Introduction
Skeletal muscle is involved in various metabolic
functions contributing to whole-body metabolism and energy
expenditure (1). Protein turnover,
the balance between muscle protein synthesis and protein breakdown,
reflects and determines the amount of skeletal muscle mass in an
individual (2). Thus, maintaining
muscle mass is crucial as an imbalance in protein turnover (e.g.,
muscle atrophy) is associated with increased morbidity and an
increased risk of developing diseases (3). Muscle atrophy is associated with a
number of chronic diseases and pathological conditions, such as
obesity, prolonged fasting, cancer, sepsis, cachexia and AIDS,
among others. In addition, treatment with synthetic glucocorticoids
(GCs) also induces muscle atrophy (4). Exogenous GCs, such as dexamethasone
(Dex) and prednisone, are used both acutely and chronically for the
treatment of a variety of inflammatory and autoimmune diseases
(e.g., cancer, asthma, rheumatoid arthritis, starvation, sepsis and
metabolic acidosis). While GCs are effective at combatting
inflammation, they do so at the expense of skeletal muscle mass by
increasing the rate of protein degradation and suppressing protein
synthesis (4-8).
GCs have been shown to exacerbate metabolic derangements caused by
a high-fat diet (HFD) including significant reductions in lean body
mass (9). Therefore, treatments to
prevent GC-induced muscle wasting are necessary in order to improve
patient survival outcomes and quality of life.
The polyunsaturated fatty-acids (PUFAs),
eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA,
22:6n-3), have emerged as key nutrients augmenting skeletal muscle
protein turnover (10-18).
PUFAs are essential nutrients involved in cell membrane structure,
membrane fluidity, cell signaling, and the regulation of gene
transcription and enzyme activity (3,19-21).
Omega-3 PUFAs also exhibit anti-inflammatory, anti-cachectic,
anti-catabolic and anabolic properties in skeletal muscle (14-16,19,22-25).
While the PUFAs, EPA and DHA, promote an anti-inflammatory
environment, the omega-6 PUFA, arachidonic acid (20:4n-6), exerts a
pro-inflammatory response (26,27).
Of recent interest is the manipulation of the n-6/n-3 ratio,
relative to the total amount of PUFAs, as a regulator of metabolic
and physiological functioning. The consumption of a HFD or
‘Western’ diet rich in saturated fatty-acids and linoleic acid
(18:2n-6) PUFAs has been linked to adverse metabolic profiles, such
as alterations in fatty acid composition, acid-base balance,
glycemic load and nutrient metabolism (3,28,29).
While the ideal ratio of n-6/n-3 is 2:1, the typical ratio in the
Western diet is ~20:1 n-6/n-3; this relatively high intake of n-6
to n-3 may exacerbate an already pro-inflammatory state in the
context of disease (3,26,30).
Previous studies have demonstrated the potential
protective effects of omega-3 PUFAs from the detrimental
side-effects of GC treatment (31-33);
however, when it comes to skeletal muscle, the results are mixed,
with some studies demonstrating n-3 treatment to decrease muscle
size and not to provide protection against GC-induced skeletal
muscle atrophy (4,34). GCs are a widely prescribed class of
drugs which are prescribed at a greater rate to obese individuals
than non-obese individuals (35).
Given the current Western diet and the increased rate of
prescriptions to obese individuals, the present study examined the
effects of GCs under the context of a HFD. To date, at least to the
best of our knowledge, no studies have examined the effects of n-3s
on skeletal muscle preservation during both GC use and the
consumption of a HFD. The present study thus aimed to elucidate
differences in GC-induced muscle atrophy when consuming a HFD rich
in either n-6 or n-3 PUFAs, using C57BL/6 male mice.
Materials and methods
Animals and experimental design
All experimental and housing protocols were approved
by the Institutional Animal Care and Use Committee of the
University of Memphis (Memphis, TN, USA; protocol no. 0830). A
total of 32 C57BL/6 male mice, 7 weeks of age, were purchased from
Envigo. All animals were kept on a 12:12-h light-dark cycle at
22±2˚C 40% humidity and had ad libitum access to food and
water during the course of the study. Following 3 days of
acclimation during which the animals received a standard chow diet,
the mice were randomly divided into two groups initially to receive
either a HFD rich in omega-6 (n-6; 45% fat; 177.5 g lard; 35%
carbohydrate; and 20% protein; n-6:n-3 PUFA, 13:1; n=16 mice), or a
HFD rich in omega-3 (n-3; 45% fat; (177.5 g Menhaden oil; 35%
carbohydrate; and 20% protein; n-6:n-3 PUFA, 1:3; n=16 mice)
(Research Diets, Inc). After 4 weeks on their respective diets, the
mice in both groups were divided, with half of the mice receiving
either a subcutaneous injection of Dex (3 mg/kg body weight)
(Peoples Custom Rx) or sterile PBS, while continuing their current
diet throughout the 5th and last week: 45% n-6 HFD + Dex (n-6 +
Dex), n=8; and 45% n-3 HFD + Dex (n-3 + Dex), n=8(36). This dosage is similar to what has
been previously used in other studies to induce muscle wasting
(4,37). Of note, 1 mouse in the n-3 + Dex
group was lost during the study, likely due to a more dominant
mouse. The dominant mouse was removed and single-housed to prevent
further aggressive behavior, as is regularly done with aggressive
mice; additionally, nesting material was provided to all cages
throughout the duration of the study for enrichment and to minimize
aggressive behaviors (36,38). The body weight, food intake and
grooming of all the mice was closely monitored daily for the
duration of the experiment. Any remaining food (from previous day
consumption) and body weight were measured three times a week
during the first 4 weeks and daily following the Dex injections. At
the end of the study period, the animals were fasted for 5 h prior
to harvesting tissue. Tissue collection was completed with the mice
anesthetized using 5% isoflurane inhalation and euthanized by
cervical dislocation and removal of the heart.
Tissue harvesting
At the end of the study period, all animals (14
weeks of age) were fasted for 5 h prior to harvesting the tissue.
Tissue collection was completed with the mouse anesthetized by
isoflurane (2-5%). The mice were euthanized by cervical dislocation
while anesthetized. Hindlimb skeletal muscles (soleus, plantaris,
gastrocnemius, tibialis anterior, extensor digitorum longus),
epididymal fat pad, heart and spleen were excised and snap-frozen
in liquid nitrogen for further analysis. The right tibialis
anterior was weighed and placed in 10% neutral-buffered formalin
before being processed for paraffin embedding. Prior to being
frozen, the gastrocnemius was divided into red and white portions,
representing portions that are high in oxidative fibers and
glycolytic fibers, respectively. Tibias were also removed and
measured as a correction factor for body size. There was no
difference in tibia lengths across all groups (data not shown).
