Introduction
In low-income countries around the globe, the
reliance on medicinal plants for the prevention and treatment of
several ailments is very high. Several reports have shown the
potential of some medicinal plants in the treatment of illness
linked to oxidative stress and inflammation. An imbalance between
oxidants and antioxidants, which results in stress, arises due to
the increasing formation of highly reactive radicals, which
outpaces the antioxidant protective mechanism, causing the harmful
consequences of highly reactive ions (1). Antioxidants are substances that
balance off the production of reactive oxygen species (ROS),
reducing the levels of oxidative stress (2). Superoxide (O2), peroxyl
(ROO), hydroxyl (OH), hydrogen peroxide
(H2O2) and other small molecules formed from
oxygen are examples of ROS, which are energetic and reactive small
molecules (3). Globally, the
occurrence of diabetes mellitus (DM) is increasing due to
exponential growth (4). Oxidative
stress is a key mechanism in the development of diabetic issues
(5). Any plant, including those
with edible fruits, that has chemicals in one or more of its organs
that have medicinal value or that serve as building blocks for the
creation of effective medications is considered medicinal. One such
plant with edible fruits is Mangifera indica L. (M.
indica) popularly known as mango, which belongs to the
Anacardiaceae family. It is one of the most economically
significant tropical fruit crops worldwide and a crucial
traditional crop (6). Various
parts of the mango tree, including the leaves and bark, are
traditionally used in folk medicine to treat a variety of ailments.
Mango leaves (MLs) have been reported to contain the following
minerals: Calcium, magnesium, iron, sodium, potassium, phosphorus,
nitrogen and some vitamins. Protein is a significant biomolecule
also found in mango leaves. Traditionally, ML extracts have been
used in the treatment of a variety of illnesses, including
diabetes, syphilis, renal disease, bronchitis, diarrhea, asthma,
scabies, respiratory issues and urinary disorders (7,8).
The phytochemicals found in ML extracts have the
potential to exhibit a wide range of biological and pharmacological
properties that inhibit inflammatory, tumor, allergy, oxidizing
agents and, diabetes, among others (9). MLs have been widely touted as an
effective ethnomedicine against DM. Inhibiting the enzymes
α-amylase and α-glucosidase, which control postprandial glucose
absorption, is one of the most efficient methods for treating DM
(10). According to previous
research, phenolics and flavonoids included in MLs provide them
with the ability to function as antioxidants (11). MLs contain phenolic compounds, such
as flavonoids and polyphenols. These compounds function as potent
antioxidants by scavenging free radicals, which are highly reactive
molecules that can cause cellular damage. The phenolic content in
MLs contributes significantly to their antioxidant capacity.
Specific flavonoids, such as quercetin, and carotenoids such as
β-carotene found in MLs contribute to their antioxidant properties.
These compounds have been found to be associated with various
health benefits, including antioxidant and anti-inflammatory
effects. MLs may contain enzymatic antioxidants, such as superoxide
dismutase and catalase. These enzymes play a role in the
detoxification of ROS, contributing to the overall antioxidant
defense system. The reducing power of MLs is indicative of their
ability to donate electrons and reduce oxidized molecules. ML
extracts may have antibacterial qualities against a range of
microorganisms, such as fungi and bacteria, according to several
studies. M. indica leaves contain phenolic compounds
including terpenes and terpenoids, as well as antinutrients, such
as saponins and glycosides as the main phytochemicals that have an
antibacterial effect (12).
Traditionally, mango bark (MB) extract has been used
in the treatment of a variety of illnesses, including anemia,
scabies, skin infections, diabetes, menorrhagia, diarrhea and
syphilis (13). MB extract has
been shown to exhibit antioxidant activity (14,15).
It is considered to have therapeutic qualities, such as
antibacterial and anti-inflammatory actions (16). MB extracts have been used
traditionally in medicine to treat infections, reduce inflammation
and speed up the healing of wounds. Of note, in the bid to increase
the therapeutic potency of the mango plant, the leaves and bark are
often combined locally to achieve potent health effects. To the
best of our knowledge, the present study is one of the first of its
kind to report a decline in biochemical activity as a result of the
antagonistic nature of combining the bark and leave extracts from
M. indica.
Materials and methods
Chemicals
The reagents used in the present study were of
laboratory grade for analyses. Ethanol,
2,2-diphenyl-l-picrylhydrazyl (DPPH), quercetin, gallic acid (GA),
aluminum chloride, sulphuric acid, ammonium molybdate, sodium
phosphate, 2,4,6-tris(2-pyridyl)-s-triazine,
2,2'-azinobis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS), NaOH,
3,5-dinitrosalicyclic acid reagent and others, were purchased from
MilliporeSigma.
Sample collection
M. indica (mango leaves and bark) were
collected from Moniya in Akinyele Local Government Area, Ibadan,
Oyo State, South-west Nigeria. Plant samples were identified at the
herbarium at the University of Ibadan, and a voucher number of
23335 was allocated. The collected samples were rinsed thoroughly
with pre-distilled water. The leaves and barks were air-dried at
room temperature for 21 days. The dried leaves and barks were then
ground using an electric blender and they were stored in foil paper
and sealed with a paper tape after weighing.
Test organism
For the present study, Fusarium solani was
used. The fungi were isolated from a soil sample and obtained from
the National Institute for Horticultural Research (NIHORT), Ibadan,
Oyo State, Nigeria.
Preparation of M. indica leaf and bark
extracts
Extraction was performed following the method
described in the study by Karigidi et al (17), with slight modifications. The mango
leaves and bark were pulverized using a blender and a total of 183
g mango leaf powder and 176.7 g mango bark powder were macerated
each in 1.8 liters of ethanol in various containers for 72 h with
stirring at intervals. The mixture was then filtered using a muslin
cloth to separate the ethanol extract from the solid plant
material. The filtered ethanol extract was transferred into a clean
container and the extract was concentrated by evaporating the
ethanol using a rotary evaporator (RE-52A), supplied by Scitek
Global Co., Ltd. at a reduced temperature of 40˚C to obtain the ML
and MB (MB) extracts. Equal quantities of leaf and bark extracts
were mixed to obtain the combined extract (MBML).
Preparation of stock solution
The stock solutions of ML and MB extracts were
prepared separately by dissolving 15 mg of each extract in 15 ml
ethanol (equivalent to 1 mg/ml).
Phytochemical and antioxidant
analysis. Total phenolic content (TPC)
The TPC of the ethanol extracts of M. indica
leaf and bark, independently and in combination, was obtained
spectrophotometrically (18) using
a UV Visible Spectrophotometer supplied by Scitek Global Co., Ltd.,
British standard (752N). Each extract (1 ml) was added to 1 ml of
10% Folin-Ciocalteu phenol reagent supplied by MilliporeSigma,
followed by 10 ml of 7% Na2CO3 solution
supplied by MilliporeSigma, after 3 min. Subsequently, 5 ml
pre-distilled water were added and thoroughly mixed. The product
was kept in a dark cupboard for 1 h and 30 min. The absorbance
reading was taken using UV Visible Spectrophotometer by Scitek
Global Co., Ltd. (752N) at 750 nm. TPC was evaluated using a
standard GA plot and expressed in µg of GA equivalent.
