and
broader applications of the supplement.
In conclusion, the present study revealed that the
use of the CHOLESTOLIVE supplement resulted in a significant
reduction of total cholesterol (-4.16%, p.s.=5%, Z=-2.518, P=0.012)
and LDL cholesterol (-5.67%) levels. The triglyceride levels
exhibited a modest reduction, although this difference did not
reach statistical significance. HDL cholesterol was not affected
throughout the study (Table II).
These findings suggest that the nutritional supplement may have
beneficial effects on reducing total cholesterol and LDL
cholesterol levels, highlighting the potential health benefits of
the bioactive compounds found in table olives, particularly
hydroxytyrosol, tyrosol and lactic acid, related to cardiovascular
well-being and metabolic health. However, further larger-scale
research is required to confirm these results and investigate the
underlying mechanisms.
Acknowledgements
The authors would like to thank Sakellaropoulos
farms (near Sparta, Laconia, Greece) for providing the Kalamon
olives and Omphax SA for undertaking the production of the
supplement.
Funding
Funding: The present study was supported by funding from
Mediakos GmbH, the CHOLESTOLIVE patent holder.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ND, EM and PM were involved in the conception of the
study. DK and CPT performed the medical examinations of the
participants. NP and CP were involved in the acquisition of data
and functioned as clinical research associates. AT, PM and ND were
involved in the design of the study and in the writing of the
manuscript. VE performed the statistical analysis. PD and PM
provided scientific input in the design and development of the
product. All authors have read and approved the final manuscript.
NP, AT and ND confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All participants were extensively briefed, before
signing an informed consent form. All collected samples were
anonymized before further processing. The research protocol was
reviewed and approved by the 55th Scientific Council of
Hippokration General Hospital during its meeting on March 22, 2021
(Reference no. ΕΣ. 55Ο/22-6-2021) and expanded without any
modifications, in accordance with institutional guidelines.
Patient consent for publication
Not applicable.
Competing interests
Although the present study was supported by funding
from Mediakos GmbH, it should be noted that the funding body had no
role in the study design, data collection, analysis, or
interpretation of the results. It should also be noted that,
although some of the authors are listed as inventors on the patent
for the nutritional supplement, they were not compensated for their
contributions to this study (32).
References
|
1
|
Tsolaki M, Lazarou E, Kozori M, Petridou
N, Tabakis I, Lazarou I, Karakota M, Saoulidis I, Melliou E and
Magiatis P: A randomized clinical trial of greek high phenolic
early harvest extra virgin olive oil in mild cognitive impairment:
The MICOIL pilot study. J Alzheimers Dis. 78:801–817.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wu MY, Du MH, Wen H, Wang WQ, Tang J and
Shen LR: Effects of n-6 PUFA-rich soybean oil, MUFA-rich olive oil
and camellia seed oil on weight and cardiometabolic profiles among
Chinese women: A 3-month double-blind randomized controlled-feeding
trial. Food Funct. 13:4375–4383. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chiavarini M, Rosignoli P, Giacchetta I
and Fabiani R: Health outcomes associated with olive oil intake: An
umbrella review of meta-analyses. Foods. 13(2619)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Darakjian LI, Rigakou A, Brannen A, Qusa
MH, Tasiakou N, Diamantakos P, Reed MN, Panizzi P, Boersma MD,
Melliou E, et al: Spontaneous in vitro and in vivo interaction of
(−)-oleocanthal with glycine in biological fluids: Novel
pharmacokinetic markers. ACS Pharmacol Transl Sci. 4:179–192.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gil AP, Kodonis I, Ioannidis A, Nomikos T,
Dimopoulos I, Kosmidis G, Katsa ME, Melliou E and Magiatis P: The
effect of dietary intervention with high-oleocanthal and oleacein
olive oil in patients with early-stage chronic lymphocytic
leukemia: A pilot randomized trial. Front Oncol.