Protein expression
Western blot analysis was performed on harvested
muscle tissue to determine differences in protein expression
levels. Portions of the red and white gastrocnemius muscle were
homogenized in Mueller buffer. The resulting protein homogenate
protein concentrations were measured using the Bradford method
(39). A total of 40 µg protein
homogenates were loaded onto 10% SDS-polyacrylamide gels. Gels were
run to separate proteins and were then and transferred overnight to
polyvinylidene difluoride membranes. Ponceau staining (RPI Research
Products) was used to stain the blots for 5 min at room temperature
to visually confirm that the gel transferred and to ensure equal
loading. The membranes were blocked in Tris-buffered saline with
0.1% Tween-20 (TBST) and 5% milk for 1 h at room temperature.
Primary antibodies for phosphorylated (p)-forkhead box O3 (FOXO3a;
cat. no. Abs554, MilliporeSigma), FOXO3a (cat. no. 12829, Cell
Signaling Technology, Inc.), p-glycogen synthase kinase (GSK)-3β
(cat. no. 5558, Cell Signaling Technology, Inc.) and GSK-3β (cat.
no. 12456, Cell Signaling Technology, Inc.) were incubated at a
ratio of 1:2,000 for 24 h in 5% TBST milk at -4˚C. Secondary rabbit
conjugated antibodies (cat. no. 7074, Cell Signaling Technology,
Inc.) were used at a ratio of 1:5,000 and were incubated for 1-2 h
in 5% TBST milk at room temperature. Enhanced chemiluminescence
(Genesee Scientific Corporation) was used to visualize the
antibody-antigen interactions and developed using a Chemidoc system
(Bio-Rad Laboratories, Inc.). The blots were analyzed by measuring
the integrated optical density of each band using ImageJ software
V1.52 (National Institutes of Health). All western blots were
normalized to the non-phosphorylated control.
RNA isolation and RT-qPCR
The following genes were analyzed for expression:
Atrogin-1, muscle RING-finger protein-1 (MuRF-1), regulated in
development and DNA damage response 1 (REDD-1) and myostatin. To
isolate RNA from mouse red and white gastrocnemius muscle, tissue
was homogenized in 3-5 ml RNA STAT-60 (Tel-Test Inc.). Total RNA
was extracted from the STAT-60 solution by the addition of
chloroform:isoamyl alcohol (24:1). The extracted RNA was dissolved
in water, reprecipitated using sodium acetate and isopropanol,
washed with 75% ethanol and quantified using a Nanodrop
spectrophotometer (Thermo Fisher Scientific, Inc.). For the qPCR
analysis of RNA transcripts, 1 µg RNA was reverse transcribed into
cDNA. cDNA was prepared using the High-Capacity cDNA Reverse
Transcription kit as per the manufacturer's instructions (Applied
Biosystems; Thermo Fisher Scientific, Inc. Cat# 4368813). The cDNA
was mixed with forward and reverse primers for the intended gene
target and PowerUp SYBR-Green master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.). qPCR was performed on a
QuantStudio 6 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The 2-ΔΔCq method was used to
determine changes in gene expression between treatment groups using
Gapdh as the housekeeping gene (40). The primer sequences used are
presented in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| Gapdh |
GTTGTCTCCTGCGACTTCA |
TGCTGTAGCCGTATTCA |
| Myostatin |
ACCCATGAAAGACGGTACAAG |
TCATCACAGTCAAGCCCAAAG |
| REDD-1 |
TGGTGCCCACCTTTCAGTTG |
GTCAGGGACTGGCTGTAACC |
| MuRF-1 |
ACCTGCTGGTGGAAAACATC |
AGGAGCAAGTAGGCACCTCA |
| Atrogin-1 |
GTTTTCAGCAGGCCAAGAAG |
TTGCCAGAGAACACGCTATG |
Histological analysis
Samples of the tibialis anterior were fixed in 10%
neutral-buffered formalin after being excised and rinsed in PBS.
Samples were fixed in formalin blocks and sectioned into
10-µm-thick sections using a microtome. Samples were deparaffinized
and stained with hematoxylin and eosin (H&E) as previously
described (41). The cross
sectional area (CSA), a measure of fiber size, was analyzed using
ImageJ software V1.52 (National Institutes of Health) as previously
described (41). Individuals
conducting the CSA analysis were blinded to the treatment groups.
Myofibers comprising both deep and superficial muscle regions were
manually traced; ~150-200 fibers were traced for each animal. This
was determined to be an appropriate fiber number as there were no
further changes in the standard deviation of myofiber areas
observed.
Statistical analysis
All data are represented as the mean ± SEM. A
two-way ANOVA was used to determine the effects of diet and
dexamethasone treatment using GraphPad Prism 8 software
(Dotmatics). Bonferroni post hoc analysis was used to examine the
interactions. A value of P<0.05 was considered to indicate a
statistically significant difference. Effect size was calculated
using Cohen's D.
Results
Muscle mass and CSA
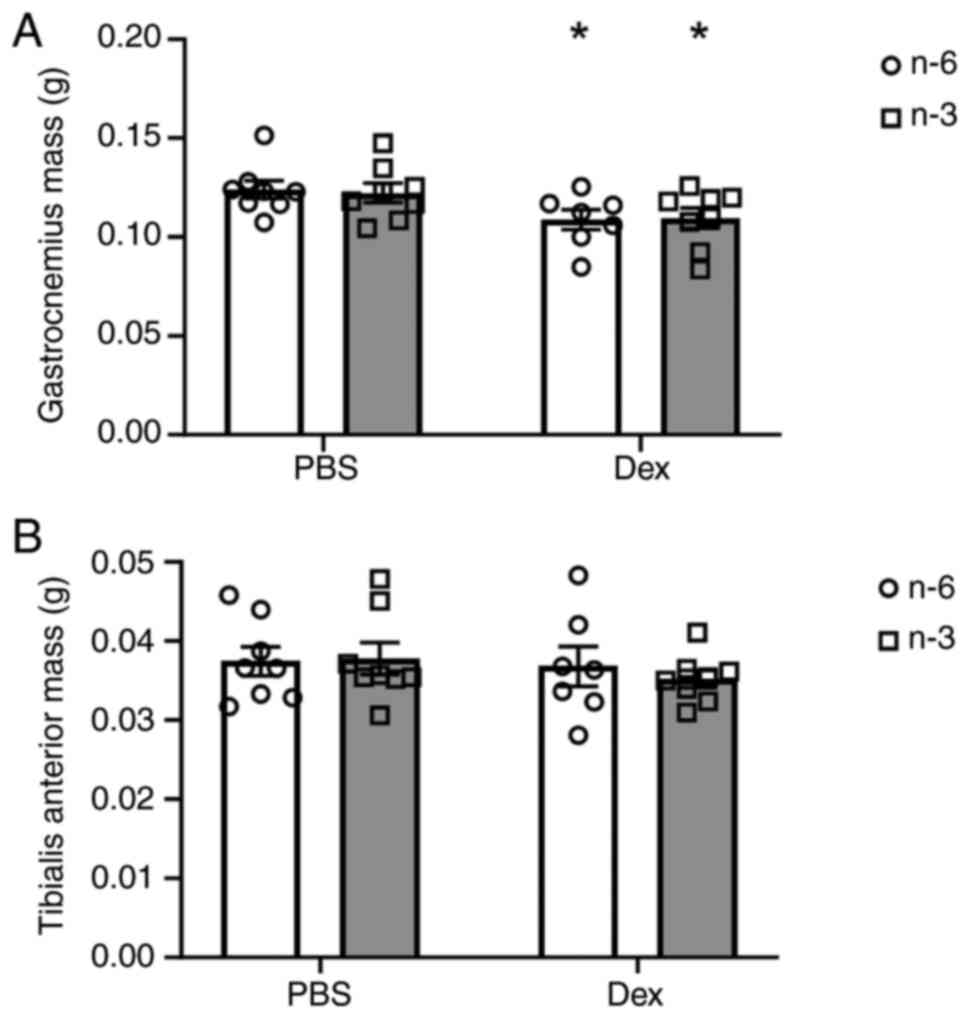
Muscle mass was measured at the time of sacrifice
and the mean weights of the mice in each group for the
gastrocnemius and the tibialis anterior muscles are presented in
Fig. 1. Dex significantly
decreased gastrocnemius weight by 12% in the mice regardless of
diet (P=0.0089; Fig. 1A); however,
there was no significant effect of diet or Dex treatment on the
mass of the tibialis anterior muscle (Fig. 1B). There was a main effect of time
to increase body weight (P<0.001). At week 5, the n-6
Dex-treated mice weighed significantly more than the n-3
Dex-treated mice, despite no differences in food intake throughout
the study (Table II). The
increased body weight was likely due to increases in fat mass, as
previously reported (32).