Total flavonoid content (TFC). The TFC was
obtained spectrophotometrically following the procedure described
in the study by Zhisten et al (19) and using the modification described
in the study by Talukdar (20). A
total of 1 ml of 2% ethanolic AlCl3 solution was mixed
with 1 ml of each extract. After leaving the mixture for 45 min on
a laboratory bench, the absorbance reading was taken using a UV
Visible Spectrophotometer by Scitek Global Co., Ltd. (752N) at 420
nm. The standard used was quercetin and the TFC was evaluated from
the standard plot and expressed as the quercetin equivalent in
µg/mg.
Total antioxidant capacity (TAC). The TAC of
each extract was obtained using phosphomolybdate (21) supplied by MilliporeSigma. A total
of 0.5 ml of each extract was added to 4 ml reagent (0.6 M
sulphuric acid, 28 Mm sodium phosphate and 4 mM ammonium
molybdate). The product formed was shaken together and heated
(95˚C) for 90 min. The mixtures formed with each extract were
allowed to cool and absorbance reading was taken using UV Visible
Spectrophotometer by Scitek Global Co., Ltd. (752N) at 765 nm. The
TAC value was obtained from the ascorbic acid standard plot and
expressed as its equivalent.
DPPH radical scavenging activity. This assay
was estimated following the procedure described in the study by
Gyamfi et al (22). A total
of 1 ml of the extracts (0.1-0.5 mg/ml) was added to 4.0 ml DPPH
(30 mg/l prepared in methanol). All samples were thoroughly mixed
and left for 35 min; the absorbance was then measured using UV
Visible Spectrophotometer by Scitek Global Co., Ltd. (752N) at 520
nm. Ascorbic acid (15 mg/15 ml) was the standard solution, while
the DPPH alone without extract was the control.
Ferric reducing antioxidant power (FRAP)
assay. FRAP assay was performed using a previously modified
method (23). Acetate buffer (300
mmol/l), TPTZ (10 mmol/l) and FeCl3·6H2O (20
mmol/l) were mixed at a ratio of 10:1:1 (v/v/v) to prepare the FRAP
reagent. After pre-heating at 37˚C, each of the extracts and FRAP
reagent was added to a 96-well plate and incubated in the dark at
37˚C for 10 min. The absorbance was recorded at 593 nm using an
Epoch2 microplate reader supplied by Thermo Fisher Scientific,
Inc.
ABTS free radical scavenging activity. The
ABTS mopping activity was carried out following the procedure
described in the study by Re et al (24). The stock solution was formed from a
mixture of ABTS aqueous solution (7 mM) with a 2.45 mM aqueous
solution of potassium persulfate in equal amounts; the product
obtained was made to stand in an unlit cupboard for twelve to
sixteen hours. Subsequently, ABTS product formed was added to 1 ml
of each extract at various concentrations (0.5 to 5 mg/ml). The
following step was to incubate at room temperature in a dark space
for ~10 min. For the control, 2 ml ABTS product were added to 1 ml
pre-distilled water. The absorbance reading was taken using UV
Visible Spectrophotometer by Scitek Global Co., Ltd. (752N) at 743
nm. Trolox was the standard used. The procedure was repeated to
obtain triplicate values. The percentage ABTS scavenged was
calculated as percentage inhibition: I%=[(Ao-As)/Ao] x100, where Ao
represents the absorbance value of the control and As represents
the absorbance value of the extract.
Antidiabetic activity. α-amylase
inhibition experiment
The inhibition experiment against α-amylase was
carried out using the dinitrosalicylic acid (DNS) method (25). Briefly, 1 ml of the extract (50,
100, or 200 µg/ml) and acarbose (100 µg/ml) was first incubated
with 1 ml α-amylase for 30 min prior to the addition of 1% w/v of 1
ml of starch solution. The product formed was then incubated at
37˚C for 10 min. The reaction was terminated by the addition of 1
ml DNS reagent, supplied by MilliporeSigma (12.0 g of sodium
potassium tartrate tetrahydrate in 8 ml of 2 M NaOH and 96 mM
3,5-dinitrosalicylic acid solution). This was followed by heating
in a boiling water bath for 5 min. The control was prepared without
the extract, and the blank was without α-amylase. The absorbance
was measured using UV Visible Spectrophotometer by Scitek Global
Co., Ltd. (752N) at 540 nm. Acarbose was used as a reference. The
inhibition was calculated as follows: Inhibition (%)
formula=ODC-OBC/ODC x100, where ODC represents the absorbance of
enzyme-substrate reaction with 30% DMSO serving as the control,
while ODB represents the absorbance of enzyme-substrate with plant
sample.
α-glucosidase inhibition experiment. The
α-glucosidase enzyme inhibitory activity was determined following
the method described in the study by Fouotsa et al (26). The α-glucosidase was first mixed
with 500 µg/ml extract in 100 mM phosphate-buffered saline (pH
6.8). Subsequently, 0.7 mM 4-nitrophenyl-α-D-glucopyranoside (pNPG)
supplied by MilliporeSigma in phosphate-buffered saline was added
as a substrate. This reaction mixture was incubated in a 96-well
microplate at 37˚C for 15 min. The α-glucosidase activity was
determined by measuring the p-nitrophenol release from the
hydrolysis of pNPG at 405 nm in a microplate with Gen5 software
supplied by BioTek Instruments. Acarbose was used as the standard
compound for this assay. The percentage of α-glucosidase inhibitory
activity was calculated as follows: % inhibition=Ao-At÷Ao x100,
where Ao is the absorbance of enzyme-substrate reaction with 30%
DMSO and At is the absorbance of enzyme substrate with plant
extract.
Antifungal assay
Antifungal assay of the extracts was performed using
Mueller Hinton agar (MHA) plates supplied by MilliporeSigma, by
agar well diffusion (27).
Fusarium solani was inoculated in Muller Hinton broth (MHB)
supplied by MilliporeSigma and incubated at 37˚C to adjust the
turbidity to 0.5 McFarland standards giving a final inoculum of
1.5x108 CFU/ml. The MHA plates were cultured with the
above maintained microbial inoculum. Extracts of 50 mg/ml
concentration were prepared in 50% DMSO. A total of five wells of 6
mm were bored in the cultured lawn media in a sterile cork borer (6
mm). The wells contained 50 µl plant extract each with the positive
control (tioconzole, 50 mg/ml) and negative control (50% DMSO).
Diffusion occurred for ~15 min at room temperature and incubation
for 18-24 h at 37˚C. Plates were observed to form a clear zone of
inhibition (ZoI) around the well and measurements were taken in mm
afterwards.