11(810249)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boskou G: Chapter 99-Antioxidant capacity
and phenolic profile of table olives from the greek market. In:
Olives and Olive Oil in Health and Disease Prevention. Preedy VR
and Watson RR (eds.) Academic Press, San Diego, pp925-934,
2010.
|
|
7
|
Grounta A, Tassou CC and Panagou EZ:
Greek-Style table olives and their functional value. In: Olives and
Olive Oil as Functional Foods. John Wiley & Sons, Ltd.,
pp325-342, 2017.
|
|
8
|
Tsantili E: Quality attributes and their
relations in fresh black ripe ‘Kalamon’ olives (lea europaea L.)
for table use-phenolic compounds and total antioxidant capacity.
International Journal of Food Science & Technology. 49:657–665.
2014.
|
|
9
|
Knaub K, Modinger Y, Wilhelm M and Schon
C: LDL-cholesterol lowering effect of hydroxytyrosol
(HTEssence®): A randomized double-blind,
placebo-controlled parallel study. J Nutr Food Sci. 10:1–8.
2020.
|
|
10
|
Fki I, Sahnoun Z and Sayadi S:
Hypocholesterolemic effects of phenolic extracts and purified
hydroxytyrosol recovered from olive mill wastewater in rats fed a
cholesterol-rich diet. J Agric Food Chem. 55:624–631.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jemai H, Fki I, Bouaziz M, Bouallagui Z,
El Feki A, Isoda H and Sayadi S: Lipid-lowering and antioxidant
effects of hydroxytyrosol and its triacetylated derivative
recovered from olive tree leaves in cholesterol-fed rats. J Agric
Food Chem. 56:2630–2636. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tabernero M, Sarriá B, Largo C,
Martínez-López S, Madrona A, Espartero JL, Bravo L and Mateos R:
Comparative evaluation of the metabolic effects of hydroxytyrosol
and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl
hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct.
5:1556–1563. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Calabriso N, Gnoni A, Stanca E, Cavallo A,
Damiano F, Siculella L and Carluccio MA: Hydroxytyrosol ameliorates
endothelial function under inflammatory conditions by preventing
mitochondrial dysfunction. Oxid Med Cell Longev.
2018(9086947)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bulotta S, Celano M, Lepore SM, Montalcini
T, Pujia A and Russo D: Beneficial effects of the olive oil
phenolic components oleuropein and hydroxytyrosol: Focus on
protection against cardiovascular and metabolic diseases. J Transl
Med. 12(219)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
D'Angelo C, Franceschelli S, Quiles JL and
Speranza L: Wide biological role of hydroxytyrosol: Possible
therapeutic and preventive properties in cardiovascular diseases.
Cells. 9(1932)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Marrugat J, Covas MI, Fitó M, Schröder H,
Miró-Casas E, Gimeno E, López-Sabater MC, de la Torre R and Farré
M: SOLOS Investigators. Effects of differing phenolic content in
dietary olive oils on lipids and LDL oxidation-a randomized
controlled trial. Eur J Nutr. 43:140–147. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
López-Villodres JA, Abdel-Karim M, De La
Cruz JP, Rodríguez-Pérez MD, Reyes JJ, Guzmán-Moscoso R,
Rodriguez-Gutierrez G, Fernández-Bolaños J and González-Correa JA:
Effects of hydroxytyrosol on cardiovascular biomarkers in
experimental diabetes mellitus. J Nutr Biochem. 37:94–100.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pirozzi C, Lama A, Simeoli R, Paciello O,
Pagano TB, Mollica MP, Di Guida F, Russo R, Magliocca S, Canani RB,
et al: Hydroxytyrosol prevents metabolic impairment reducing
hepatic inflammation and restoring duodenal integrity in a rat
model of NAFLD. J Nutr Biochem. 30:108–115. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chandramohan R and Pari L:
Antihyperlipidemic effect of tyrosol, a phenolic compound in
streptozotocin-induced diabetic rats. Toxicol Mech Methods.