 | Table IIbody weight and food intake. |
Table II
body weight and food intake.
| | n-6, PBS | n-3, PBS | n-6, Dex | n-3, Dex |
|---|
| Week | Body weight
(g) | Food intake
(g/mouse/day) | No. of mice | Body weight
(g) | Food intake
(g/mouse/day) | No. of mice | Body weight
(g) | Food intake
(g/mouse/day) | No. of mice | Body weight
(g) | Food intake
(g/mouse/day) | No. of Mice |
|---|
| 1 | 22.15±0.54 | 2.85±0.05 | 8 | 23.28±0.33 | 2.86±0.05 | 8 | 22.93±0.31 | 2.97±0.07 | 8 | 23.05±0.63 | 3.09±0.39 | 8 |
| 4 | 27.39±0.82 | 2.73±0.20 | 8 | 26.30±0.42 | 2.39±0.26 | 8 | 27.78±0.55 | 2.78±0.10 | 8 | 26.34±0.99 | 3.48±0.33 | 8 |
| 5 | 26.83±0.59 | 2.42±0.26 | 8 | 26.21±0.30 | 2.56±0.15 | 8 |
27.69±0.67a | 3.02±0.52 | 7 | 25.41±0.77 | 3.19±0.00 | 8 |
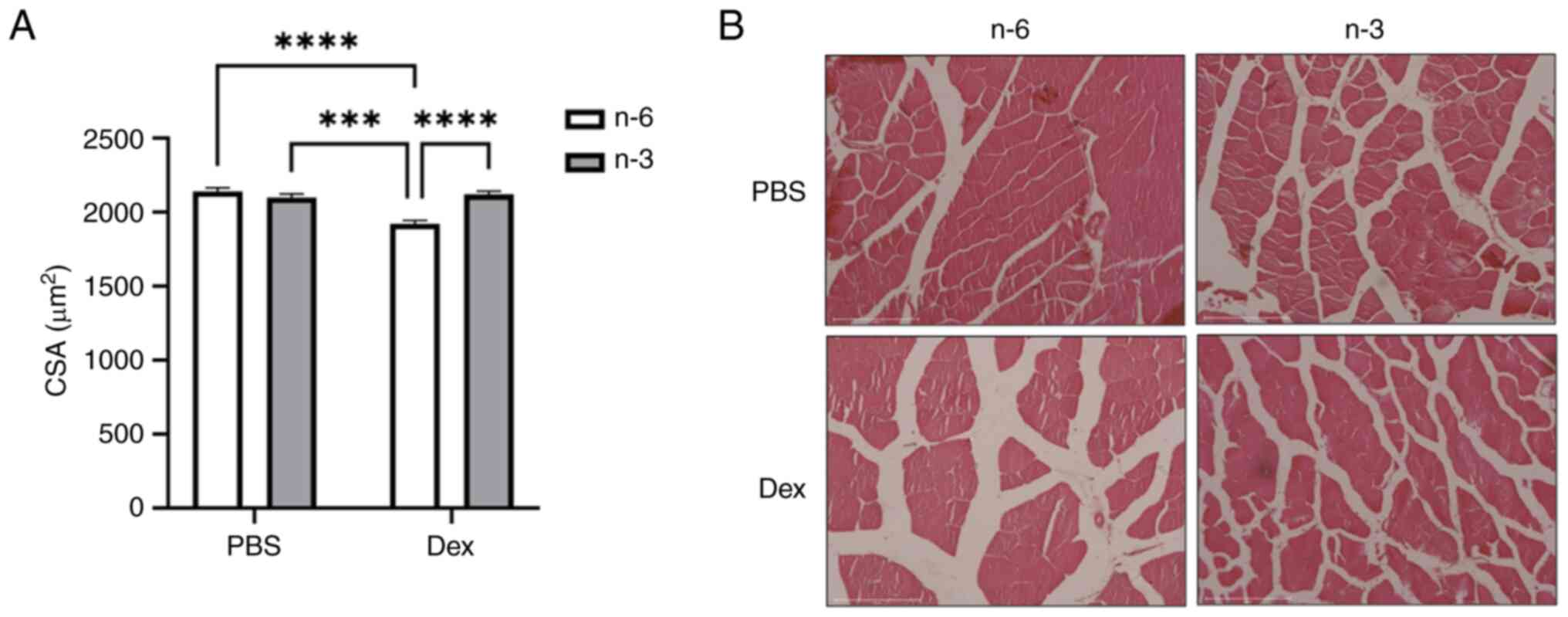
The CSA, a marker of fiber size, measured in the
tibialis anterior muscle revealed that Dex reduced the average
fiber size compared to PBS in mice fed the n-6-rich diet
(P<0.0001). This reduction was attenuated in the mice treated
with Dex fed the n-3-rich diet, suggesting that n-3 may offer some
protection against Dex-induced muscle atrophy (Fig. 2).