Determination of MIC and MBC. For the
determination of MIC and MBC, the broth microdilution technique was
used (28). For this purpose,
2-fold serial dilutions of tbe extracts were prepared directly in
sterile 96-well microdilution plates with flat bottom wells
containing MHB to obtain various concentrations. The bacterial
inoculum was added at a final concentration of 106
CFU/ml by diluting 1:100 the 0.5 McFarland turbidity culture in
MHB. Finally, 5 µl bacteria were added to the wells apart from
those of the negative control. Tioconazole was used as a positive
control. The plate was covered with a sterile lid and incubated for
24 h at 37˚C. Resazurin (0.003%) was added to each well of the
microtiter plate and was incubated at 37˚C for 3-4 h. The wells
showing bacterial growth exhibited a pink color; however, the wells
without bacterial growth remained blue. The lowest concentration of
the extract that completely inhibited bacterial growth was taken as
the MIC. The MBC was also determined with incubation for 18 h at
37˚C after streaking well content on nutrient agar plates.
Gas chromatrography mass spectroscopy
(GC-MS)
GC-MS was carried out as previously described by
Sermakkani and Thangapandian (29). Identification of the extracts was
determined by their molecular structure and the value of their
weight. The spectral peaks obtained were compared with mass spectra
database of National Institute of Standards and Technology.
Statistical analyses
Data analyses were performed using one-way analysis
of variance (ANOVA) with the least significant difference using
Tukey's post hoc test. Values are presented as the mean ± SD
(triplicate readings). A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Yield of ethanol extracts
The dry weight of M. indica leaves prior to
extraction was 183.04 g and the final weight after extraction was
84.4 g. The percentage yield of M. indica leaves was 46.15%.
The dry weight of M. indica bark was 176.71 g and the final
weight after extraction was 19.22 g. The percentage yield of M.
indica bark was 10.88%.
Antioxidant activities. TPC
The TPC present in the ethanol extracts of M.
indica leaves (ML) and bark (MB) and a mixture of Mangifera
indica leaves and bark (MBML) is illustrate din Fig. 1A. The bark extract exhibited the
highest phenolic content (0.79±0.015) followed by the leaf extract
(0.54±0.004) and the extract combination (0.56±0.002). The result
of the bark extract was significantly (P<0.05) higher than the
other extracts.
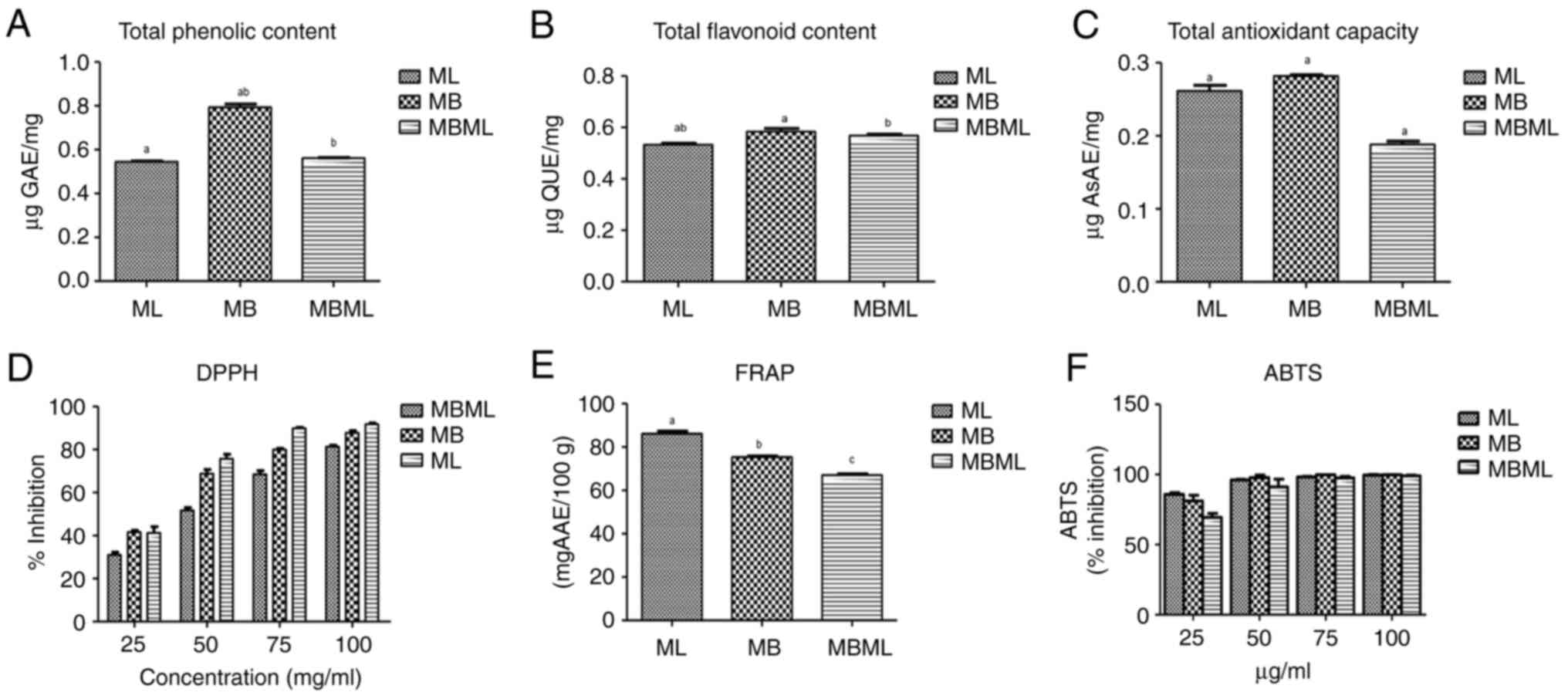 | Figure 1Phytochemicals and antioxidant
activities of ethanol extracts of Mangifera indica leaf,
bark and the combined leaf and bark extracts. (A) Total phenolic
content, (B) total flavonoid content, (C) total antioxidant
capacity, (D) DPPH scavenging activity, (E) FRAP, and (F) ABTS.
Values are presented as the mean ± standard deviation of triplicate
readings, n=3. Bars with the same superscript letter indicate
significant differences (P<0.05). ML, Mangifera indica
leaf; MB, Mangifera indica bark; MBML, Mangifera
indica leaf and bark combination; DPPH,
2,2-diphenyl-l-picrylhydrazyl; FRAP, ferric reducing antioxidant
power; ABTS, 2,2'-azinobis(3-ethylbenzothiazoline-6-sulphonic
acid. |
TFC. The TFC of the ethanol extracts ML, MB,
and MBML is presented in Fig. 1B.
The MB extract exhibited the highest flavonoid presence
(0.58±0.013), followed by MBML (0.57±0.006) and ML (0.53±0.006).
The TFC of ML was significantly lower (P<0.05) than that of MB
and MBML; the difference between MB and MBML was not
significant.