31:507–516. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Boronat A, Mateus J, Soldevila-Domenech N,
Guerra M, Rodríguez-Morató J, Varon C, Muñoz D, Barbosa F, Morales
JC, Gaedigk A, et al: Cardiovascular benefits of tyrosol and its
endogenous conversion into hydroxytyrosol in humans. A randomized,
controlled trial. Free Radic Biol Med. 143:471–481. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chang CY, Huang IT, Shih HJ, Chang YY, Kao
MC, Shih PC and Huang CJ: Cluster of differentiation 14 and
toll-like receptor 4 are involved in the anti-inflammatory effects
of tyrosol. J Functional Foods. 53:93–104. 2019.
|
|
22
|
Berrougui H, Ikhlef S and Khalil A: Extra
virgin olive oil polyphenols promote cholesterol efflux and improve
HDL functionality. Evid Based Complement Alternat Med.
2015(208062)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Perona JS, Cabello-Moruno R and
Ruiz-Gutierrez V: The role of virgin olive oil components in the
modulation of endothelial function. J Nutr Biochem. 17:429–445.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Priore P, Siculella L and Gnoni GV: Extra
virgin olive oil phenols down-regulate lipid synthesis in
primary-cultured rat-hepatocytes. J Nutr Biochem. 25:683–691.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sarna LK, Sid V, Wang P, Siow YL, House JD
and Karmin O: Tyrosol attenuates high fat diet-induced hepatic
oxidative stress: Potential involvement of cystathionine β-synthase
and cystathionine γ-lyase. Lipids. 51:583–590. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mousouri E, Melliou E and Magiatis P:
Isolation of megaritolactones and other bioactive metabolites from
‘megaritiki’ table olives and debittering water. J Agric Food Chem.
62:660–667. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Karkoula E, Skantzari A, Melliou E and
Magiatis P: Quantitative measurement of major secoiridoid
derivatives in olive oil using qNMR. Proof of the artificial
formation of aldehydic oleuropein and ligstroside aglycon isomers.
J Agric Food Chem. 62:600–607. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Visseren FLJ, Mach F, Smulders YM,
Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM,
Capodanno D, et al: 2021 ESC guidelines on cardiovascular disease
prevention in clinical practice. Eur Heart J. 42:3227–3337.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guasch-Ferré M, Salas-Salvadó J, Ros E,
Estruch R, Corella D, Fitó M and Martínez-González MA: PREDIMED
Investigators. The PREDIMED trial, Mediterranean diet and health
outcomes: How strong is the evidence? Nutr Metab Cardiovasc Dis.
27:624–632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Laffond A, Rivera-Picón C, Rodríguez-Muñoz
PM, Juárez-Vela R, Ruiz de Viñaspre-Hernández R, Navas-Echazarreta
N and Sánchez-González JL: Mediterranean diet for primary and
secondary prevention of cardiovascular disease and mortality: An
updated systematic review. Nutrients. 15(3356)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Albini A, Albini F, Corradino P, Dugo L,
Calabrone L and Noonan DM: From antiquity to contemporary times:
How olive oil by-products and waste water can contribute to health.
Front Nutr. 10(1254947)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Katsarou MS, Magiatis P, Melliou E and
Drakoulis N: Olive-derived compositions. WO Patent 2022090192A1.
Filed October 26, 2021; issued May 5, 2022.
|
|
33
|
Cai T, Abel L, Langford O, Monaghan G,
Aronson JK, Stevens RJ, Lay-Flurrie S, Koshiaris C, McManus RJ,
Hobbs FDR and Sheppard JP: Associations between statins and adverse
events in primary prevention of cardiovascular disease: Systematic
review with pairwise, network, and dose-response meta-analyses.
BMJ. 374(n1537)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
CDC: Statins and Diabetes: What you should
know. Diabetes, 2024.
|
|
35
|
Han S, Jiao J, Xu J, Zimmermann D,
Actis-Goretta L, Guan L, Zhao Y and Qin L: Effects of plant stanol
or sterol-enriched diets on lipid profiles in patients treated with
statins: Systematic review and meta-analysis. Sci Rep.
6(31337)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Moreyra AE, Wilson AC and Koraym A: Effect
of combining psyllium fiber with simvastatin in lowering
cholesterol. Arch Intern Med. 165:1161–1166. 2005.PubMed/NCBI View Article : Google Scholar
|