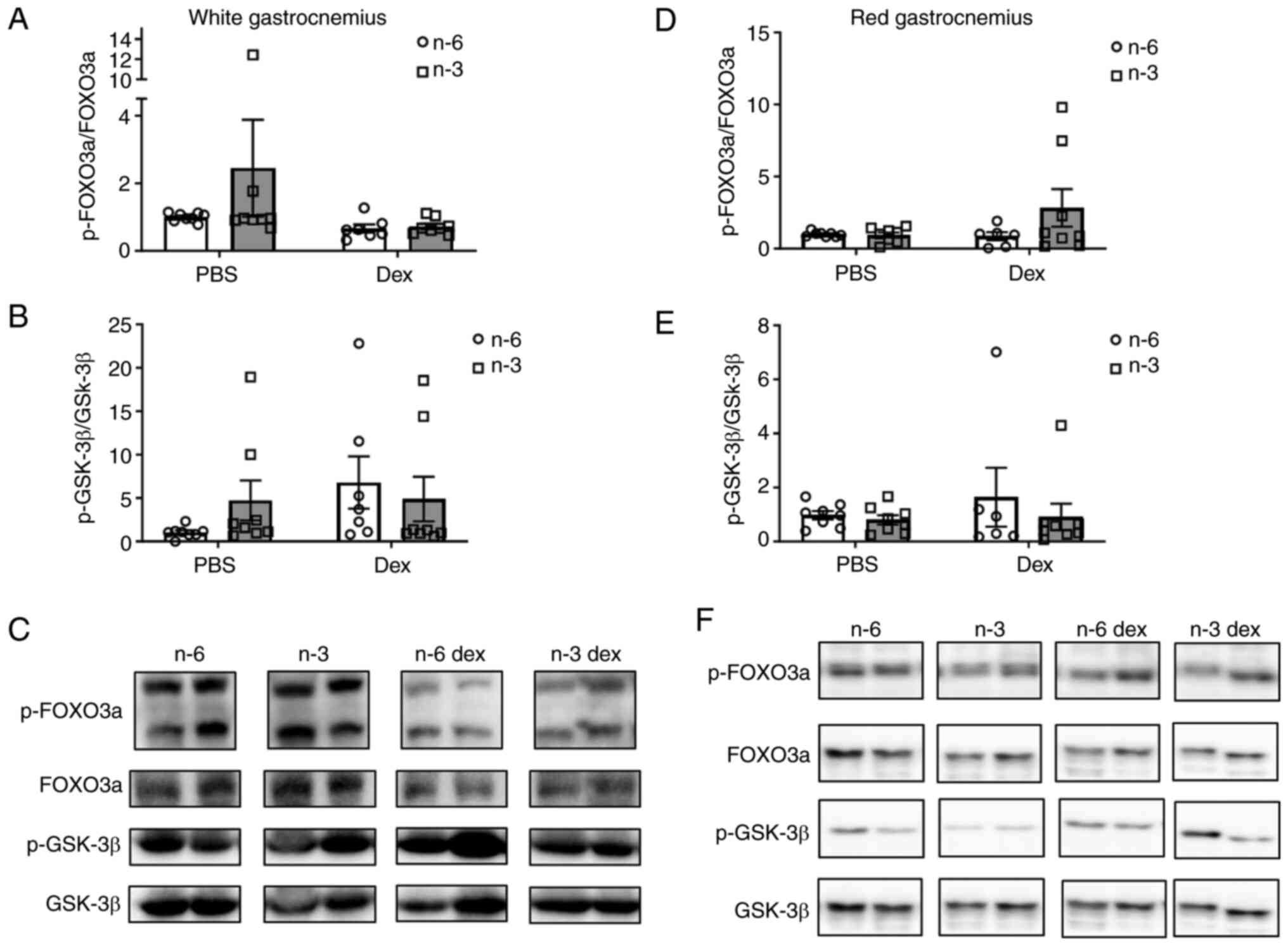
Protein degradation
To determine the effects of an omega-3-rich diet on
GC-induced muscle atrophy, markers of muscle atrophy were measured
in both the red and white gastrocnemius muscle. The phosphorylation
levels of FOXO3a, a marker of both proteasomal degradation and
autophagic degradation, and GSK-3β, a factor involved in the GC
suppression of protein synthesis, were measured using western blot
analysis (Fig. 3). In the white
gastrocnemius, the phosphorylation of FOXO3a was not significantly
altered by diet or Dex; however, there was marked effect of Dex
(Cohen's d=1.42) toward a decreased phosphorylation of FOXO3a
compared with the PBS group (Fig.
3A and C). Although the
phosphorylation of GSK-3β was not significantly (P=0.19) altered by
diet or Dex, Dex exhibited a large effect size (Cohen's d=1.42)
toward the increased phosphorylation of GSK-3β (Fig. 3B and C). In the red gastrocnemius, there was no
effect of either diet or GCs to alter the phosphorylation of FOXO3a
or GSK-3 β (Fig. 3D and F).
The present study then examined the gene expression
of REDD-1, myostatin, MuRF-1 and atrogin-1. There was no marked
difference in REDD-1 expression with diet or Dex treatment in the
white gastrocnemius (Fig. 4A). The
level of myostatin, a negative regulator of muscle mass, was not
significantly altered by diet or Dex treatment; however, there was
a notable effect of Dex (Cohen's d=2.1) towards the upregulation of
myostatin expression (Fig. 4B). As
markers of the proteasomal degradation pathway, the E3 ligases
MuRF-1 and atrogin-1 were measured. There was no significant effect
of either diet or dexamethasone (P=0.15) on MuRF-1 expression.
However, Dex (Cohen's d=6.47) increased MuRF-1 expression
regardless of diet in the white gastrocnemius (Fig. 4C). Atrogin-1 expression was
significantly higher with Dex (P=0.0035) in the mice fed either
diet when compared to the control. Additionally, within the n-6-fed
group, Dex significantly increased atrogin-1 expression (P=0.04;
Fig. 4D). In the red
gastrocnemius, Dex decreased REDD-1 expression (Fig. 4E). Dex also increased myostatin
(Fig. 4F), MuRF-1 (Fig. 4G) and atrogin-1 (Fig. 4H), regardless of diet. These data
suggest that Dex induces muscle atrophy via the upregulation of the
ubiquitin pathway, independently of HFD composition. Additionally,
Dex may target more oxidative fibers.