TAC. As demonstrated in Fig. 1C, the ML and MB extracts had a high
antioxidant potential. The TAC of the ethanol extract of M.
indica bark (0.28±0.002) was higher than that of the ethanol
extracts of the mixture of M. indica leaf and bark extract
(0.19±0.005) and M. indica leaf extract (0.26±0.008). The
TAC of MB and ML was significantly higher (P<0.05) than that of
MBML, while that of MB was significantly higher (P<0.05) than
that of ML
DPPH radical scavenging activity. As
demonstrated in Fig. 1D, among the
three extracts studied, ML had the highest percentage scavenging
activity at almost all concentrations (25-100 mg/ml) of the ethanol
extract, followed by MB and MBML. This assay indicated that the ML
extract exhibited the highest radical scavenging activity, followed
by the MB extract and the mixture (MBML). However, the differences
were not statistically significant (P>0.05).
FRAP. As presented in Fig. 1E, the ethanol extract of ML had the
highest reducing power, which was significantly (P<0.05) higher
than the reducing power of the other extracts. However, the FRAP of
the MB extract was higher than that of the mixture (MBML), although
the difference was not statistically different (P>0.05).
ABTS radical scavenging ability. As
illustrated in Fig. 1F, when
compared with 25 mg/ml ascorbic acid, ML had the highest
antioxidant activity, followed by MB and MBML. At 50 mg/ml, MB had
the highest scavenging ability, followed by ML, and then MBML. In
addition, at 75 mg/ml, the highest scavenging ability of MB was
slightly higher than that of ML, with MBML being the lowest. At 100
mg/ml, the three extracts were only slightly different from one
another: ML > MB > MBML. These results suggest that at 25
mg/ml, ML had the highest ability to scavenge the ABTS radical. At
50 mg/ml, MB had the highest ability to scavenge the ABTS radical
than ML; this was also observed at 75 mg/ml. At 100 mg/ml, the
three extracts had similar antioxidant activities. However, the
differences observed were not statistically different
(P>0.05).
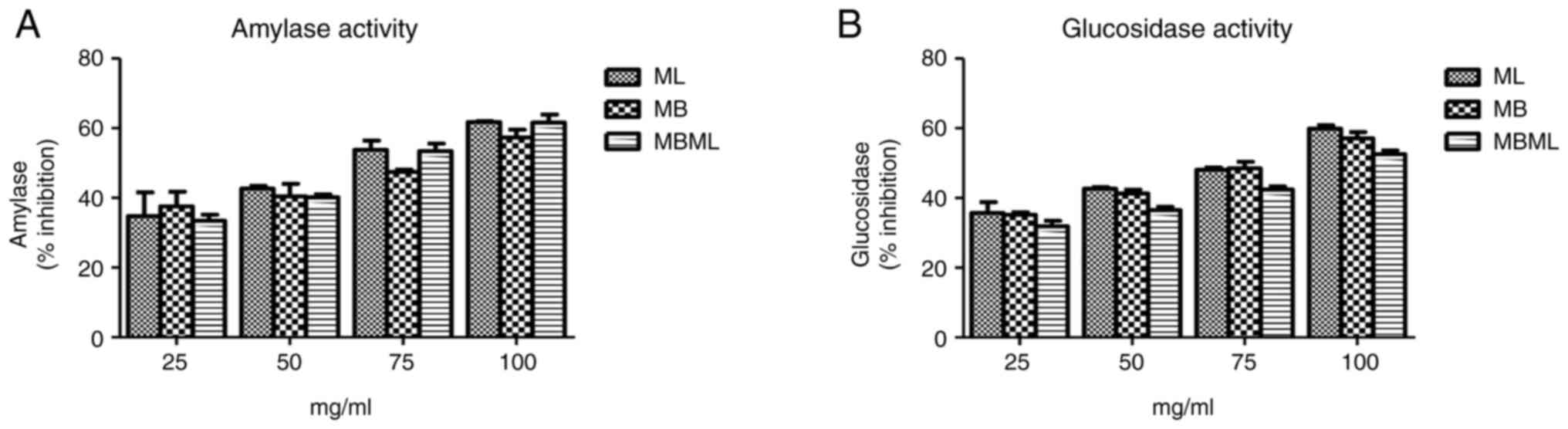
Antidiabetic activities. α-amylase
inhibitory activity
Based on the results of α-amylase assays presented
in Fig. 2A, the ethanol extracts
of MB effectively inhibited the enzyme activity compared to the
other extracts at 25 mg/ml. At 50 mg/ml, the ML extract was more
effective at inhibiting the α-amylase than the MB extract, which
was also similar to the result observed at 75 mg/ml. At 100 mg/ml,
ML was still effective at inhibiting α-amylase followed by MBML;
however, the MB extract had lost some of its inhibitory activity.
Therefore, the inhibitory activities of these extracts, ML, MB and
MBML against α-amylase vary depending on the concentration. More
so, the differences in activity observed were not statistically
significant (P>0.05).
α-glucosidase inhibition activity
The result of the assay for the α-glucosidase
inhibitory activity of the extracts is illustrated in Fig. 2B. Among the ethanol extracts, the
ML extract exhibited the most potent activity, followed by the MB
and MBML extracts, against the α-glucosidase enzyme as compared to
acarbose at 25, 50 and 100 mg/ml. At 75 mg/ml, the MB extract
exhibited the most potent activity, followed by the ML and MBML
extracts. However, these differences were not statistically
significant (P>0.05).
Antifungal activities
Clear zones of inhibition were observed on plates
with a higher concentration of the M. indica leaf extract
compared to culture plates with lower concentrations. The zone of
inhibition was measured in mm, as presented in Table I. The results indicated a
concentration-dependent inhibition, where higher concentrations of
the extracts led to larger inhibition zones. The concentrations of
the M. indica leaf extract were 0.25, 12.5, 25, 50 and 100
mg/ml, in which the inhibition ranges were 15, 18, 20, 24 and 28
mm, respectively.
 | Table IInhibition zone of Mangifera
indica leaf extract indicating the inhibition of the growth of
Fusarium solani. |
Table I
Inhibition zone of Mangifera
indica leaf extract indicating the inhibition of the growth of
Fusarium solani.
| Concentration
(mg/ml) | Fusarium
solani (mm) |
|---|
| 100 | 28 |
| 50 | 24 |
| 25 | 20 |
| 12.5 | 18 |
| 0.25 | 15 |
| DMSO | - |
| Tioconazole
(70%) | 36 |
MIC. The MIC values for Mangifera
indica leaf extract are presented in Table II. The M. indica leaf
extract inhibited the growth of Fusarium solani with MIC of
0.25 mg/l. This result demonstrated that at a concentration as low
as 0.25 mg/l, no growth was observed.
 | Table IIMinimum inhibitory concentration
(MIC) of Mangifera indica leaf extract. |
Table II
Minimum inhibitory concentration
(MIC) of Mangifera indica leaf extract.
| Concentration
(mg/ml) | Fusarium
solani |
|---|
| 100 | - |
| 50 | - |
| 25 | - |
| 12.5 | - |
| 0.25 | - |
MBC. As shown by the results presented in
Table III, the M. indica
leaf extract killed Fusarium solani at a concentration as
low as 12.5 mg/ml. Growth was only observed at 0.25 mg/ml. The
phytocompounds present in the extract may have been responsible for
the impressive bactericidal activity of the extract.
 | Table IIIMinimum bactericidal concentration of
Mangifera indica leaf extract, |
Table III
Minimum bactericidal concentration of
Mangifera indica leaf extract,
| Concentration
(mg/ml) | Fusarium
solani |
|---|
| 100 | - |
| 50 | - |
| 25 | - |
| 12.5 | - |
| 0.25 | + |
GC-MS analysis
The GC-MS analysis of the ethanol extract of M.
indica leaf, bark, and mixture of leaf and bark revealed the
presence of 16, five and six phytocompounds, respectively as
presented in Tables IV, V and VI, while the mass spectra images of the
extracts are illustrated in Fig.