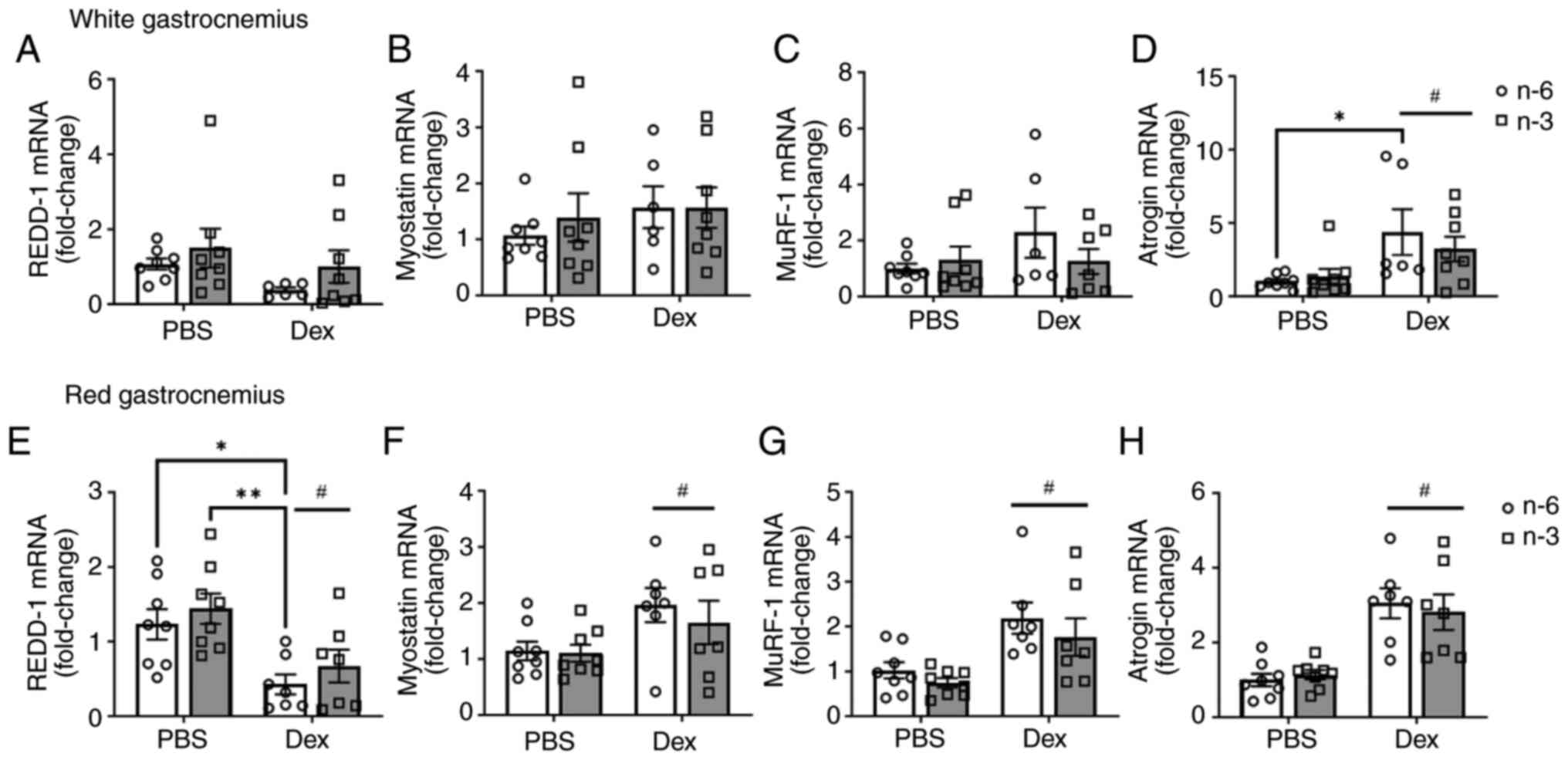 | Figure 4Transcriptional markers of protein
degradation pathways. The mRNA expression of (A) REDD-1, (B)
myostatin, (C) MuRF-1, and (D) atrogin in white gastrocnemius
muscle and (E) REDD-1, (F) myostatin, (G) MuRF-1, and (H) atrogin
in red gastrocnemius muscle was measured in mice fed either a
high-fat diet rich in lard (n-6) or Menhaden oil (n-3) with Dex
injection. All data are presented as the mean ± SEM.
*P<0.05 and **P<0.01;
#P<0.05, significant effect of Dex,. REDD-1,
regulated in development and DNA damage response 1; MuRF-1, muscle
RING-finger protein-1; Dex, dexamethasone. |
Discussion
Supplementation with omega-3 PUFAs has well-known
benefits on skeletal muscle protein turnover (25,42).
In various models of muscle atrophy (i.e., sepsis, arthritis,
starvation, cancer and cachexia), studies investigating n-3s have
demonstrated that supplementation with either n-3s or altering the
n-6/n-3 ratio maintains or increases protein synthesis and inhibits
muscle atrophy. Exogenous GCs are administered as a drug therapy in
a variety of muscle wasting and inflammatory conditions; however,
chronic GC usage is linked to increased muscle atrophy. Recent
studies investigating both n-3 supplementation and GC-induced
muscle atrophy have found conflicting results compared to the
aforementioned benefits observed in other atrophy conditions
(4,34). Additionally, studies have shown
that a HFD contributes to muscle wasting by altering protein
synthesis and increasing markers of ubiquitination-proteasome
system (UPS) degradation (43,44).
Altering the n-6/n-3 ratio could alleviate the deleterious effects
of consuming a high n-6 to n-3 diet. However, little is known of
the effects of n-3s on GC-induced muscle atrophy while consuming a
HFD. Therefore, the present study sought to induce muscle atrophy
by Dex and observe the effects of an omega-3-rich vs. an
omega-6-rich HFD on markers of protein degradation. It was observed
that Dex administration increased muscle atrophy by significantly
reducing muscle weight and upregulating several atrogenes; however,
a HFD rich in n-3s did not attenuate these alterations. These
findings were not surprising, as it is well-established that
GC-induced atrophy is fiber-type specific, namely affecting
glycolytic fibers, such as the gastrocnemius (45). Notably, in the present study, there
was no change in muscle weight of the primarily glycolytic tibialis
anterior muscle with Dex treatments, although there were minimal
changes in CSA. While not fully established in mice, Bass et
al (46) demonstrated that in
humans some muscles, such as the tibialis anterior appear to be
more atrophy-resistant compared to the gastrocnemius. This could
suggest that a larger stimulus or a longer atrophy stimulus may be
required to fully induce atrophy in the tibialis anterior compared
to the gastrocnemius.
Several studies have found n-3 PUFAs to have a
protective, anabolic and anti-catabolic effect on skeletal muscle
mass in healthy and diseased conditions (10,14-17,47,48).
By contrast, Fappi et al (4) investigated the effects of n-3s on
GC-induced muscle atrophy and reported that while Dex decreased
gastrocnemius muscle weight, concomitant n-3 supplementation did
not alter these reductions. The results obtained in the present
study are consistent with those of the study by Fappi et al
(4); indeed, Dex significantly
reduced gastrocnemius mass; however, a HFD rich in n-3s did not
attenuate muscle loss (Fig. 2). A
HFD rich in saturated fat and n-6 PUFAs has been shown to reduce
muscle mass by decreasing myofibrillar proteins via the
overactivation of the UPS, myonuclear apoptosis and oxidative
stress (44,49). It was hypothesized that n-3
supplementation alone is not sufficient to negate the effects of
both GCs and a HFD acting on the UPS and other cell signaling
mechanisms regulating protein turnover. There is increasing
evidence to indicate that n-3 supplementation may amplify anabolic
stimulation, such as post-prandial and post-exercise, and it may
help with muscle recovery (50-52).