3.
 | Table IVGC-MS analysis of the
phytoconstituents of ethanol extracts of Mangifera indica
leaf. |
Table IV
GC-MS analysis of the
phytoconstituents of ethanol extracts of Mangifera indica
leaf.
| Serial no. | Retention time
(min) | Compound name | Molecular
formula | Molecular
weight | Peak area (%) |
|---|
| 1 | 4.300 | Phenol |
C6H5OH | 94.1 | 9.39 |
| 2 | 6.669 | 3-Phenoxypropionic
acid |
C9H10O | 166 | 1.69 |
| 3 | 6.795 | Benzene,
ethoxy- |
C8H10O | 122.16 | 3.00 |
| 4 | 7.362 | Pyrimidine,
5-methyl- |
C5H6N2 | 94.1 | 1.12 |
| 5 | 7.488 |
3-Methylpyridazine |
C6H7N2 | 108.13 | 1.32 |
| 6 | 8.100 |
1H-Cycloprop[e]azulene, 1a,2,3,4,
4a,5,6,7b-octahydro-1,1,4,7-tetramethyl-, [1aR-(1a.alpha.,
4.alpha.,4a.beta.,7b.alpha.)]- |
C15H24 | 204.35 | 15.13 |
| 7 | 8.226 |
Bicyclo[7.2.0]undec-4-ene,
4,11,11-trimethyl-8-methylene-, [1R-(1R*, 4 Z,9S*)]- |
C15H24 | 204.35 | 4.99 |
| 8 | 8.603 | Humulene |
C15H24 | 204.35 | 1.62 |
| 9 | 8.678 | 1-Octen-3-yne |
C8H12 | 108.2 | 1.24 |
| 10 | 9.050 | Naphthalene,
1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-2-(1-methylet henyl)-,
[2R-(2.alpha., 4a.alpha.,8 a.beta.)]- |
C18H28 | 236.41 | 3.23 |
| 11 | 10.932 | Benzoic acid,
3-hydrazino-4-methyl |
C8H11ClN2O2 | 202.64 | 1.22 |
| 12 | 10.967 | 1-Propyne,
1,1'-thiobis- |
C6H6S | 114.18 | 1.39 |
| 13 | 11.945 |
5-Ethyl-2-methyl-pyridin-4-amine |
C8H12N2 | 136.19 | 1.16 |
| 14 | 11.979 | Phenol,
2-methylthioacetyl- |
C7H8OS | 140.2 | 1.28 |
| 15 | 13.347 | Benzene,
4-methyl-1,2-dinitro- |
C7H6N2O4 | 182.13 | 2.24 |
| 16 | 13.747 |
1H-Pyrrole-2-carboxylic acid,
4-formyl-3,5-dimethyl- |
C8H9NO3 | 167.16 | 1.49 |
 | Table VGC-MS analysis of the
Phytoconstituents of ethanol extracts of Mangifera indica
bark. |
Table V
GC-MS analysis of the
Phytoconstituents of ethanol extracts of Mangifera indica
bark.
| Serial no. | Retention time
(min) | Compound name | Molecular
formula | Molecular
weight | Peak area (%) |
|---|
| 1 | 5.639 | Phenol |
C6H5OH | 94.1 | 35.91 |
| 2 | 8.105 |
3-Methylpyridazine |
C6H7N2 | 108.13 | 4.49 |
| 3 | 9.295 | Naphthalene,
1,2,3,4,4a,5,6,8a-octahydro-4a,8-dimethyl-2-(1-methylethenyl)-,
[2R-(2.alpha.,4a.alpha.,8 a.beta.)]- |
C18H28 | 236.41 | 16.34 |
| 4 | 10.783 |
Borolo[1,2-a]borine, octahydro- |
C8H15B | 122.02 | 2.79 |
| 5 | 14.972 |
4H-Furo[3,2-b]pyrrole-5-carboxylic acid,
4-(2-oxopropyl)- |
C7H5NO3 | 151.12 | 3.88 |
 | Table VIGC-MS analysis of the
phytoconstituents of ethanol extracts of Mangifera indica
leaf and bark mixture. |
Table VI
GC-MS analysis of the
phytoconstituents of ethanol extracts of Mangifera indica
leaf and bark mixture.
| Serial no. | Retention time
(min) | Compound name | Molecular
formula | Molecular
weight | Peak area (%) |
|---|
| 1 | 5.679 | Phenol |
C6H5OH | 94.1 | 17.66 |
| 2 | 7.739 | Cyclobutane
carbonitrile, 3-methyl-3-phenoxy- |
C12H13NO | 187.26 | 3.39 |
| 3 | 8.109 | Aromandendrene |
C15H24 | 204.35 | 15.55 |
| 4 | 8.157 |
1H-Pyrrolo[3,4-d]pyrimidine-2,5-dione,
4,6-bis (4-hydroxyphenyl)-1-me thyl-3,4,6,7-tetrahydro- |
C19H17N3O4 | 351.12 | 2.67 |
| 5 | 8.609 | 3-Phenoxypropionic
acid |
C9H10O | 166 | 2.98 |
| 6 | 9.742 | 1-Octen-3-yne |
C8H12 | 108.2 | 2.72 |
Discussion
Plant-based diets contain phenolic chemicals, which
have been reported to be potent antioxidants. The antioxidant
capacity and antidiabetic effects of M. indica, as well as
its potential to ward off chronic illnesses, such as diabetes and
other neurological conditions, have been shown to be greatly
enhanced by phytochemicals, such as phenolic compounds (9,30),
which makes the delicious fruit-bearing plant not only nutritious,
but capable of providing medicinal functions. In the present study,
the TPC of the ethanol extract of M. indica bark
(0.79±0.015) was higher than that of the ethanol extracts of M.
indica leaves (0.54 and the mixture of M. indica leaves
and bark (0.56±0.002). However, the total phenolic content of the
ethanol extracts of the mixture of M. indica leaves and bark
was higher than that of M. indica leaves.