However, research on the effects of n-3 on protein synthesis is
conflicting when coupling n-3 with atrophic stimuli, and may be the
result of a myriad of differentially regulated signaling pathways
depending on the atrophic stimulus. REDD-1, a known GC target
(53,54) and negative regulator of protein
synthesis, is also required for the proper functioning of
mitochondrial capacity and insulin sensitivity, both of which
influence protein turnover (55,56).
Moreover, REDD-1 deletion has been reported to prevent Dex-induced
muscle loss (57). The results of
the present study suggest a homeostatic role for REDD-1 and
demonstrate that Dex-induced muscle atrophy occurs independently of
REDD-1 upregulation with no effect of fatty acid composition.
The UPS and autophagic systems are activated in
catabolism and function synergistically to promote degradation. In
any condition inducing muscle atrophy, atrogenes such as MuRF-1 and
atrogin-1 are modulated by GCs, insulin/IGF-1 and myostatin-SMAD2/3
pathways, and FOXO and NF-κb transcription factors (5,37,58).
Although a HFD is one factor involved in disrupting protein
turnover processes, PUFAs have been shown to positively affect
Akt/FOXO signaling. In some muscle wasting conditions, n-3
supplementation increases the phosphorylation of FOXO, thus
decreasing atrogin-1 and MuRF-1 expression (13,18,19).
By contrast, GCs induce muscle atrophy by activating FOXO to
upregulate the transcription of MuRF-1 and atrogin-1. In the
present study, there was an effect of Dex to decrease the
phosphorylation of FOXO3a and increase myostatin, regardless of
diet, increasing atrogin-1 expression. Other studies have shown n-3
supplementation to aggravate Dex-induced atrophy by further
increasing the activation of atrogin-1 and MuRF-1 compared to Dex
alone (4,34); however, the present study found no
notable differences in mice fed a HFD high in n-3s vs. a HFD high
in n-6s with concomitant dexamethasone administration; however
additional markers of muscle atrophy need to be examined in the
future.
To the best of our knowledge, the present study is
the first to investigate the effects of a HFD rich in omega-3 vs.
omega-6 on GC-induced muscle atrophy. It was demonstrated that Dex
administration reduced gastrocnemius weight; however a HFD rich in
n-3s did not elicit any protective effects on muscle mass compared
with a HFD rich in n-6s. There was an effect of Dex to decrease the
phosphorylation of FOXO3a; however, a HFD rich in n-3s did not
attenuate this. Dex administration significantly increased
atrogin-1 expression, regardless of HFD composition or muscle type.
There was a marked effect of Dex to increase MuRF-1 and myostatin
gene expression, with no attenuation by a HFD rich in n-3s. The
present study corroborates the potentially harmful effects of GCs
on skeletal muscle. As GCs are used as a therapeutic treatment for
a variety of inflammatory, autoimmune and cancerous conditions, any
nutritional approach to negate their negative effects would be
beneficial to the affected population. However, the present study
demonstrates that omega-3 supplementation in a HFD does not protect
skeletal muscle from the detrimental effects of GC-induced atrophy.
Further studies are warranted to examine the interaction with other
metabolically active tissues. It is possible that even in the
context of a HFD, omega-3 PUFAs provide metabolic protection to
active tissues, which could still confer some protective effects
from the development of metabolic syndrome that is associated with
long term treatment with GCs.
One limitation of the present study may be the use
of a mouse model; generally, rats are more sensitive to GC-induced
muscle atrophy compared to mice. Markedly lower doses of Dex
(<0.5 mg/kg) will cause notable atrophy in a rat over a period
of 7 days vs. a higher dose of 1-3 mg/kg required to observe the
same effects in a mouse (45).
This may be the reason why Fappi et al (4) demonstrated more robust effects of Dex
on markers of protein degradation than was observed herein.
Additionally, the sensitivity of humans to GCs needs to be
examined. Another limitation of the present study is that there is
no standard dosage for Dex among studies. Dex was administered at 3
mg/kg over a 7-day period; perhaps given a longer duration, greater
effects may have been observed. Further studies are required to
consider the effect of omega-3 supplementation in a HFD with other
nutritional supplements to combat GC-induced muscle atrophy. The
present study analyzed FOXO3a as a regulator involved in the
activation of atrogenes, as well as the subsequent activation of
genes regulated by FOXO, such as MuRF-1 and atrogin. Additional
genes that are known to regulate muscle mass, such as REDD-1 and
myostatin were also included, which have GC response elements.
However, the authors were only able to examine these
transcriptionally. There is the possibility that the genes and
protein levels would differ. The inflammatory environment may play
a role in a number of the pathways examined; thus, further studies
are required to examine the inflammatory cytokine and fatty acid
profile changes in comparison to a low-fat diet control group.
In conclusion, the present study demonstrates that
the negative effects of Dex are not prevented by an n-3 high-fat
diet. The data presented herein support the detrimental effects of
Dex on muscle atrophy and report mild benefits of an n-3 high-fat
diet.
Acknowledgements
The authors would like to thank Dr Karyl Buddington,
the veterinarian at the University of Memphis (Memphis, TN, USA),
and all of the animal care staff for their assistance with this
project.
Funding
Funding: The present study was funded through the College of
Health Sciences Faculty Research Grant.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MP and KB conceptualized the present study. KB, APe,
APr, JL, DC, NW and MP were involved in data investigation and data
curation. KB was involved in the writing and preparation of the
original draft of the manuscript. NW and MP were involved in the
writing, reviewing and editing of the manuscript. MP, KB, APe and
NW were involved in visualization. All authors have read and agreed
to the published version of the manuscript. MP and KB confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The animal study protocol was approved by the
Institutional Review Board of The University of Memphis (Memphis,
TN, USA; protocol no. 0830; date of approval: October 25,
2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wolfe RR: The underappreciated role of
muscle in health and disease. Am J Clin Nutr. 84:475–482.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sandri M: Protein breakdown in muscle
wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int J
Biochem Cell Biol. 45:2121–2129. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jeromson S, Gallagher IJ, Galloway SD and
Hamilton DL: Omega-3 fatty acids and skeletal muscle health. Mar
Drugs. 13:6977–7004. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fappi A, Godoy TS, Maximino JR, Rizzato
VR, Neves Jde C, Chadi G and Zanoteli E: The effects of omega-3
fatty acid supplementation on dexamethasone-induced muscle atrophy.