Phenolic components can also be grouped into several
categories, including flavonoids, which have potent antioxidant
activities and are known to be effective scavengers of most
oxidizing molecules and free radicals (31). As shown by the results of the
present study, flavonoids were present in the extracts based on the
amount of total flavonoid present in each. The flavonoid content of
the ethanol extract of M. indica bark extract (MB)
(0.58±0.013) was higher than that of the ethanol extracts of the
mixture of M. indica leaves and bark (MBML) (0.57±0.006) and
M. indica leaves (ML) (0.53±0.006). However, the flavonoid
content of the ethanol extracts of mixture of M. indica
leaves and bark was higher than that of M. indica leaves.
The TAC refers to the overall ability of the extracts to neutralize
free radicals and prevent oxidative damage. The TACy of MB
(0.28±0.002) was higher than that of ML (0.26±0.008); however, the
MBML extract had the lowest activity (0.19±0.005). This result is
supported by the findings of previous studies, suggesting that
phenolic compounds significantly contribute to the antioxidant
capacity of mango bark extracts (32,33).
DPPH is used for the evaluation of the antioxidant
activity (34). DPPH, is a free
radical donor that accepts an electron or hydrogen to become a
stable diamagnetic molecule (22).
As demonstrated in the present study, all three extracts were able
to scavenge DPPH; however, the ML extract had the highest activity,
which was followed by the MB and then MBML extracts, although the
difference was not statistically significant. This further
demonstrates the reduction in the therapeutic potential of M.
indica when the bark and the leaf extracts are combined.
FRAP assay quantifies the ability of an antioxidant
to reduce the Fe3+/tripyridyl-striazine complex. The
reducing capacity serves as a potent indicator of its antioxidant
activity. The reducing power of antioxidants is a key indicator of
potential antioxidant activity. The reductones can exert
antioxidant activity by donating a hydrogen atom and breaking the
free radical chains (35). Herein,
the reducing powers of the extracts were assessed based on their
ability to reduce Fe3+ to Fe2+ and the
results in ascorbic acid equivalent. This result indicated that the
ethanol extracts of M. indica leaf possessed the highest
antioxidant activity. However, combining ML and MB caused a decline
in their ferric reducing power.
The ABTS assay uses ABTS radicals formed by
oxidation of ABTS with potassium persulphate. ABTS radical is
soluble in water and organic solvents, which enables the
determination of the antioxidant capacity of both hydrophilic and
lipophilic compounds (36). Based
on the results of the present study, as the concentration
increased, the ability of MBML to scavenge ABTS radicals improved,
enabling it to reach similar values to those of the ML and MB
extracts. To the best of our knowledge, no published study to date
has previously compared the antioxidant activities of a mixture of
leaves and bark extract of M. indica with the individual
parts.
Compounds such as ferulic acid, resveratrol,
catechin, anthocyanins and quercetin, which are plant-based
flavonoids and phenolics, are involved in regulating glycemia
through increased glucose uptake, insulin secretion, lipid
peroxidation inhibition, and the inhibition of enzymes such as
α-amylase and α-glucosidase (37).
The similar inhibitory activity across all extracts (MB, ML and
MBML) suggests that the active compounds in ML and MB which have
comparable efficacy in inhibiting α-amylase, may be able to reduce
post-prandial blood glucose amounts and therefore be potent
candidates in the prevention or treatment of diabetes mellitus.
The increasing inhibition with concentration for
α-glucosidase inhibitory activity suggests a
concentration-dependent effect for all extracts. Research has shown
that various parts of the mango plant exhibit antidiabetic
properties due to bioactive compounds, such as mangiferin,
flavonoids and phenolic acids. The study by Aderibigbe et al
(38) indicated that MLs exerted
significant hypoglycemic activity in diabetic rats.
Previous studies have also shown that MLs possess
significant antimicrobial properties due to the presence of
bioactive compounds, such as mangiferin, quercetin and other
phenolic compounds. The findings from the present study corroborate
the findings obtained in the study by Prakash et al
(39), which demonstrated that ML
extract exhibited antimicrobial activity against various bacterial
and fungal strains, including Fusarium species, with
inhibition zones ranging from 10 to 25 mm, depending on the
concentration and the microbial strain. Fusarium solani is a
common plant pathogen; herein, the leaf extract exhibited
significant inhibition of Fusarium solani. The
microorganism, Fusarium solani, was susceptible to
tioconzole, which was used as a positive control, while the
microorganism showed resistance to DMSO, which was the negative
control. The study by Meera et al (40) also reported similar inhibition
zones for MLs against various pathogens, including Fusarium
solani. These findings support the concentration-dependent
antifungal activity observed in the present study.
Phytoconstituents are basically responsible for the
antioxidant, antidiabetic and antifungal properties of ML and MB
extracts. The GC-MS analysis of the ethanol extract of M.
indica leaves revealed several bioactive compounds, including
phenol, 3-phenoxypropionic acid, benzene, ethoxy-, pyrimidine,
5-methyl-, and 3-methylpyridazine, among others. Previous research
has consistently identified phenolic compounds in M. indica
leaf extracts. Adeyinka et al (41) highlighted the presence of phenolic
compounds, which contribute significantly to the plant's
antioxidant properties. Similarly, Amaechi et al (42) reported the identification of
phenolic compounds in their GC-MS analysis, emphasizing their role
in the bioactivity of the plant. Both studies identified various
sesquiterpenes and hydrocarbons. Adeyinka et al (41) found compounds, such as
1H-cyclopropa[e]azulene and caryophyllene, which is associated with
the sesquiterpenes identified in the present study. The GC-MS
analysis of the ethanol extract of MB also revealed several key
compounds, including phenol, 3-methylpyridazine, naphthalene,
borolo[1,2-a]borne, and 4H-furo[3,2-b]pyrrole-5-carboxylic acid. In
line with previous work by Sharma et al (43) and Singh et al (44), the presence of phenol was
significant in the bark extract. They reported the range of
phenolics contributing to the antioxidant activity and the
anti-inflammatory properties, respectively. Phenolic compounds,
known for their antioxidant properties, were consistently found in
high concentrations across different parts of the plant. The
detection of 3-methylpyridazine in the bark extract is consistent
with earlier findings in the leaf extracts, where
nitrogen-containing heterocycles such as pyrimidine were
identified. These compounds are associated with various biological
activities, including antimicrobial and anti-cancer properties,
underscoring their importance in medicinal applications. The
identification of 4H-furo[3,2-b]pyrrole-5-carboxylic acid aligns
with findings from previous studies, suggesting common biosynthetic
pathways for these metabolites within the plant. The GC-MS analysis
of a mixture of M. indica bark and leaves revealed a diverse
range of phytochemicals, including phenolic compounds,
nitrogen-containing compounds, terpenoids, hydrocarbons, esters,
and carboxylic acid derivatives. The presence of phenol, a major
constituent in both plant parts, is notable, suggesting its
significance in the plant's metabolic processes. The detection of
nitrogen-containing compounds and terpenoids/hydrocarbons across
all analyses highlights the plant's metabolic diversity. However,
there was a noticeable reduction in the number of compounds
detected in the MBML mixture compared to the single extracts of ML
and MB. This reduction in the mixture could be responsible for the
antagonistic attributes observed in the antioxidant activities and
anti-diabetic activity, especially the α-glucosidase inhibition
activity. Antagonistic interactions often results in a reduced sum
of effects of the individual compounds (45). Such a reduction in activity could
only be possible if some of the compounds present in the single
extracts are no longer available in the mixture. This deletion of
compounds may result from the complex interaction of plant
components that can be elucidated by more detailed metabolomic
techniques.
The possible mechanisms underlying the effect of
M. indica extracts as reported in the present study includes
the inhibition of α-amylase and α-glucosidase, whose increased
activities can result in the elevation of blood glucose
(hyperglycemia). It has previously been reported that some
phytochemicals, paraticularly phenols and flavonoids can limit the
release of glucose and speed up its uptake, thereby helping to
ameliorate high blood sugar in type 2 diabetes (46). More so, the antioxidant activities
observed showed that the plant extracts are capable of neutralizing
free radicals, thereby protecting against oxidative stress.
Clearly, plants compounds such as carotenoids, flavonoids and other
phenolic components present in M. indica could have been
responsible for the antioxidant, antidiabetic and antifungal
activities of the extracts.
The findings of the present study can influence
future plant-based drug development and clinical practices, as the
findings demonstrate that different parts of a plant could solely
possess favorable health-promoting potentials, but may not
necessarily be as potent when combined. Therefore, further research
is essential for combination therapy when developing drug
candidates traditionally from medicinal plants.
In conclusion, the present study investigated the
biochemical activities and phytoconstituents of the ethanol
extracts from the leaves and bark of M. indica, both
individually and in combination. The phytochemical analysis using
GC-MS revealed numerous bioactive compounds, such as phenols,
sesquiterpenes, terpenoids, fatty acids and esters. The results
demonstrated that the ethanol extract of M. indica bark (MB)
exhibited the highest total phenolic and flavonoid contents,
indicating its superior antioxidant potential, which was closely
followed by the leaf extract (ML). The mixture of leaves and bark
(MBML) had the least antioxidant activity. The antidiabetic assays
suggested a strong potential for MB and ML in regulating enzymes
linked to diabetes and revealed that both the leaf and the bark
have strong inhibitory effect on α-amylase and α-glucosidase
enzymes which are crucial in managing diabetes while the
antimicrobial tests highlighted the efficacy of the leaf extract
(ML) in inhibiting Fusarium solani. The ethanol extracts of
M. indica leaf and bark exhibit considerable antioxidant,
antidiabetics and antifungal activities, supporting their
traditional medicinal uses individually. These findings pave the
way for further pharmacological research and development of natural
therapeutics from mango leaves or its bark.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MEK designed, supervised the study and reviewed the
manuscript. OEF carried out the laboratory experiments and assays,
while KOK analyzed the results and reviewed the manuscript. All
authors have read and approved the final manuscript. MEK, KOK and
OEF confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yaribeygi H, Farrokhi FR, Rezaee R and
Sahebkar A: Oxidative stress induces renal failure: A review of
possible molecular pathways. J Cell Biochem. 119:2990–2998.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu Z, Ren Z, Zhang J, Chuang CC,
Kandaswamy E, Zhou T and Zuo L: Role of ROS and nutritional
antioxidants in human diseases. Front Physiol.
9(477)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jha JC, Banal C, Chow BS, Cooper ME and
Jandeleit-Dahm K: Diabetes and kidney disease: Role of oxidative
stress. Antioxid Redox Signal. 25:657–684. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mayer-Davis EJ, Lawrence JM, Dabelea D,
Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S,
Pettitt DJ, et al: Incidence trends of type 1 and type 2 diabetes
among youths, 2002-2012. N Engl J Med. 376:1419–1429.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yaribeygi H, Butler AE, Barreto GE and
Sahebkar A: Antioxidative potential of antidiabetic agents: A
possible protective mechanism against vascular complications in
diabetic patients. J Cell Physiol. 234:2436–2446. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Batool N, Ilyas N, Shabir S, Saeed M and
Mazhar R: Mini-review-A mini-review of therapeutic potential of
Mangifera indica L. Pak J Pharm Sci. 31:1441–1448.
2018.PubMed/NCBI
|
|
7
|
Kulkarni VM and Rathod VK: Exploring the
potential of Mangifera indica leaves extract versus
mangiferin for therapeutic application. Agric Nat Resour.
52:155–161. 2018.
|
|
8
|
Ruiz-Montañez G, Ragazzo-Sánchez JA,
Calderón-Santoyo M, Velázquez-de la Cruz G, de León JA and
Navarro-Ocaña A: Evaluation of extraction methods for preparative
scale obtention of mangiferin and lupeol from mango peels
(Mangifera indica L.). Food Chem. 159:267–272.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mirza B, Croley CR, Ahmad M, Pumarol J,
Das N, Sethi G and Bishayee A: Mango (Mangifera indica L.):
A magnificent plant with cancer preventive and anticancer
therapeutic potential. Crit Rev Food Sci Nutr. 61:2125–2151.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nair SS, Kavrekar V and Mishra A: In vitro
studies on alpha amylase and alpha glucosidase inhibitory
activities of selected plant extracts. Eur J Exp Biol. 3:128–132.
2013.
|
|
11
|
Kumar Y, Kumar V and Sangeeta :
Comparative antioxidant capacity of plant leaves and herbs with
their antioxidative potential in meat system under accelerated
oxidation conditions. J Food Meas Charact. 14:3250–3262. 2020.
|
|
12
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: A review on ethnopharmacological applications, pharmacological
activities, and bioactive compounds of Mangifera indica
(Mango). Evid Based Complement Altern Med.
2017(6949835)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scartezzini P and Speroni E: Review on
some plants of Indian traditional medicine with antioxidant
activity. J Ethnopharmacol. 71:23–43. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Martínez G, Delgado R, Pérez G, Garrido G,
Núñez Sellés AJ and León OS: Evaluation of the in vitro antioxidant
activity of Mangifera indica L. extract (Vimang). Phytother
Res. 14:424–427. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Martínez Sánchez G, Candelario-Jalil E,
Giuliani A, León OS, Sam S, Delgado R and Núñez Sellés AJ:
‘Mangifera indica L. extract (QF808) reduces
ischaemia-induced neuronal loss and oxidative damage in the gerbil
brain’. Free Radic Res. 35:465–473. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rymbai H, Srivastav M, Sharma RR, Patel CR
and Singh AK: Bio-active compounds in mango (Mangifera
indica L.) and their roles in human health and plant defence-a
review. J Hortic Sci Biotech. 88:369–379. 2013.
|
|
17
|
Karigidi ME, Olotu O, Adegoke AM, Olugbami
JO and Odunola OA: Cymbopogon citratus extracts inhibit
inflammation and oxidative damage in mice colon by downregulating
IL-6, HSP 70 and upregulating APC protein. Arch Basic Appl Med.