Biomed Res Int. 2014(961438)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schakman O, Kalista S, Barbe C, Loumaye A
and Thissen JP: Glucocorticoid-induced skeletal muscle atrophy. Int
J Biochem Cell Biol. 45:2163–2172. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bodine SC, Latres E, Baumhueter S, Lai VK,
Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K,
et al: Identification of ubiquitin ligases required for skeletal
muscle atrophy. Science. 294:1704–1708. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Braun TP and Marks DL: The regulation of
muscle mass by endogenous glucocorticoids. Front Physiol.
6(12)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
McGlory C, Calder PC and Nunes EA: The
influence of omega-3 fatty acids on skeletal muscle protein
turnover in health, disuse, and disease. Front Nutr.
6(144)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Auvinen HE, Coomans CP, Boon MR, Romijn
JA, Biermasz NR, Meijer OC, Havekes LM, Smit JW, Rensen PC and
Pereira AM: Glucocorticoid excess induces long-lasting changes in
body composition in male C57Bl/6J mice only with high-fat diet.
Physiol Rep. 1(e00103)2013.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Gingras AA, White PJ, Chouinard PY, Julien
P, Davis TA, Dombrowski L, Couture Y, Dubreuil P, Myre A, Bergeron
K, et al: Long-chain omega-3 fatty acids regulate bovine whole-body
protein metabolism by promoting muscle insulin signalling to the
Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol. 579 (Pt
1):269–284. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kamolrat T and Gray SR: The effect of
eicosapentaenoic and docosahexaenoic acid on protein synthesis and
breakdown in murine C2C12 myotubes. Biochem Biophys Res Commun.
432:593–598. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Khal J and Tisdale MJ: Downregulation of
muscle protein degradation in sepsis by eicosapentaenoic acid
(EPA). Biochem Biophys Res Commun. 375:238–240. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Chen F, Odle J, Lin X, Zhu H, Shi
H, Hou Y and Yin J: Fish oil increases muscle protein mass and
modulates Akt/FOXO, TLR4, and NOD signaling in weanling piglets
after lipopolysaccharide challenge. J Nutr. 143:1331–1339.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Murphy RA, Mourtzakis M, Chu QS, Baracos
VE, Reiman T and Mazurak VC: Nutritional intervention with fish oil
provides a benefit over standard of care for weight and skeletal
muscle mass in patients with nonsmall cell lung cancer receiving
chemotherapy. Cancer. 117:1775–1782. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ryan AM, Reynolds JV, Healy L, Byrne M,
Moore J, Brannelly N, McHugh A, McCormack D and Flood P: Enteral
nutrition enriched with eicosapentaenoic acid (EPA) preserves lean
body mass following esophageal cancer surgery: Results of a
double-blinded randomized controlled trial. Ann Surg. 249:355–363.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Smith GI, Atherton P, Reeds DN, Mohammed
BS, Rankin D, Rennie MJ and Mittendorfer B: Dietary omega-3 fatty
acid supplementation increases the rate of muscle protein synthesis
in older adults: A randomized controlled trial. Am J Clin Nutr.
93:402–412. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smith GI, Julliand S, Reeds DN, Sinacore
DR, Klein S and Mittendorfer B: Fish oil-derived n-3 PUFA therapy
increases muscle mass and function in healthy older adults. Am J
Clin Nutr. 102:115–122. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
You JS, Park MN, Song W and Lee YS:
Dietary fish oil alleviates soleus atrophy during immobilization in
association with Akt signaling to p70s6k and E3 ubiquitin ligases
in rats. Appl Physiol Nutr Metab. 35:310–318. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Castillero E, Martin AI, Lopez-Menduina M,
Villanua MA and Lopez-Calderon A: Eicosapentaenoic acid attenuates
arthritis-induced muscle wasting acting on atrogin-1 and on
myogenic regulatory factors. Am J Physiol Regul Integr Comp
Physiol. 297:R1322–R1331. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee JM, Lee H, Kang S and Park WJ: Fatty
Acid desaturases, polyunsaturated fatty acid regulation, and
biotechnological advances. Nutrients. 8(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Murphy RA, Mourtzakis M and Mazurak VC:
n-3 polyunsaturated fatty acids: The potential role for
supplementation in cancer. Curr Opin Clin Nutr Metab Care.
15:246–251. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Beck SA, Smith KL and Tisdale MJ:
Anticachectic and antitumor effect of eicosapentaenoic acid and its
effect on protein turnover. Cancer Res. 51:6089–6093.
1991.PubMed/NCBI
|
|
23
|
Magee P, Pearson S and Allen J: The
omega-3 fatty acid, eicosapentaenoic acid (EPA), prevents the
damaging effects of tumour necrosis factor (TNF)-alpha during
murine skeletal muscle cell differentiation. Lipids Health Dis.
7(24)2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Whitehouse AS and Tisdale MJ:
Downregulation of ubiquitin-dependent proteolysis by
eicosapentaenoic acid in acute starvation. Biochem Biophys Res
Commun. 285:598–602. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Taheri M, Chilibeck PD and Cornish SM: A
brief narrative review of the underlying mechanisms whereby omega-3
fatty acids may influence skeletal muscle: From cell culture to
human interventions. Nutrients. 15(2926)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Robinson LE, Buchholz AC and Mazurak VC:
Inflammation, obesity, and fatty acid metabolism: influence of n-3
polyunsaturated fatty acids on factors contributing to metabolic
syndrome. Appl Physiol Nutr Metab. 32:1008–1024. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Ruxton CH, Reed SC, Simpson MJ and
Millington KJ: The health benefits of omega-3 polyunsaturated fatty
acids: A review of the evidence. J Hum Nutr Diet. 17:449–459.
2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Buettner R, Scholmerich J and Bollheimer
LC: High-fat diets: Modeling the metabolic disorders of human
obesity in rodents. Obesity (Silver Spring). 15:798–808.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cordain L, Eaton SB, Sebastian A, Mann N,
Lindeberg S, Watkins BA, O'Keefe JH and Brand-Miller J: Origins and
evolution of the Western diet: Health implications for the 21st
century. Am J Clin Nutr. 81:341–354. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Simopoulos AP, Leaf A and Salem N Jr:
Workshop on the essentiality of and recommended dietary intakes for
omega-6 and omega-3 fatty acids. J Am Coll Nutr. 18:487–489.
1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mark PJ, Wyrwoll CS, Zulkafli IS, Mori TA
and Waddell BJ: Rescue of glucocorticoid-programmed adipocyte
inflammation by omega-3 fatty acid supplementation in the rat.
Reprod Biol Endocrinol. 12(39)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Son W, Brown K, Persinger A, Pryke A, Lin
J, Powell Z, Wallace N, van der Merwe M and Puppa M: Effect of
omega-3 rich high-fat diet on markers of tissue lipid metabolism in
glucocorticoid-treated mice. Int J Mol Sci.