12:68–75. 2024.
|
|
18
|
Kim KH, Tsao R, Yang R and Cui SW:
Phenolic acid profiles and antioxidant activities of wheat bran
extracts and the effect of hydrolysis conditions. Food Chem.
95:466–473. 2006.
|
|
19
|
Zhisten J, Mengcheng T and Jianming W: The
determination of flavonoid contents in mulberry and their
scavenging effects on superoxide radicals. Food Chem. 64:555–559.
1999.
|
|
20
|
Talukdar D: Arsenic-induced oxidative
stress in the common bean legume, Phaseolus vulgaris L. seedlings
and its amelioration by exogenous nitric oxide. Physiol Mol Biol
Plants. 19:69–79. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Prieto P, Pineda M and Aguilar M:
Spectrophotometric quantitation of antioxidant capacity through the
formation of a phosphomolybdenum complex: Specific application to
the determination of vitamin E. Anal Biochem. 269:337–341.
1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gyamfi M, Yonamine M and Aniya Y:
Free-radical scavenging action of medicinal herbs from Ghana:
Thonningia sanguinea on experimentally-induced liver injuries. Gen
Pharmacol. 32:661–667. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Benzie IF and Strain JJ: The ferric
reducing ability of plasma (FRAP) as a measure of ‘antioxidant
power’: The FRAP assay. Anal Biochem. 239:70–76. 1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Re R, Pellegrini N, Proteggente A, Pannala
A, Yang M and Rice-Evans C: Antioxidant activity applying an
improved ABTS radical cation decolorization assay. Free Radic Biol
Med. 26:1231–1237. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bhutkar MA and Bhise SB: In vitro assay of
alpha amylase inhibitory activity of some indigenous plants. Int J
Chem Sci. 10:457–462. 2012.
|
|
26
|
Fouotsa HA, Lannang M, Mbazoa CD, Rasheed
S, Marasini BP, Ali Z, Devkota KP, Kengfack AE, Shaheen F,
Choudhary MI and Sewald N: Xanthones inhibitors of α-glucosidase
and glycation from Garcinia nobilis. Phytochem Lett. 5:236–239.
2012.
|
|
27
|
Balouiri M, Sadiki M and Ibnsouda SK:
Methods for in vitro evaluating antimicrobial activity: A review. J
Pharm Anal. 6:71–79. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Eloff JN: The antibacterial activity of 27
southern African members of the Combretaceae. S Afr J Sci.
95:148–152. 1999.
|
|
29
|
Sermakkani M and Thangapandian V: GC-MS
analysis of Cassia italica leaf methanol extract. Asian J Pharm
Clin Res. 5:90–94. 2012.
|
|
30
|
Rasouli H, Farzaei MH and Khodarahmi R:
Polyphenols and their benefits: A review. Int J Food Prop.
20:1700–1741. 2017.
|
|
31
|
Nunes XP, Silva FS, Guedes da Silva
Almeida JR, de Lima JT, Ribeiro LAA, Quintans Júnior LJ and Filho
JMB: Biological oxidations and antioxidant activity of natural
products. In: Rao V (ed). Phytochemicals as nutraceuticals-global
approaches to their role in nutrition and health. Intech, pp1-20,
2012.
|
|
32
|
Kim H, Moon JY, Kim H, Lee DS, Cho M, Choi
HK, Kim YS, Mosaddik A and Cho SK: Antioxidant and
antiproliferative activities of mango (Mangifera indica L.)
flesh and peel. Food Chem. 121:429–436. 2010.
|
|
33
|
Ribeiro SMR, Barbosa LCA, Queiroz JH,
Knödler M and Schieber A: Phenolic compounds and antioxidant
capacity of Brazilian mango (Mangifera indica L.) varieties.
Food Chem. 110:620–626. 2008.
|
|
34
|
Zhou X, Shang J, Wang J, Jiang B and Wang
Q: Antioxidant activity of extracts from the aril of Torreya
fargesii Franch. And its protection on the oxidation of DHA algal
oil. CyTA J Food. 16:381–389. 2018.
|
|
35
|
Liu S, Sun J, Yu L, Zhang C, Bi J, Zhu F,
Qu M and Yang Q: Antioxidant activity and phenolic compounds of
Holotrichia parallela Motschulsky extracts. Food Chem.
134:1885–1891. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Magalhães LM, Segundo MA, Reis S and Lima
JLFC: Methodological aspects about in vitro evaluation of
antioxidant properties. Anal Chim Acta. 613:1–19. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Barone E, Calabrese V and Mancuso C:
Ferulic acid and its therapeutic potential as a hormetin for
age-related diseases. Biogerontology. 10:97–108. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Aderibigbe AO, Emudianughe TS and Lawal
BA: Antihyperglycaemic effect of Mangifera indica in rat.
Phytother Res. 13:504–507. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Prakash O, Mishra A, Mishra A and Singh R:
Antimicrobial activity of Mangifera indica (mango) leaves
against bacterial and fungal pathogens. J Pharmacogn Phytochem.
3:45–49. 2011.
|
|
40
|
Meera CR, Sneha SS and Sunil CJ:
Antifungal activity of Mangifera indica leaf extracts
against Fusarium species. Int J Pharm Sci Res. 10:100–106.
2019.
|
|
41
|
Adeyinka OA, Bankole IAS and John R: GC-MS
bioprospecting of phytochemicals in the ethanolic extract of fresh
Mangifera indica leaves. J Chem Soc Nigeria.
47(798)2022.
|
|
42
|
Amaechi D, Ekpe IP, Yisa BN and Adamgbe
TF: Phytochemical profiling (GC-MS) of the leaf extract of
Mangifera indica and its hypolipidemic effect on serum lipid
profile in streptozotocin-induced diabetic Wistar rats. Asian Sci
Bull. 2:17–23. 2024.
|
|
43
|
Sharma A, Gaur V, Sharma R and Kumar N:
Chemical composition and antioxidant activities of Mangifera
indica bark extracts. J Med Plant Res. 12:154–162. 2018.
|
|
44
|
Singh R, Gupta A and Kumar A: GC-MS
analysis of Mangifera indica bark extracts for
anti-inflammatory compounds. Phytomedicine. 21:367–374. 2017.
|
|
45
|
Vaou N, Stavropoulou E, Voidarou CC,
Tsakris Z, Rozos G, Tsigalou C and Bezirtzoglou E: Interactions
between medical plant-derived bioactive compounds: Focus on
antimicrobial combination effects. Antibiotics (Basel).
11(1014)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ngo DH, Ngo DN, Vo TTN and Vo TS:
Mechanism of action of Mangifera indica leaves for
anti-diabetic activity. Sci Pharm. 87(13)2019.
|