24(11492)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hill JL, Wyman JM, Godwin SM, Beech LA,
Buddington RM, Sutter TR, Ringwald-Smith K and van der Merw M:
Dietary omega-3 fatty acids reduce adiposity and alter
glucocorticoid-associated transcripts in epididymal white adipose
tissue of C57BL/6 male mice raised on a high fat diet. J Obes
Chronic Dis. 4:13–22. 2020.
|
|
34
|
Fappi A, Neves JC, Kawasaki KA, Bacelar L,
Sanches LN, P da Silva F, Larina-Neto R, Chadi G and Zanoteli E:
Omega-3 multiple effects increasing glucocorticoid-induced muscle
atrophy: autophagic, AMPK and UPS mechanisms. Physiol Rep.
7(e13966)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Savas M, Wester VL, Staufenbiel SM, Koper
JW, van den Akker ELT, Visser JA, van der Lely AJ, Penninx BWJH and
van Rossum EFC: Systematic evaluation of corticosteroid use in
obese and non-obese individuals: A multi-cohort study. Int J Med
Sci. 14:615–621. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lidster K, Owen K, Browne WJ and Prescott
MJ: Cage aggression in group-housed laboratory male mice: An
international data crowdsourcing project. Sci Rep.
9(15211)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gilson H, Schakman O, Combaret L, Lause P,
Grobet L, Attaix D, Ketelslegers JM and Thissen JP: Myostatin gene
deletion prevents glucocorticoid-induced muscle atrophy.
Endocrinology. 148:452–460. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Weber EM, Zidar J, Ewaldsson B, Askevik K,
Udén E, Svensk E and Törnqvist E: Aggression in group-housed male
mice: A systematic review. Animals (Basel). 13(143)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Baltgalvis KA, Berger FG, Pena MM, Mark
Davis J, White JP and Carson JA: Activity level, apoptosis, and
development of cachexia in Apc(Min/+) mice. J Appl Physiol (1985).
109:1155–1161. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Therdyothin A, Phiphopthatsanee N and
Isanejad M: The effect of omega-3 fatty acids on sarcopenia:
Mechanism of action and potential efficacy. Mar Drugs.
21(399)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Abrigo J, Rivera JC, Aravena J, Cabrera D,
Simon F, Ezquer F, Ezquer M and Cabello-Verrugio C: High fat
diet-induced skeletal muscle wasting is decreased by mesenchymal
stem cells administration: Implications on oxidative stress,
ubiquitin proteasome pathway activation, and myonuclear apoptosis.
Oxid Med Cell Longev. 2016(9047821)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sishi B, Loos B, Ellis B, Smith W, du Toit
EF and Engelbrecht AM: Diet-induced obesity alters signalling
pathways and induces atrophy and apoptosis in skeletal muscle in a
prediabetic rat model. Exp Physiol. 96:179–193. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bodine SC and Furlow JD: Glucocorticoids
and skeletal muscle. Adv Exp Med Biol. 872:145–176. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bass JJ, Hardy EJO, Inns TB, Wilkinson DJ,
Piasecki M, Morris RH, Spicer A, Sale C, Smith K, Atherton PJ and
Phillips BE: Atrophy resistant vs. atrophy susceptible skeletal
muscles: ‘aRaS’ as a novel experimental paradigm to study the
mechanisms of human disuse atrophy. Front Physiol.
12(653060)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Smith GI, Atherton P, Reeds DN, Mohammed
BS, Rankin D, Rennie MJ and Mittendorfer B: Omega-3 polyunsaturated
fatty acids augment the muscle protein anabolic response to
hyperinsulinaemia-hyperaminoacidaemia in healthy young and
middle-aged men and women. Clin Sci (Lond). 121:267–278.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Therdyothin A, Prokopidis K, Galli F,
Witard OC and Isanejad M: The effects of omega-3 polyunsaturated
fatty acids on muscle and whole-body protein synthesis: A
systematic review and meta-analysis. Nutr Rev. 83:e131–e143.
2025.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bhatt BA, Dube JJ, Dedousis N, Reider JA
and O'Doherty RM: Diet-induced obesity and acute hyperlipidemia
reduce IkappaBalpha levels in rat skeletal muscle in a fiber-type
dependent manner. Am J Physiol Regul Integr Comp Physiol.
290:R233–R240. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Witard OC, Banic M, Rodriguez-Sanchez N,
van Dijk M and Galloway SDR: Long-chain n-3 PUFA ingestion for the
stimulation of muscle protein synthesis in healthy older adults.
Proc Nutr Soc: Nov 21, 2023 (Epub ahead of print).
|
|
51
|
Lopez-Seoane J, Martinez-Ferran M,
Romero-Morales C and Pareja-Galeano H: N-3 PUFA as an ergogenic
supplement modulating muscle hypertrophy and strength: A systematic
review. Crit Rev Food Sci Nutr. 62:9000–9020. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lopez-Seoane J, Jimenez SL, Del Coso J and
Pareja-Galeano H: Muscle hypertrophy induced by N-3 PUFA
supplementation in absence of exercise: A systematic review of
randomized controlled trials. Crit Rev Food Sci Nutr. 63:6536–6546.
2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shimizu N, Yoshikawa N, Ito N, Maruyama T,
Suzuki Y, Takeda S, Nakae J, Tagata Y, Nishitani S, Takehana K, et
al: Crosstalk between glucocorticoid receptor and nutritional
sensor mTOR in skeletal muscle. Cell Metab. 13:170–182.
2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang H, Kubica N, Ellisen LW, Jefferson LS
and Kimball SR: Dexamethasone represses signaling through the
mammalian target of rapamycin in muscle cells by enhancing
expression of REDD1. J Biol Chem. 281:39128–39134. 2006.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Horak P, Crawford AR, Vadysirisack DD,
Nash ZM, DeYoung MP, Sgroi D and Ellisen LW: Negative feedback
control of HIF-1 through REDD1-regulated ROS suppresses
tumorigenesis. Proc Natl Acad Sci USA. 107:4675–4680.
2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Williamson DL, Li Z, Tuder RM, Feinstein
E, Kimball SR and Dungan CM: Altered nutrient response of mTORC1 as
a result of changes in REDD1 expression: Effect of obesity vs.
REDD1 deficiency. J Appl Physiol (1985). 117:246–256.
2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Britto FA, Begue G, Rossano B, Docquier A,
Vernus B, Sar C, Ferry A, Bonnieu A, Ollendorff V and Favier FB:
REDD1 deletion prevents dexamethasone-induced skeletal muscle
atrophy. Am J Physiol Endocrinol Metab. 307:E983–E993.
2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang R, Jiao H, Zhao J, Wang X and Lin H:
Glucocorticoids enhance muscle proteolysis through a
myostatin-dependent pathway at the early stage. PLoS One.
11(e0156225)2016.PubMed/NCBI View Article : Google Scholar
|